Abstract
Background:
The discovery of somatic acquired mutations of JAK2 (V617F) in Philadelphia-negative myeloproliferative neoplasms (Ph-negative MPNs) including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) has not only improved rational disease classification and prognostication but also brings new understanding insight into the pathogenesis of diseases. Dosage effects of the JAK2 (V617F) allelic burden in Ph-negative MPNs may partially influence clinical presentation, disease progression, and treatment outcome.
Material and Methods:
Pyrosequencing was performed to detect JAK2 (V617F) and MPL (W515K/L) and capillary electrophoresis to identify CALR exon 9 mutations in 100 samples of Ph-negative MPNs (38.0 PV, 55 ET, 4 PMF, and 3 MPN-U).
Results:
The results showed somatic mutations of JAK2 (V617F) in 94.7% of PV, 74.5% of ET, 25.0% of PMF, and all MPN-U. A high proportion of JAK2 (V617F) mutant allele burden (mutational load > 50.0%) was predominantly observed in PV when compared with ET. Although a high level of JAK2 (V617F) allele burden was strongly associated with high WBC counts in both PV and ET, several hematological parameters (hemoglobin, hematocrit, and platelet count) were independent of JAK2 (V617F) mutational load. MPL (W515K/L) mutations could not be detected whereas CALR exon 9 mutations were identified in 35.7% of patients with JAK2 negative ET and 33.3% with JAK2 negative PMF.
Conclusions:
The JAK2 (V617F) allele burden may be involved in progression of MPNs. Furthermore, a high level of JAK2 (V617F) mutant allele appears strongly associated with leukocytosis in both PV and ET.
Keywords: Philadelphia-negative myeloproliferative neoplasms, JAK2 (V617F), MPL, CALR
Introduction
According to the revised 2008 WHO classification of myeloid malignancies, polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) were recognized as classical Philadelphia-negative myeloproliferative neoplasms (Ph-negative MPN). This criteria was mainly incorporating of several parameters including clinical presentations, hematological findings (e.g. white and red blood cell count, hemoglobin and hematocrit levels, and platelet number), bone marrow study, and genetic analysis (Vardiman et al., 2009; Choi et al., 2015; Ahmed et al., 2016). During the last two decades, several research groups attempted to identify genetic alterations that involve in the pathogenesis and could be the potential therapeutic target to eradicate the disease. The discovery of a unique base substitution on exon 14 of JAK2 in classical MPN in 2005 by four independent groups has improved the understanding in disease-causing mutation in Ph-negative MPNs. Additionally, this discovery has significantly improved the diagnostic criteria of MPNs. JAK2 (V617F) is frequently identified in almost PV cases (~95%) and approximately half of ET, and PMF patients (50-60%) (Baxter et al., 2005; Levine et al., 2005; Jekarl et al., 2010). However, the observed frequency of this mutation is affected by the selected MPN samples as well as the number of cases in each study. In addition, there are evidences indicating that approximately 5% of PV who were nonmutated JAK2 (V617F) harbor the mutations spanning through JAK2 exon 12 (Scott et al., 2007; Lakey et al., 2010). Furthermore, about 3–5% of JAK2-unmutated ET and 5–8% of PMF patients have point mutation on exon 10.0 of MPL (myeloproliferative leukemia virus oncogene) that encodes thrombopoietin receptor (Pardanami et al., 2006; Ahmed et al., 2016). Moreover, other variable mutations in epigenetic regulator genes such as TET2, IDH1/2, DNMT3, ASXL1, and EZH2 are occasionally found in MPNs patients with and without JAK2 and MPL mutations (Vainchenker et al., 2011). To date, there are several reports revealing frequency of JAK2 (V617F), JAK2 exon 12, and MPL (W515K/L) mutations in Ph-negative MPNs. However, there is still less clear about the molecular mechanism underlining those somatic mutations in the pathogenesis of MPNs which is necessary to further investigate.
JAK2 is a critical component of several cellular signaling pathways that involves in many processes such as proliferation, differentiation, survival, and stem cell self-renewal. Additionally, JAK2 is responsible for cellular signaling from various hematopoietic growth factors. Hence, the genetic alterations directly affected JAK2 encoding sequence or related JAK2 proteins (e.g. MPL receptor) may allow or introduce mutated hematopoietic stem cell hypersensitive to several cytokines which involve in hematopoiesis [(thrombopoietin (TPO), erythropoietin (EPO), insulin-like growth factor 1 (IGF1), stem cell factors (SCF) and granulocyte colony-stimulating factors (GCSF)] (Rampal et al., 2014). In consequence, pre-leukemic clone with mutated JAK2 were originated and subsequently fully transformed into malignant cells by accumulating of additionally genetic events.
Recently, the genetic alterations in exon 9 of calreticulin (CALR) encoding gene have been identified in ET (67%) and PMF (88%) patients who are negative for JAK2 and MPL (Klampfl et al., 2013; Nangalia et al., 2013). The C-terminus of CALR contains Ca2+ binding property which involves in several cellular mechanisms such as Ca2+ homeostasis, immune response to tumor, cell-cell interaction, phagocytosis, and cellular signaling (Tefferi et al., 2014b). Common mutations of CALR are frameshift mutations by deletion or insertion of nucleotide sequences on exon 9 resulting in the production of abnormal CALR protein. There are two types of CALR mutations frequently observed in patients including; type 1 (L367fs*46) which caused by 52 base pairs deletion (50% of CALR mutated cases) and (K385fs*47) which caused by 5 base pairs TTGTC insertion (30% of CALR mutated cases). Interestingly, recent studies revealed that CALR mutations were associated with lower hemoglobin level, lower leukocyte count, higher platelet count, lower incident of thrombosis, and better survival in ET as well as in PMF (Klampfl et al., 2013). These suggest the potential applications of the detection of CALR mutations for differential diagnosis of MPN as well as the establishment of laboratory guideline for risk-assessment of Ph-negative MPNs. The study of clinicohematological data and risk stratification in Pakistan patients showed that ET was observed in a relatively young population comparing to the West patients and risk stratification revealed predominance of high risk disease (Sultan and Irfan, 2015a). In this work, we performed pyrosequencing technique to determine the mutational statuses and mutated allele burden of JAK2 (V617F) and MPL (W515K/L) as well as capillary gel electrophoresis to detect frameshift mutations in exon 9 of CALR in 100 MPN patients. Moreover, we further investigated the impact of JAK2 (V617F) mutant allele burden on disease phenotypes, patient individuals, clinical and hematological characteristics.
Materials and Methods
Patient Samples
A hundred genomic DNA (gDNA) samples isolated from blood or bone marrow of newly diagnosed Ph-negative MPN patients were collected from 2012 to 2015 at the Human Genetic Laboratory, Department of Pathology, Ramathibodi Hospital, Bangkok, Thailand. DNA isolation were performed by using QIAamp DNA Blood mini kit (Qiagen, Germany) and subsequently quantified by the Nanodrop 2000 spectrophotometer (Thermo Scientific, USA). Patients’ data including medical history, clinical manifestation, and hematological finding (e.g. leukocyte, red blood cell, and platelet counts, hemoglobin and hematocrit levels) were recorded. The disease classification and inclusion criteria were based on the revised 2008 WHO classification of myeloid neoplasms and acute leukemia (Vardiman et al., 200). All samples were routinely analyzed JAK2 (V617F) statuses using allele specific polymerase chain reaction (AS-PCR) according to previously published protocol (Jones et al., 2005). This work was approved by ethic committee on human right related to research involving human subjects, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Thailand and performed according to the principles of the Declaration of Helsinki (ID 06-58-58).
Pyrosequencing analysis of JAK2 (V617F) and MPL (W515K/L) allele burden
Specific PCR reactions for both JAK2 (V617F) and MPL (W515K/L) mutations were performed using specific oligonucleotide primes as described in Table 1.0. The optimal PCR reaction of both JAK2 (V617F) and MPL (W515K/L) were performed in a 25 μl total volume of PyroMark PCR kit (QIAGEN) with final concentrations of 1X PyroMark PCR Mastermix, 1x CollaLoad Concentrate, 0.2 μM of each primer and 40 ng of gDNA. The amplification was carried out in Veriti® Thermal Cycler (Applied Biosystems, Foster City, CA). For detection of JAK2 (V617F), the optimal PCR condition was following; initial PCR activation at 94°C for 7 minutes, 50 cycles of denaturing at 94 °C for 30 seconds, annealing at 58°C for 30 seconds and extension at 72°C for 30 seconds and final extension at 72°C for 7 minutes, respectively. For detection of MPL (W515K/L), the optimal PCR condition was following; initial PCR activation at 95°C for 10 minutes, 40 cycles of denaturing at 95 °C for 20 seconds, annealing at 59°C for 30 seconds and extension at 72°C for 30 seconds and final extension at 72°C for 5 minutes, respectively. PCR products were subsequently detected with 2 % agarose gel electrophoresis prior to pyrosequencing analysis. DNA templates (single stranded) were immobilised on the streptavidin-coated Sepharose high-performance beads (GE Health care, Sweden) using the PyroMark Q24 Vacuum Workstation (QIAGEN) as the manufacturer instruction. Pyrosequencing reactions with specific biotinylated sequencing primers (Table 1) were performed using the PyroGold Reagents (Biotage) and worked on the Pyromark Q24 instrument (Biotage) according to the manufacturer instruction. Pyrogram outputs were analyzed by using PyroMark Q24 software (Biotage). The percentage of mutant versus wild type alleles (percent of mutant allele burden) were analyzed using allele quantification (AQ) software.
Table 1.
Primer sets for PCR Amplification, Pyrosequencing, and Fragment Analysis
| Mutation | Primer name | Primer sequence (5’-3’) | Amplicon length (bp) |
|---|---|---|---|
| JAK2 (V617F) | JAK2-forward | TATGATGAGCAAGCTTTCTCACAAG | 102 |
| JAK2-reverse | biotin-AGAAAGGCATTAGAAAGCCTGTAGTT | ||
| JAK2-sequencing | GGTTTTAAATTATGGAGTATGT | ||
| MPL (W515K/L) | MPL-forward | CCGCTCTGCATCTAGTGCT | 79.0 |
| MPL-reverse | biotin-CTGTAGTGTGCAGGAAACTG | ||
| MPL-sequencing | TGCTGCTGCTGAGGT | ||
| CALR exon 9 | CALR-forward | 6-FAM-GGCAAGGCCCTGAGGTGT | - |
| CALR-reverse | GGCCTCAGTCCAGCCCTG |
PCR fragment analysis for detection of CALR exon 9 Mutations
PCR fragment analysis according to previous publication (Klampfl et al., 2013) was performed to determine CALR exon 9 frame-shift mutation status on gDNA samples isolated from patients who had no JAK2 (V617F) mutation. The PCR primer details were described in Table 1. Amplification was conducted on Veriti® Thermal Cycler (Applied Biosystems) with following hot start at 95°C, for 10 min, 10 cycles of 94°C for 15 seconds, 55°C for 15 seconds, 72°C for 30 seconds, 20 cycles of 89 °C for 15 seconds, 55°C for 15 seconds, 72°C for 30 seconds and final extension at 72°C for 20 minutes, respectively. PCR products were diluted to 1:25 with sterile water and sized on 3130xl Genetic Analyzer (Applied Biosystem, USA). The results were analyzed using Gene Mapper software version 4 (Applied Biosystems).
Statistical analysis
The analysis of obtained data was analyzed using SPSS version 16 software (SPSS Inc., USA). The comparison of demographic data and laboratory findings among groups was performed using the Mann-Whitney U test (2 groups) or Kruskal-Wallis test (>2 groups). Chi-squared test was used for comparison of sex and JAK2 (V617F) mutational status among disease subtypes. The p-value of less than 0.05 was considered to indicate statistical significance (all tests were two-tailed).
Results
The establishment of pyrosequencing technique for the detection of JAK2 (V617F) allele burden. We performed the highly sensitive and specific pyrosequencing method to analyse the level of JAK2 (V617F) allele burden in DNA samples extracted from Ph-negative MPN patients. To determine the minimal threshold (limitation) of the established pyrosequencing method, we prepared serial dilution of gDNA of homozygous JAK2 (V617F) mutation in gDNA of homozygous JAK2 (V617F) wild type (0-100% of mutant in wild type DNA) and subsequently determined JAK2 (V617F) allele burden using established protocol. The assay was able to detect a minimum of 5% mutant in wild type DNA (R2 = 0.9959) (Figure 1). The result indicated that the established method had very high sensitivity, specificity, and was suitable for detection of tiny mutant JAK2 (V617F) cells in wild type population.
Figure 1.
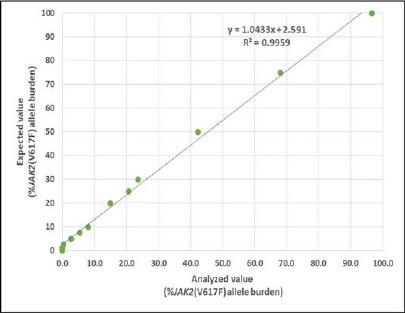
Linear Relationship Between Percentages of Expected and Analyzed Level of JAK2 (V617F) Mutant Allele Burden by the Established Pyrosequencing Technique (R2 = 0.9959)
JAK2 (V617F) allele burden and patient demographic data and clinical findings
In this report, we re-analyzed the data from the routine laboratory service for detection of JAK2 (V617F) by AS-PCR from 2012-2015. We observed that the prevalence of JAK2 (V617F) was 94.7% (36/38) in PV, 74.5% (41/55) in ET, 25% (1/4) in PMF, and 100% (3/3) in MPN-U (Table 2). Furthermore, we analyzed demographic data and laboratory findings of individual patients such as sex, age, WBC counts, hemoglobin and hematocrit level (Table 3). There was no significant difference of sex and age between PV and ET patients (p=0.078 and 0.829, respectively). Additionally, the majority of blood parameters including WBC counts, hemoglobin, and hematocrit of PV (14.9±1.1 x103/±L, 17.6±0.5 g/dL, and 55.1±1.5%, respectively) were significantly higher than ET group (11.7±0.8 x103/±L; p<0.018; 12.3±0.3 g/dL; p<0.005 and 37.7±0.8%; p<0.005). Whereas, an average number of platelets was significantly lower in PV than ET group (471.8±35.5 x103/±L and 793.3±37.6 x103/±L; p<0.005). Interestingly, an average level (percentage) of JAK2 (V617F) mutant allele burden in PV was significantly higher than in ET (63.0±4.5% in PV and 25.1±2.9 % in ET, p<0.005) (Figure 2).
Table 2.
Demographic, Clinical, and Laboratory Features of Ph-Negative MPNs.
| Characteristics | PV | ET | PMF* | MPN-U* |
|---|---|---|---|---|
| Number of patient, N (%) | 38.0 (38.0%) | 55.0 (55.0%) | 4.0 (4.0%) | 3.0 (3.0%) |
| Frequency of JAK2 (V617F) positive, N (%) | 36.0 (94.7%) | 41.0 (74.5%) | 1.0 (25.0%) | 4.0 (100.0%) |
| Frequency of CALR exon 9 positive, N (%) | N/A | 5.0 (35.7%)* | 1.0 (33.3%)* | N/A |
| Frequency of MPL (W515K/L) positive, N (%) | N/A | 0.0 | 0.0 | N/A |
| Age (years) Mean (±SD) | 62.9 (±2.2) | 62.2 (±2.5) | 65.0 (47.0-77.8) | 76.0(73.0-82.0) |
| Male (% of total) | 23.0 (60.5%) | 23.0 (41.8%) | 2.0(50.0%) | 2.0 (66.7%) |
| WBC count (x103/µL) Mean (±SD) | 14.9 (±1.1) | 11.7 (±0.8) | 13.6 (5.2-21.1) | 57.6 (33.0-101.9) |
| Hb (g/dL) Mean (±SD) | 17.6 (±0.5) | 12.3 (±0.3) | 9.8 (6.2-12.0) | 10.6 (7.0-11.5) |
| Hct (%) Mean (±SD) | 55.1 (±1.5) | 37.7 (±0.8) | 31.2 (19.2-36.8) | 33.9 (22.4-35.4) |
| Platelet count (x103/µL) Mean (±SD) | 471.8 (±35.5) | 793.3 (±37.6) | 471.5 (147.0-814.0) | 338.0 (105.0-453.0) |
| JAK2 (V617F) allele burden (%) Mean (±SD) | 63.0 (±4.5) | 25.1 (±2.9) | 2.5 (0.25-5.3) | 50.0 (46.0-97.0) |
| Thrombotic events, N (%) | 7.0 (18.4%) | 15.0 (27.3%) | 2.0 (50.0%) | 2.0 (66.7%) |
| Bleeding events, N (%) | 1.0 (2.6%) | 5.0 (9.1%) | 1.0 (25.0%) | 0.0 |
| Splenomegaly, N (%) | 2 (5.3%) | 2.0 (3.6%) | 3.0 (75.0%) | 0.0 |
| History of MF transformation, N (%) | 0.0 | 4.0 (7.3%) | 4.0 (100.0%) | 0.0 |
N/A, Not analyzed;
Data of PMF and MPN-U group were revealed by Median (Range) values
Table 3.
Comparison of Clinicohematologic Characteristics of PV and ET Patients.
| Characteristics | PV | ET | p value |
|---|---|---|---|
| Age (years) Mean (±SD) | 62.9 (±2.2) | 62.2 (±2.5) | 0.829 |
| Male (% of total) | 23.0 (60.5%) | 23 (41.8%) | 0.078 |
| WBC count (x103/µL) Mean (±SD) | 14.9 (±1.1) | 11.7 (±0.8) | 0.018 |
| Hb (g/dL) Mean (±SD) | 17.6 (±0.5) | 12.3 (±0.3) | < 0.005 |
| Hct (%) Mean (±SD) | 55.1 (±1.5) | 37.7 (±0.8) | < 0.005 |
| Platelet count (x103/µL) Mean (±SD) | 471.8 (±35.5) | 793.3 (±37.6) | < 0.005 |
| JAK2 (V617F) allele burden (%) Mean (±SD) | 63.0 (±4.5) | 25.1 (±2.9) | < 0.005 |
Figure 2.
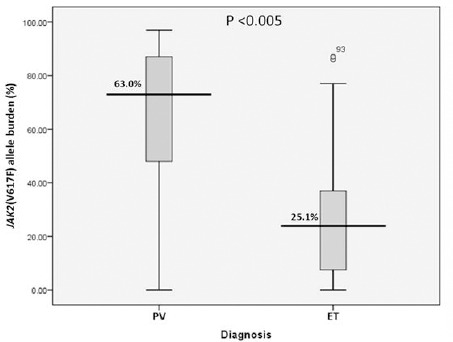
The Comparison of Mean Level (Percentage) of JAK2 (V617F) Mutant Allele Burden Between PV and ET Patients
To further intensely investigate the impact of JAK2 (V617F) mutant allele burden and disease phenotypes, we divided PV and ET patients into 3 groups according to the level of JAK2 (V617F) mutant allele burden; wild type (allele burden <5%), low (allele burden 5-50%), and high (allele burden >50%), respectively and subsequently compared with hematological parameters. We observed that the distribution of high mutant allele burden was frequently observed in PV (30.1%) more than ET (4.3%) (p<0.001) (Figure 3). Nevertheless, low level of JAK2 (V617F) and wild type allele were predominantly identified in ET when compared with PV (Table 3) (p<0.005). While the number of WBC counts was strongly associated with high level of JAK2 (V617F) in both PV and ET groups (p=0.013 and 0.032, respectively), other hematological parameters were independent from JAK2 (V617F) allele burden status; hemoglobin (p = 0.750 in PV and p = 0.215 in ET), hematocrit (p = 0.521 in PV and p = 0.205 in ET), and platelets (p = 0.116 in PV and p = 0.315 in ET) (Data not shown). To gather, we demonstrated the distribution of JAK2 (V617F) in Ph-negative MPN patients and studied the impact of JAK2 (V617F) allele burden on several hematological parameters.
Figure 3.
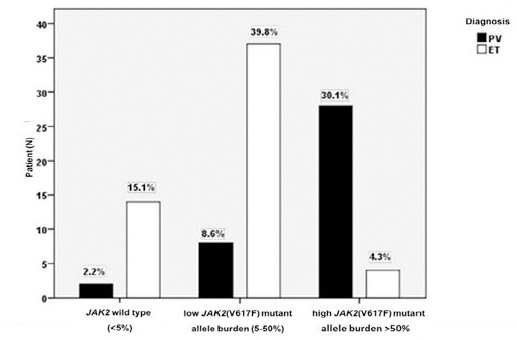
The Distribution of JAK2 (V617F) Allele Burden Levels in PV and ET Patients
The distribution of MPL (W515K/L) and CALR exon 9 mutations
To further investigate the distribution of other common somatic mutations in Ph-negative MPNs, we performed pyrosequencing technique for detection of MPL (W515K/L) and capillary electrophoresis (fragment analysis) to analyze CALR exon 9 mutations on gDNA samples of JAK2 (V617F)-nonmutated MPN (ET n = 14 and PMF n = 3). Although we could not detect MPL (W515K/L) mutation in this study, CALR exon 9.0 mutations were identified in 5.0 of 14.0 (35.7%) of nonmutated JAK2 (V617F) ET and in 1.0 of 3.0 (33.3%) of nonmutated JAK2 (V617F) PMF patients (Table 2). Additionally, all of positive CALR mutations were the most common type 1 CALR mutation with 52-bp deletion (c.1092_1143del; L367fs*46). In further analysis of ET patients with CALR mutations, we found that there was no significant difference in sex, WBC counts, hemoglobin and hematocrit levels, and platelet counts among ET patients with JAK2 (V617F) and CALR wild type, JAK2 (V617F) mutation, and CALR mutation (sex p = 0.4, WBC p = 0.2, Hb p = 0.1, Hct p = 0.1, platelet p = 0.1, respectively). Nevertheless, the age of ET patients with CALR mutation was significantly higher than JAK2/CALR wild type (p = 0.006) and JAK2 (V617F) positive ET patients (p = 0.048) (Figure 4). To gather, we demonstrated the distribution and analyzed common hematological parameters in CALR exon 9 positive ET and PMF patients who were negative for JAK2 (V617F).
Figure 4.
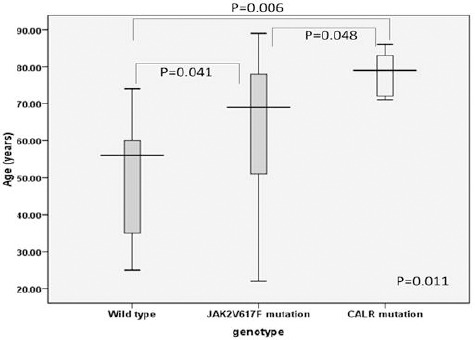
The Comparison of Mean Age in ET Patients with JAK2/CALR Wild Type, JAK2 (V617F), and CALR Mutations
Discussion
During the two decades, causative genetic mutations in hematological malignancy have been discovered and well-defined their molecular mechanisms in the pathogenesis of the disease. Recently, the revision on WHO classification of myeloproliferative neoplasm and acute leukemia (Arber et al., 2016) and the previous well-recognized version (the 2008 criteria) (Vardiman et al., 2009) have included several genetic mutations into the major criteria for the classification of particular diseases such as the present of somatic mutations on JAK2, CALR, and MPL in Ph-negative MPNs. In addition, some of those mutations become prognostic and monitoring markers and could be potential therapeutic targets for the treatment. Therefore, the establishment and standardization of molecular genetic testing to detect those mutations are necessary.
We attempted to set up pyrosequencing technique which is a very high robust, sensitive, and specific method to detect the level of JAK2 (V617F) and MPL (W515K/L) mutations as well as PCR fragment analysis for identification of frameshift mutation on CALR exon 9 in Ph-negative MPNs (PV, ET, and PMF). For the detection of JAK2 (V617F) allele burden level, the established pyrosequencing assay was able to detect the minimum of 5% mutant allele in wild type which was similar to the previous reports using the same technique (Jelinek et al., 2005; Jones et al., 2005). While pyrosequencing method requires two rounds of PCR conditions and needs a special analysis software, more sensitive real-time PCR technique (the sensitivity is less than 5% mutant: wild type) (Huijsmans et al., 2011) could complete the analysis within a single PCR reaction. Nevertheless, real-time PCR is necessary to have two different probes that are able to discriminate mutant and wild type alleles (easy to get a false positive result by real-time PCR). Another advantage of using pyrosequencing for detection of causative genetic mutations in several cancers and genetic diseases is that the test is not expensive compared to other reliable methods such as real-time PCR, SnapShot, and Sanger sequencing. Therefore, pyrosequencing technique becomes more well-established in several molecular diagnosis laboratories. For detection of CALR exon 9 frameshift mutations, we were able to perform PCR fragment analysis (capillary gel electrophoresis) by following the published protocol (Klampfl et al., 2013). While this protocol was able to detect almost common mutations on CALR exon 9 coding sequence, other mutations spanning outside/nearby exon 9 of CALR need to be investigated to better understand the molecular pathology of MPNs driving by CALR mutation. Furthermore, in particular mutation patterns (e.g., new mutations, ambiguous pattern), subsequent experiments such as target sequencing are very important.
We further reported the distribution of JAK2 (V617F), CALR, and MPL (W515K/L) mutations in Ph-negative MPNs. We found that the frequency of JAK2 (V617F) mutation was 94.7% in PV, 74.5% in ET, 25.0% in PMF. The result was similar to previously published articles in which JAK2 (V617F) is commonly identified in PV (approximately to 80.0-95.0%) and ranged from about 20% to 70% in ET and PMF depending on the tested samples (Baxter et al., 2005; Jones et al., 2005; Sultan and Irfan et al., 2005a; Sultan and Irfan, 2015b; Kim et al, 2010; Sultan et al., 2016). Interestingly, we observed that JAK2 (V617F) mutation is predominantly positive in MPN-U patients (3 of 3). Similar to PV, ET and PMF, the observed frequencies of JAK2 (V617F) in MPN-U were different in several research groups such as 14.3 % from Jekarl et al (2010), 54% from Duletic et al (2012), and 77.5% from Lin et al (2015), respectively. This may also be affected by the number and selection of tested samples. For the analysis of CALR exon 9 frameshift mutations, we could identify 35.7% and 33.3% of CALR mutations in nonmutated JAK2 (V617F) ET and PMF, respectively. Our findings were similar to previouly publications that CALR mutations are predominantly identified in ET and PMF but not positive in patients with PV (Klampal et al., 2013; Rumi et al., 2014; Kim et al., 2015a; Grinsztejn et al., 2016). The result indicated that CALR represents as a potential genetic marker for the differential diagnosis of ET and PMF from other MPNs. While several studies could identify genetic mutations of MPL (W515L/K) in Ph-negative MPNs (3-5%) (Pardanami et al., 2006; Ruan et al. 2010), we were not able to detect MPL (W515L/K) mutation in this study.
Prospective on the level of JAK2 (V617F) mutant allele burden in MPN patients, we demonstrated that overall patients with PV have higher level of JAK2 mutant allele than in ET which was similar to previous reports (Larsen et al., 2007; Ha et al., 2012). Furthermore, in PV patients with high JAK2 mutation load (>50%) exhibited high hemoglobin level, white blood cell count, lower platelet level, and increase a chance of thrombosis. Interestingly, elevation of platelet level was correlated with high JAK2 mutation load in ET. These results indicated the clinical significance of monitoring JAK2 mutation level for disease classification and prognosis. Similar to several studies, ET patients with CALR mutations result in predominantly increasing in platelet level (Rumi et al., 2014; Tefferi et al., 2014; Kim et al., 2015a; Kim et al., 2015b;). While hemoglobin, hematocrit levels, leukocyte count, and chance of thrombosis did not significantly change in those patients.
In conclusion, recent revised WHO classification of MPN requires both phenotypic and genetic data for diagnosis and classification of the disease. Here, we established a routine pyrosequencing technique to measure mutation levels of both JAK2 (V617F) and MPL (W515K/L) in Ph-negative MPNs. Additionally, we performed fragment gel electrophoresis to analyze frameshift mutations of CALR in ET and PMF patients. Mutation spanning of JAK2 (V617F), MPL (W515K/L), and CALR (those three so-called triple markers) were reported in Thai patients with MPNs. Finally, mutation level of JAK2 (V617F) and CALR mutation patterns were analyzed and correlated with patient’s hematological data.
Acknowledgements
We thank Dr. Suporn Chuncharunee and staffs at Division of Hematology, Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University for their valuable helps especially for the ALL samples and clinical data. The authors would like to thank Ramathibodi Cancer Center for the support of reagents and chemicals.
References
- Ahmed RZ, Rashid M, Ahmed N, Nadeem M, Shamsi TS. Coexisting JAK2V617F and CALR exon 9 mutations in myeloproliferative neoplasms - Do they designate a new subtype? Asian Pac J Cancer Prev. 2016;17:923–6. doi: 10.7314/apjcp.2016.17.3.923. [DOI] [PubMed] [Google Scholar]
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- Choi CW, Bang SM, Jang S, et al. Guidelines for the management of myeloproliferative neoplasms. Korean J Intern Med. 2015;30:771–88. doi: 10.3904/kjim.2015.30.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duletić AN, Dekanic A, Hadzisejdić I, et al. JAK2-v617F mutation is associated with clinical and laboratory features of myeloproliferative neoplasms. Coll Antropol. 2012;36:859–65. [PubMed] [Google Scholar]
- Grinsztejn E, Percy MJ, McClenaghan D, et al. The prevalence of CALR mutations in a cohort of patients with myeloproliferative neoplasms. Int J Lab Hematol. 2016;38:102–6. doi: 10.1111/ijlh.12447. [DOI] [PubMed] [Google Scholar]
- Ha JS, Kim YK, Jung SI, et al. Correlations between Janus kinase 2 V617F allele burdens and clinicohematologic parameters in myeloproliferative neoplasms. Ann Lab Med. 2012;32:385–91. doi: 10.3343/alm.2012.32.6.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijsmans CJ, Poodt J, Savelkoul PH, et al. Sensitive detection and quantification of the JAK2V617F allele by real-time PCR blocking wild-type amplification by using a peptide nucleic acid oligonucleotide. J Mol Diagn. 2011;13:558–64. doi: 10.1016/j.jmoldx.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekarl DW, Han SB, Kim M, et al. JAK2 V617F mutation in myelodysplastic syndrome, myelodysplastic syndrome/myeloproliferative neoplasm, unclassifiable, refractory anemia with ring sideroblasts with thrombocytosis, and acute myeloid leukemia. Korean J Hematol. 2010;45:46–50. doi: 10.5045/kjh.2010.45.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek J, Oki Y, Gharibyan V, et al. JAK2 mutation. 1849G>T is rare in acute leukemias but can be found in CMML, Philadelphia chromosome-negative CML, and megakaryocytic leukemia. Blood. 2005;106:3370–3. doi: 10.1182/blood-2005-05-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AV, Kreil S, Zoi K, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106:2162–8. doi: 10.1182/blood-2005-03-1320. [DOI] [PubMed] [Google Scholar]
- Kim JT, Cho YG, Choi SI, et al. JAK2 V617F and exon 12 geneticvariations in Korean patients with BCR/ABL1-negative myeloproliferative neoplasms. Korean J Lab Med. 2010;30:567–74. doi: 10.3343/kjlm.2010.30.6.567. [DOI] [PubMed] [Google Scholar]
- Kim BH, Cho YU, Bae MH, et al. JAK2 V617F, MPL, and CALR mutations in Korean patients with essential thrombocythemia and primary myelofibrosis. J Korean Med Sci. 2015a;30:882–8. doi: 10.3346/jkms.2015.30.7.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Im K, Park SN, et al. CALR, JAK2, and MPL mutation profiles in patients with four different subtypes of myeloproliferative neoplasms: primary myelofibrosis, essential thrombocythemia, polycythemia vera, and myeloproliferative neoplasm, unclassifiable. Am J Clin Pathol. 2015b;143:635–44. doi: 10.1309/AJCPUAAC16LIWZMM. [DOI] [PubMed] [Google Scholar]
- Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–90. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- Lakey MA, Pardonani A, Hoyer JD, et al. Bone marrow morphologic features in polycythemia vera with JAK2 exon 12 mutations. Am J Clin Pathol. 2010;133:942–8. doi: 10.1309/AJCP3Z2AKUWRGTNM. [DOI] [PubMed] [Google Scholar]
- Larsen TS, Pallisgaard N, Møller MB, Hasselbalch HC. The JAK2 V617F allele burden in essential thrombocythemia, polycythemia vera and primary myelofibrosis--impact on disease phenotype. Eur J Haematol. 2007;79:508–15. doi: 10.1111/j.1600-0609.2007.00960.x. [DOI] [PubMed] [Google Scholar]
- Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–97. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Lin Y, Liu E, Sun Q, et al. The prevalence of JAK2, MPL, and CALR mutations in Chinese patients with BCR-ABL1-negative myeloproliferative neoplasms. Am J Clin Pathol. 2015;144:165–71. doi: 10.1309/AJCPALP51XDIXDDV. [DOI] [PubMed] [Google Scholar]
- Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369:2391–405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108:3472–6. doi: 10.1182/blood-2006-04-018879. [DOI] [PubMed] [Google Scholar]
- Rampal R, Al-Shahrour F, Abdel-Wahab O, et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. 2014;123:123–33. doi: 10.1182/blood-2014-02-554634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan GR, Jiang B, Li LD, et al. MPL W515L/K mutations in 343 Chinese adults with JAK2V617F mutation-negative chronic myeloproliferative disorders detected by a newly developed RQ-PCR based on TaqMan MGB probes. Hematol Oncol. 2010;28:33–9. doi: 10.1002/hon.899. [DOI] [PubMed] [Google Scholar]
- Rumi E, Harutyunyan AS, Pietra D, et al. CALR exon 9 mutations are somatically acquired events in familial cases of essential thrombocythemia or primary myelofibrosis. Blood. 2014;123:2416–9. doi: 10.1182/blood-2014-01-550434. [DOI] [PubMed] [Google Scholar]
- Sultan S, Irfan SM. Acquired JAK-2 V617F mutational analysis in Pakistani patients with essential thrombocythemia. Asian Pac J Cancer Prev. 2015a;16:7327–30. doi: 10.7314/apjcp.2015.16.16.7327. [DOI] [PubMed] [Google Scholar]
- Sultan S, Irfan SM. JAK-2 V617F mutational analysis in primary idiopathic myelofibrosis: experience from Southern Pakistan. Asian Pac J Cancer Prev. 2015b;16:7889–92. doi: 10.7314/apjcp.2015.16.17.7889. [DOI] [PubMed] [Google Scholar]
- Sultan S, Irfan SM, Khan SR. Somatic JAK-2 V617F mutational analysis in polycythemia rubra vera: a tertiary care center experience. Asian Pac J Cancer Prev. 2016;17:1053–5. doi: 10.7314/apjcp.2016.17.3.1053. [DOI] [PubMed] [Google Scholar]
- Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–68. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A, Wassie EA, Lasho TL, et al. Calreticulin mutations and long-term survival in essential thrombocythemia. Leukemia. 2014a;28:2300–3. doi: 10.1038/leu.2014.148. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Lasho TL, Finke CM, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014b;28:1472–7. doi: 10.1038/leu.2014.3. [DOI] [PubMed] [Google Scholar]
- Vainchenker W, Delhommeau F, Constantinescu SN, Bernard OA. New mutations and pathogenesis of myeloproliferative neoplasms. Blood. 2011;118:1723–35. doi: 10.1182/blood-2011-02-292102. [DOI] [PubMed] [Google Scholar]
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]


