Abstract
Background:
The Glasgow Prognostic Score (GPS) is calculated from measured CRP and albumin levels. We here evaluated the significance of the GPS in patients with resected pulmonary adenocarcinoma.
Materials and Methods:
The present study included 156 patients with lung adenocarcinoma who underwent lobectomy at Kanazawa Medical University between 2002 and 2012.
Classification was into three groups:
Those with normal albumin (>=3.5 g/dl) and C-reactive protein (CRP) (<=1.0 mg/dl) levels were classified as GPS 0 (n =136), those with low albumin (<3.5 g/dl) or elevated CRP (>1.0 mg/dl) levels as GPS 1 (n = 16), and those with low albumin (<3.5 g/dl) and elevated CRP (>1.0 mg/dl) levels as GPS 2 (n = 4). We retrospectively investigated relationships between the patient characteristics including the GPS, and disease-free survival and cancer-specific survival.
Results:
The pathological stages of the patients were as follows: IA (n=78, 50%), IB (n=31, 19.9%), IIA (n=20.0, 12.8%), IIB (n=9.0, 5.7%), and IIIA (n=18.0, 11.5%). Lobectomy was performed in all cases. The average GPS was 0.15 (0-2) and showed significant relationships with stage and tumor size. The 2-year survival rates in patients with GPS0, 1 and 2 were 81.4%, 38.4%, and 25.0%, respectively. Clear correlations were noted with both cancer-specific survival and disease-free survival. Furthermore, multivariate analysis revealed that GPS was a significant prognostic factor.
Conclusions:
The GPS could be a prognostic factor for patients with resected pulmonary adenocarcinoma.
Keywords: Lung adenocarcinoma, glasgow prognostic score, prognosis
Introduction
Lung cancer is the leading cause of cancer-related death worldwide (Jemal A et al., 2011). Lung adenocarcinoma, the most common type of primary lung cancer, is diagnosed in 1 million patients each year (Siegel R et al., 2014). Targeted therapy has been successful in a subset of lung adenocarcinoma patients with driver oncogenic mutations (Lynch TJ et al., 2004). However, the effect is still insufficient, and lung adenocarcinoma is still associated with a high rate of mortality.
Studies have found that the prognosis of cancer is related to inflammation (Mei Z et al., 2014). Inflammation-based prognostic scores have been developed to predict the prognosis of cancer patients. The Glasgow Prognostic Score (GPS), which is based on serum albumin and C-reactive protein (CRP) levels, is the most commonly used scoring system (Al Murri AM et al., 2006). Recently, the GPS has been reported to be a prognostic factor for patients with cancer (Mimatsu K et al., 2014: La Torre M et al., 2012: Horino K et al., 2013: Vashist YK et al., 2011). An elevated GPS has been shown to be associated with a worse prognosis in patients with inoperable non-small cell lung cancer (NSCLC) (Forrest LM et al., 2003: Forrest LM et al., 2005). Furthermore, Tomita et al. reported that the preoperative GPS may be useful to predict the prognosis of patients with NSCLC (Tomita M et al., 2014); however, the report did not focus patients with lung adenocarcinoma, which is relatively homogeneous group. We therefore examined postoperative survival, according to the GPS, in patients with resected pulmonary adenocarcinoma.
Materials and Methods
Patients
We retrospectively reviewed a database of 156.0 continuous patients (75.0 men and 81.0 women) who underwent lobectomy at Kanazawa Medical University between 2002 and 2012. All patients underwent pulmonary lobectomy and had histologically-confirmed primary pulmonary adenocarcinomas. Tumors were classified according to the TNM classification (7th edition) (Goldstraw P et al., 2007). The study was approved by the Institutional Review Board of Kanazawa Medical University, Ishikawa, Japan (I045).
Measurement of Glasgow prognostic score
The blood sample collection was performed on admission. The GPS definitions were as follows: Patients with a CRP level of < 10 mg/L and an albumin level of >35 g/L were allocated to GPS 0. If only the CRP was increased or the albumin level was decreased, the patients were allocated to GPS1. The patients in whom the CRP level was >10 mg/L and the albumin level was <35 g/L were classified as GPS 2. This study is a retrospective design.
Statistical analysis
The prognostic factors were examined using both univariate and multivariate analyses. The disease-free survival (DFI) rates and overall survival (OS) rates were calculated using the Kaplan-Meier method and compared using the log-rank test. Hazard ratios and confidence intervals were estimated with the use of a stratified Cox proportional-hazards model. P values of <0.05 were considered to indicate statistically significance. All statistical analyses were performed using the StatView software program (SAS Institute Inc, Cary, NC).
Results
The baseline patient characteristics are shown in Table 1. The median age of the patients was 65.2 years (range: 31-83). The GPS of the study patients were as follows: GPS0 (n=136, 87.2%), GPS1 (n=16, 10.3%), and GPS2 (n=4.0, 2.5%). Seventy-five patients (48.1%) were male and Eighty-nine patients (57.1%) were smokers. The pathological stages of the patients were as follows: IA (n=78, 50%), IB (n=31, 19.9%), IIA (n=20, 12.8%), IIB (n=9, 5.7%), IIIA (n=18.0, 11.5%). Forty-nine (31.4%) patients had comorbidities. Relationships between GPS and clinicopathological features of the patients are shown in Table 2. Stage and tumor size show significant relationship with GPS.
Table 1.
The Patients’ Characteristics
| No. of Patients | % | ||
|---|---|---|---|
| Age | >=65 | 86 | 55.1 |
| <65 | 70 | 44.9 | |
| Gender | male | 75 | 48.1 |
| female | 81 | 51.9 | |
| Smoking | + | 89 | 57.1 |
| - | 67 | 42.9 | |
| Stage | IA | 78 | 50.0 |
| IB | 31 | 19.9 | |
| IIA | 20 | 12.8 | |
| IIB | 9 | 5.7 | |
| IIIA | 18 | 11.5 | |
| Tumor Size | >=3.0cm | 57 | 36.5 |
| <3.0cm | 99 | 63.5 | |
| LN metastasis | + | 33 | 21.2 |
| - | 123 | 78.8 | |
| Differentiation | 1.2 | 142 | 91.0 |
| 3.4 | 14 | 9.0 | |
| GPS | 0.0 | 136 | 87.2 |
| 1.0 | 16 | 10.3 | |
| 2.0 | 4 | 2.5 |
Table 2.
Clinicopathological Characteristics of Study Participants
| GPS0 | GPS1/2 | p value | ||
|---|---|---|---|---|
| Gender | male | 62 | 13 | 0.167 |
| female | 74 | 7 | ||
| Smoking | + | 81 | 8 | 0.159 |
| - | 55 | 12 | ||
| Age | >=65 | 72 | 14 | 0.234 |
| <65 | 64 | 6 | ||
| Stage | I | 102 | 7 | 0.001 |
| II/IIIA | 34 | 13 | ||
| Tumor size | >=3cm | 45 | 12 | 0.037 |
| < 3cm | 91 | 8 | ||
| LN metastasis | + | 25 | 8 | 0.055 |
| - | 111 | 12 | ||
| Differentiation | 1.2 | 124 | 18 | 1.000 |
| 3.4 | 10 | 2 |
The 5-year survival rates of patients with GPS 0, 1 and 2 were 86.7%, 56.3% and 25%, respectively (Figure 1). The 5-year survival rates of GPS 1/2 was significant.
Figure 1.
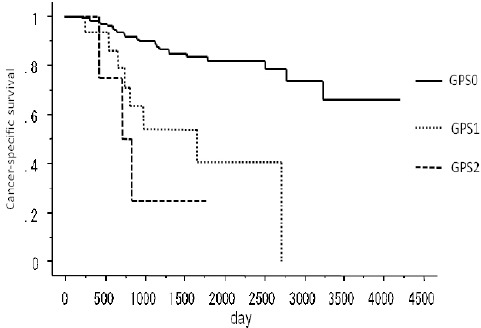
The Cancer-Specific Survival of GPS0, 1 and 2 in Lung Adenocarcinoma Patients
The median follow-up period of the survivors was 48.0 months. During the follow-up period, 33 (21.2%) patients died. A Kaplan-Meyer method revealed a significant association between the GPS and the disease-free survival (p=0.001) (Figure 2), and overall survival (p<0.001) (Figure 3). Gender (p=0.011), Smoking (p<0.001), Tumor size (p=0.001), Lymph node metastasis (p<0.001), Differentiation (G3/4) (p=0.047) and GPS (p<0.001) were significantly associated with cancer-specific survival in a univariate analysis (Table 3). The multivariate analysis of these significant variables showed the GPS (HR: 2.9 95% CI: 1.25 -6.67 p=0.013), smoking and tumor size to be independently associated with cancer-specific survival (Table 4).
Figure 2.
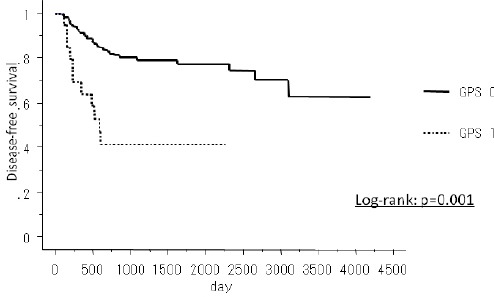
The Disease-Free Survival of GPS0 and GPS1/2 in Lung Adenocarcinoma Patients
Figure 3.
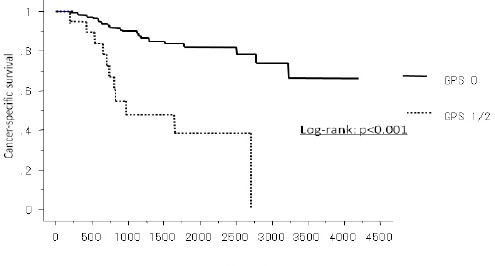
The Cancer-Specific Survival of GPS0 and GPS1/2 in Lung Adenocarcinoma Patients
Table 3.
A Univariate Survival Analysis Examining the Association between the Glasgow Prognostic Scores (GPS) and Survival
| Parameter | HR | 95% CI | P value |
|---|---|---|---|
| Age(>=65) | 1.5 | 0.7-3.1 | 0.249 |
| Gender | 0.4 | 0.2-0.8 | 0.011 |
| Smoking | 3.9 | 1.8-8.3 | <0.001 |
| Tumor Size(>=3cm) | 4.3 | 2.0-9.1 | 0.001 |
| LN Metastasis | 4.6 | 2.3-9.3 | <0.001 |
| Differentiation (G3.4) | 2.7 | 1.0-6.9 | 0.047 |
| GPS | 5.5 | 2.6-11.7 | <0.001 |
Table 4.
The Results of a Multivariate Survival Analysis Examining the Association between the Glasgow Prognostic Scores (GPS) and Survival
| Palameter | HR | 95% CI | p value |
|---|---|---|---|
| Gender | 1.6 | 0.6-4.7 | 0.372 |
| Smoking | 3.1 | 1.1-9.1 | 0.04 |
| Tumor Size(>=3cm) | 2.9 | 1.3-6.7 | 0.011 |
| LN metastasis | 2.0 | 0.9-4.5 | 0.904 |
| Differentiation (G3.4) | 0.6 | 0.1-4.4 | 0.63 |
| GPS | 2.9 | 1.2-6.7 | 0.013 |
Discussion
Many cancers arise from sites of infection, chronic irritation and inflammation (Coussens LM et al., 2002). The association between inflammation and lung cancer has been extensively reported (Tauler J et al., 2009). Recently, There are reports about the relationship between the GPS and resected lung cancer (Tomita M et al., 2014: Miyazaki T et al., 2015). However, there are no reports on relationship between GPS and resected lung adenocarcinoma. We therefore investigated the relationship between the GPS and resected lung adenocarcinoma.
The GPS was found to be correlated with the Stage and tumor size in the present study. Cancer progression releases inflammatory cytokines and growth factors. These are increased as part of the systemic inflammatory response (Canna K et al., 2008) and lead to the production of CRP. When the inflammatory reaction advances, the systemic nutritional status worsens, and the albumin value decreases. Thus, the GPS seemed to be related to the progression, which is reflected by the stage and tumor size. Because GPS may be involved to the LN factor, the LN factor was not associated in survival by multivariable analysis.
In the present study, we found that the GPS predicted the prognosis as well as the pathological stage of 156.0 patients who underwent lobectomy for adenocarcinoma. The GPS was associated significantly shorter disease-free survival rates and overall survival in resected lung adenocarcinoma. Moreover, a multivariate analysis revealed that the GPS was a significant prognostic factor for overall survival. Our findings suggest that, similar to other cancers, the GPS could be a very important factor for predicting the prognosis of patients with resected adenocarcinoma of the lung. However, the results should be interpreted with caution because the present study involved a short observation period and a small number of cases.
Recently, in NSCLS, it was reported that GPS were positive correlatived with cytokeratin 19 fragment antigen 21-1(CYFRA21-1), carcinoembryonic antigen (CEA) and tissue polypeptide specific antigen (TPS) in patients with advanced NSCLS (Jiang AG et al., 2015). GPS of score 2 predicts a prolonged ICU stay in patients undergoing pneumonectomy (Petrella F et al., 2016). Furthermore, in the other cancers, the presence of a systemic inflammatory response, as evidenced by the GPS, appears to be superior to the subjective assessment of a patient’s performance status (PS) in predicting the response to platinum-based chemotherapy in patients with advanced gastroesophageal cancer (Crumley AB et al., 2008). The GPS was also found to be an independent negative prognostic factor for overall survival in esophageal cancer patients treated with the combination chemotherapy of docetaxel and nedaplatin as a second-line chemotherapy (Kawabata R et al., 2011). We therefore suggest that the GPS is useful index that is simple and easy to implement in the clinical setting, not only surgery cases but also in chemotherapy cases. These characteristics will make the GPS an important index for various fields.
This study has same limitations. This study is retrospective design. In future, prospective study will be necessary. In conclusion, the GPS was demonstrated to be correlated with poor survival. The GPS is therefore important in predicting the prognosis of lung adenocarcinoma.
Conflict of interest
The authors declare no conflicts of interest in association with this study.
Abbreviation
GPS: Glasgow Prognostic Score
CRP: C-reactive protein
NSCLC: non-small cell lung cancer
HR: hazard ratio
References
- Al Murri AM, Bartlett JM, Canney PA, et al. Evaluation of an inflammation-based prognostic score in patients with metastaic breast cancer. Br J Cancer. 2006;94:227–30. doi: 10.1038/sj.bjc.6602922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canna K, Hilmy M, McMillan DC, et al. The relationship between tumour proliferative activity, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Colorectal Dis. 2008;10:663–7. doi: 10.1111/j.1463-1318.2007.01416.x. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumley AB, Stuart RC, McKernan M, et al. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG-ps) in patients receiving palliative chemotherapy for gastroesophageal cancer. J Gastroenterol Hepatol. 2008;23:325–9. doi: 10.1111/j.1440-1746.2007.05105.x. [DOI] [PubMed] [Google Scholar]
- Forrest LM, McMillan DC, McArdle CS, et al. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J cancer. 2003;89:1028–30. doi: 10.1038/sj.bjc.6601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest LM, McMillan DC, McArdle CS, et al. A prospective longitudinal study of performance status, an inflammation-based score (GPS) and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2005;92:1834–6. doi: 10.1038/sj.bjc.6602591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC lung cancer staging project: proposals for revison of the TNM stage grouping in the forthcoming (seventh) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- Horino K, Beppu T, Kuroki H, et al. Glasgow Prognostic Score as a prognostic factor for hepatectomy for hepatocellular. Int J Cli Oncol. 2013;18:829–38. doi: 10.1007/s10147-012-0451-3. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jiang AG, Chen HL, Lu HY. The relationship between Glasgow Prognostic Score and serum tumor markers in patients with advanced non-small cell lung cancer. BMC cancer. 2015;15:386. doi: 10.1186/s12885-015-1403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata R, Imamura H, Kishimoto T, et al. Second-line combination chemotherapy with docetaxel and nedaplatin for metastatic or recurrent squamous cell carcinoma of the esophagus refractory to chemotherapy with 5-fluorouracil plus cisplatin. Esophagus. 2011;8:179–85. [Google Scholar]
- La Torre M, Nigri G, Cavallini M, et al. The Glasgow Prognostic Score as a predictor of survival in patients with potentially resectable pancreatic adenocarcinoma. Ann Sur Oncol. 2012;19:2917–23. doi: 10.1245/s10434-012-2348-9. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;35:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Mei Z, Liu Y, Liu C, et al. Tumor-infiltrating inflammation and prognosis in colorectal acncer. Br J Cancer. 2014;110:1595–605. doi: 10.1038/bjc.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimatsu K, Oida T, Fukino N, et al. GPS is a useful predictive factor of outcome after palliative gastrectomy for stage IV gastric cancer. AntiCaner Res. 2014;34:3131–6. [PubMed] [Google Scholar]
- Miyazaki T, Yamasaki N, Tsuchiya T, et al. Inflammation-based scoring is a useful prognostic predictor of pulmonary resection for elderly patients with clinical stage I non-small-cell lung cancer. Eur J Cardiothorac Surg. 2015;47:140–5. doi: 10.1093/ejcts/ezu514. [DOI] [PubMed] [Google Scholar]
- Petrella F, Radice D, Casiraghi M, et al. Glasgow prognostic score class 2 predicts prolonged Intensive Care Unit Stay In Patients Undergoing Pneumonectomy. Ann Thorac Surg. 2016;16:30667–1. doi: 10.1016/j.athoracsur.2016.05.111. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, et al. Cancer statistics. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Tauler J, Mulshine JL. Lung cancer and inflammation: interaction of chemokines and hnRNPs. Curr Opin Pharmacol. 2009;9:384–8. doi: 10.1016/j.coph.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Tomita M, Ayabe T, Chosa E, et al. Prognostic significance of pre and postoperative glasgow prognostic score for patients with NSCLC. Anticancer Res. 2014;34:3137–40. [PubMed] [Google Scholar]
- Vashist YK, Loos J, Dedow J, et al. Glasgow prognostic score is a predictor of perioperative and long-term outcome in patients with only surgically treated esophageal cancer. Ann Sur Oncol. 2011;18:1130–8. doi: 10.1245/s10434-010-1383-7. [DOI] [PubMed] [Google Scholar]


