Abstract
Background:
There are substantial differences in the mortality rates of stomach cancer among the 47 prefectures in Japan, and Aomori prefecture is one of the most severely impacted. The aims of this study were to determine the incidence and mortality rates of stomach cancer in Aomori prefecture in comparison with Japan as a whole and cast light on reasons underlying variation.
Methods:
Data on stomach cancer cases were extracted from the Aomori Cancer Registry Database. Incidence rates for specific stages at the time of diagnosis were cited from Monitoring of Cancer Incidence in Japan, and mortality rates for stomach cancer in Aomori prefecture and the whole of Japan were obtained from Vital Statistics. Age-standardised incidence and mortality rates were calculated using the direct method.
Results:
The age-standardised incidence rate of stomach cancer in Aomori prefecture was higher than in the whole of Japan for males but lower for females. However, the age-standardised mortality rates were higher in Aomori prefecture in both sexes. The proportion of localised cancers was lower in Aomori prefecture than in the whole of Japan for most age groups.
Conclusions:
The lower rate for localised cancer suggests that higher age-standardised mortality rates are due to delays in diagnosis, despite an attendance rate for stomach cancer screening was higher in Aomori prefecture than in the whole of Japan. One plausible explanation for the failure of successful early detection might be poor quality control during screening implementation that impedes early detection.
Keywords: Stomach cancer, delays in diagnosis, age-standardised mortality rate
Introduction
Stomach cancer is one of the most common malignancies worldwide (Forman and Brewster, 2014), despite decreasing incidence rates in industrialised countries in the past four decades. The incidence of stomach cancer was reported to be linked to several environmental factors such as Helicobacter pylori infection and a diet rich in salt and poor in fruits and vegetables (Abnet and Corley, 2015; Graham, 2015), in addition to unknown endogenous factors. Incidence rates of stomach cancer among second-generation Japanese immigrants in the USA were lower than those among first generation immigrants (Kamineni and Williams, 1999; Locke and King, 1980), highlighting that a change in lifestyle was influential in decreasing incidence. Geographical variations in incidence is mainly affected by differences in lifestyle; however, the variability of stomach cancer mortality rates remains complicated due to many contributing factors, such as incidence, rate of early diagnosis and surgery and proficiency of surgeons and endoscopy specialists. Thus, the mechanisms of the high mortality rate of this disease should be carefully investigated to reveal the source of unfavourable geographical distributions affecting mortality.
In Japan, cancer has been the leading cause of death since the 1980s, and stomach cancer is the second leading cause of mortality among all cancers (Vital Statistics of Japan, 2014). Although the mortality rate of stomach cancer has been decreasing similar to that in other industrialised countries, the magnitude and impact of this disease remain because of the ageing Japanese population. Aomori prefecture (Population: 1,368,197.0, Area: 9,645.59.0km2 in 2010), located in Northeast Japan, continues to experience the highest cancer-related mortality rates since the 2,000.0s. This region was the seventh among 47.0 prefectures of Japan for stomach cancer-related mortality in 2002, which has been worsening since 2003. Among several prefectures with substantial differences, Aomori prefecture remains one of the highly impacted regions. Thus, it is of utmost importance to reveal the barriers to alleviation of this grim picture and reverse the tide in favour of improving cancer-related mortality rates in Aomori prefecture.
Thus, the aims of this study were to determine the incidence and mortality rates of stomach cancer in Aomori prefecture and whole of Japan and to determine the factors contributing to the higher mortality rates in Aomori prefecture compared to other prefectures in Japan.
Material and Methods
Cases with stomach cancer (ICD-10 code, C16) that were diagnosed between 2009 and 2012 were extracted from the Aomori Cancer Registry Database. Date of birth, date of diagnosis and stage at diagnosis for all cases were also extracted from the database. Although the Aomori Cancer Registry was established in 1989, data of patients diagnosed before 2009 were not reliable due to poor ascertainment (%DCO>40). From 2009 onward, we recognise that the quality assurance of the Aomori Cancer Registry is adequate for analysis, because % DCO between 2009 and 2012 were 5.1, 5.1, 2.6, and 2.0, respectively. Incidence of stomach cancer in whole of Japan between 2009 and 2012 were obtained from the Monitoring of Cancer Incidence in Japan (MCIJ). The MCIJ is data collection of cancer incidence stratified by prefectural units in Japan. Although Cancer Registry Act was not enforced, cancer incidence data in 2012 were gathered from all prefectures by MCIJ, which was managed by National Cancer Center of Japan. The population of Aomori prefecture in 2009 was calculated with interpolation, and those in 2011 and 2012 were determined using extrapolation based on the population data from the National Census of Japan in 2005 and 2010. Age-standardised incidence rates (AIRs) in Aomori prefecture were calculated with a direct method using the World Health Organization (WHO) world standard population figures during the period between 2009 and 2012. AIRs in whole of Japan were retrieved from the MCIJ for the period from 1995 to 2012. The number of deaths from stomach cancer by sex and for each of the 5-year age groups between 0.0 and 85.0+ (e.g. 0–4, 5–9, 10–14, …, 75–79, 80–84, 85+) in Aomori prefecture and whole of Japan were obtained from the Vital Statistics of Japan for the period between 1995 and 2014. Age-standardised mortality rates (AMRs) due to stomach cancer in each year was calculated by the same method described for AIR. Age-specific incidence and mortality rates were presented as means for the period between 2010 and 2012 according to the 5-year age groups. AIRs and AMRs were reported as per 100,000 person-years.
Stage at diagnosis was classified as localised, regional, distant or unknown according to the MCIJ. Localised cancer was confined to the gastric wall with negative lymph nodes. Those diagnosed with carcinoma in situ were excluded from this study. Tumours with spread to regional lymph nodes and/or immediately adjacent tissues were classified as regional cancer. Distant cancer was defined as tumours with distant extension or metastases. Tumours with insufficient information on stage were classified as those of unknown stage.
Percentages of specific stages at diagnosis in Aomori prefecture were reported in nine age groups: < 50, 50–54, 55–59, …, 75–89, 80–84, 85+ years. Percentages of specific stages at diagnosis in whole of Japan were reported as averages for all ages and both sexes as determined from the MCIJ. All values were presented as means in the period from 2010 to 2012.
Results
1-Age-standardised incidence and mortality rates
Figure 1a and 1b show age-standardised incidence and mortality rates of stomach cancer in Aomori prefecture and whole of Japan. AMRs of 4-year (2009-2012) averages and 95% confidence intervals in Aomori prefecture and whole of Japan were 22.6 (21.4-23.8) and 18.7 (18.6-18.9), respectively, among males, and 8.2 (7.5-8.9) and 7.0 (6.9-7.1), respectively, among females. AMRs of stomach cancer among males in Aomori prefecture and whole of Japan in 1995 were 34.4 and 30.4, respectively; AMRs remained higher in Aomori prefecture from 1995 onward. AMRs of stomach cancer among females in Aomori prefecture and whole of Japan were 12.2 and 12.6, respectively; similar to that observed among males, these rates remained higher from 1998 onward among females in Aomori prefecture. In 2014, AMRs among males were 22.1 and 16.6 in Aomori prefecture and whole of Japan, respectively, whereas AMRs among females were 6.5 and 6.3, respectively, for the same year in Aomori prefecture and whole of Japan. AIRs of 4-year (2009-2012) averages and 95.0% confidence intervals in Aomori prefecture and whole of Japan were 53.4 (51.0-55.8) and 55.5 (55.3-55.7), respectively, among males, and 17.7 (16.4-19.0) and 20.2 (20.1-20.3), respectively, among females. In 2012, in Aomori prefecture and whole of Japan, AIRs among males were 59.5 and 55.4, respectively, whereas AIRs among females were 18.0 and 20.0, respectively. An increase of incidence rates among males could be caused by improvement of data ascertainment.
Figure 1a.
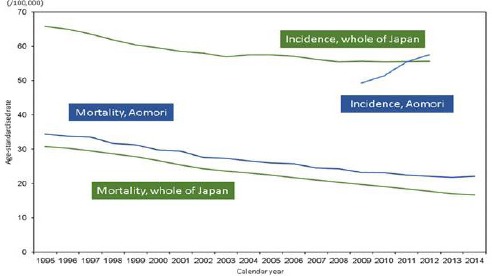
Age-Standardised Incidence and Mortality Rates of Stomach Cancer Among Males in Aomori Prefecture and Whole of Japan
Figure 1b.
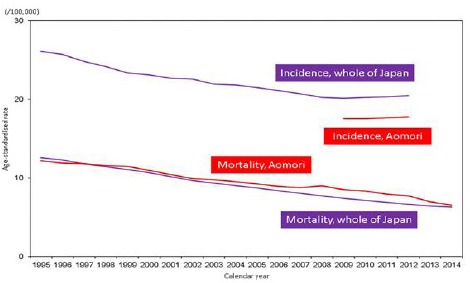
Age-Standardised Incidence and mortality Rates of Stomach Cancer Among Females in Aomori Prefecture and Whole of Japan
2. Age-specific incidence and mortality rates
In males, there were no noticeable differences in age-specific incidence rates among males aged 50–54 years or younger between Aomori prefecture and whole of Japan. Incidence rates among males aged 55–59 years or older were lower in Aomori prefecture than in whole of Japan, except for those between the ages of 60–64 and 80–84 years (Figure 2a). Age-specific mortality rates among males aged 45.0–49.0 years or older were higher in Aomori prefecture than in whole of Japan; in contrast, differences in mortality rates among males aged 40–44 years or younger were very small (Figure 2b)
Figure 2a.
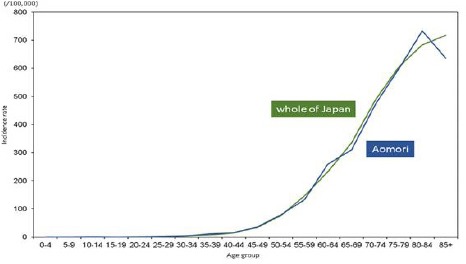
Age-Specific Incidence Rates of Stomach Cancer among Males in Aomori Prefecture and Whole of Japan (2010-2012)
Figure 2b.
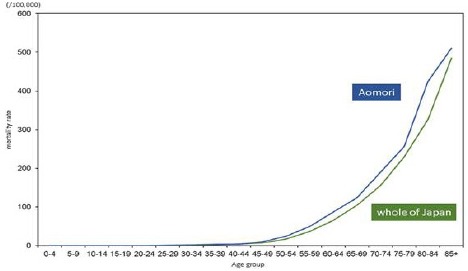
Age-Specific Mortality Rates of Stomach Cancer among Males in Aomori Prefecture and Whole of Japan (2010-2012)
In females, there were no noticeable differences in age-specific incidence rates among those aged 25–29 years or younger between Aomori prefecture and whole of Japan. Incidence rates among females aged 30–34 years or older were lower in Aomori prefecture than in whole of Japan (Figure 3a). Age-specific mortality rates among females aged 30–34 years or older were higher in Aomori prefecture than in whole of Japan, except for those between the ages of 35–39 and 65–69 years; however, the differences among those aged 25–29 years or younger were very small (Figure 3b).
Figure 3a.
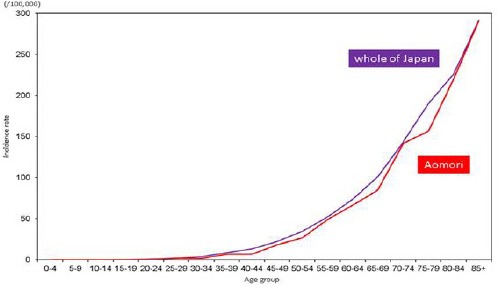
Age-Specific Incidence Rates of Stomach Cancer among Females in Aomori Prefecture and Whole of Japan (2010-2012)
Figure 3b.
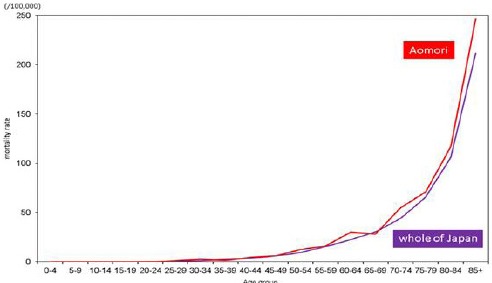
Age-Specific Mortality Rates of Stomach Cancer among Females in Aomori Prefecture and Whole of Japan (2010-2012)
3. Age-specific percentages of specific stages at diagnosis
Figure 4a and 4b show age-specific percentages of specific stages at diagnosis in Aomori prefecture and whole of Japan among males and females, respectively. Specifically, In Aomori prefecture, the percentages of localised, regional and distant cancer cases in males were 45.6%, 22.3% and 21.4%, respectively, and those in females were 45%, 21.1% and 20.9%, respectively. The percentages of localised cancer cases among males and females were lower in Aomori prefecture than in whole of Japan (53%). In males, the percentage of localised cancer cases in each age group was lower in Aomori prefecture than in whole of Japan, with the highest percentage of 52.5% observed among the 70–74- year age group. Whereas the percentage of localised cancer cases was lowest (25.9%) among males aged 85+ years, that age group also had the highest percentage of cases with unknown stage (34.7%). Among males aged < 50 years, the percentage of localised cancer cases was the second lowest (35.2%), and that of cancer cases with unknown stage accounted for 11.1%. In females, the percentages of localised cancer in each age group were also lower in Aomori prefecture than in whole of Japan, except for that among those aged 75–79 years (54%). The second highest percentage of localised cancer cases were observed among females aged 70–74 years (52.3%). Among those aged 50–54 years, the percentage of distant cancer cases was the highest (36.7%) and that of localised cancer was the lowest in Aomori prefecture.
Figure 4a.
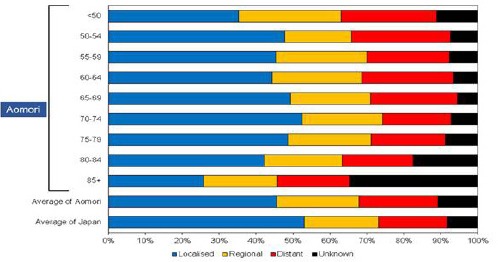
Age-Specific Percentages of Specific Stages of Stomach Cancer at Diagnosis Among Males (2010-2012)
Figure 4b.
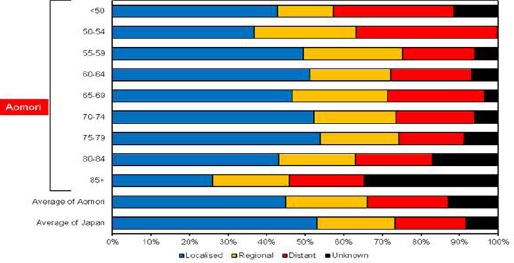
Age-Specific Percentages of Specific Stages of Stomach Cancer at Diagnosis Among Females (2010-2012)
Discussion
We found that the AIRs of stomach cancer were slightly higher among males and lower among females in Aomori prefecture than in whole of Japan; however, the AMRs of stomach cancer were higher for both sexes in Aomori prefecture than in whole of Japan. Furthermore, our analysis revealed that age-specific mortality rates were higher in Aomori prefecture than in whole of Japan for most age groups, thus indicating that the higher AMRs in Aomori prefecture were not caused by substantially high age-specific mortality rates in certain age groups. The lower percentages of localised cancer cases in most age groups suggested that higher AMRs were associated with delays in diagnosis.
Screening for specific cancers was adopted as part of national health initiatives in many countries, with an aim at reducing cancer-related mortality through early detection. In Japan, screening for stomach cancer with double-contrast barium X-ray radiography has been conducted nationwide for all residents aged 40 years and older since 1983 as stomach cancer has been the leading cause of cancer-related deaths over a long period of time. Given the recognised efficacy of double-contrast barium X-ray radiography as a screening tool for stomach cancer (Inaba and Hirayama, 1999; Mizoue and Yoshimura, 2003), proper implementation of this initiative can potentially reduce the mortality. Attendance rate is one of the most reasonable and frequently used indicators of quality control of screening tools. Among a total of 47 prefectures in Japan, the attendance rate for stomach cancer screening in Aomori prefecture was the fourth and the third highest in males and females, respectively (Cancer Registry and Statistics, 2016). Among several critical requirements for effective screening are the enrolment of all residents in the target age group, high sensitivity and specificity and high compliance of follow-up examinations (Rabeneck and Lansdorp-Vogelaar, 2015). One or more of these factors might be contributing to the failure of early detection of stomach cancer cases despite high screening attendance rates in Aomori prefecture. For example, poor achievement in quality control of screening implementation could impede early detection despite high attendance rates.
Several previous studies reported the incidence, mortality and percentage of patients with stomach cancer of specific stages. Zheng et al, (2014) reported the statistics on the stage and survival rates of patients with stomach cancer in Shanghai, China; however, the ratio of cases with cancer of unknown stage was more than 50%. Lambert et al, (2002) demonstrated differences in AIRs, AMRs and ratio of stomach cancer cases of specific stages in Osaka prefecture (Japan), Slovenia and the USA for the period between 1975 and 1995. In 1995, AMRs of stomach cancer among males and females were higher in Osaka prefecture than whole of Japan, notwithstanding the lower AIRs in Osaka prefecture. A combination of lower AIRs and higher AMRs was observed both in Osaka prefecture in 1995 and in Aomori prefecture in this study. Data collection for population-based cancer registries was poorer in 1995 than in 2012; thus, the AIRs in 1995 in Japan could be lower than predicted. Given that the AIRs in 1995 were not reliable, the overall findings in the present study were unique and underlined the importance of early detection.
Stage at diagnosis for stomach cancer in Osaka prefecture was previously reported (Verdecchia and Mariotto, 2003). Localised stomach cancer cases accounted for 30% of all cases during the period from 1978 to 1989 in Osaka prefecture and was the highest in four areas reported in that study. The screening programmes for stomach cancer could have contributed to the increase in early-diagnosed cases in Osaka prefecture, as the other areas in that study, Iowa (USA), Varese (Italy) and Campinas (Brazil), did not adopt the programme. In our study, the percentage of localised stomach cancer cases in Aomori prefecture accounted for more than 40% of all cases in the period from 2010 to 2012. The difference in the percentage of localised cases between the two studies could be explained by longitudinal factors, such as the widespread use of other diagnostic methods including upper gastrointestinal endoscopy over the course of 30.0 years, and cross-sectional factors, such as differences in the screening attendance rates. Stage distribution of cardia and non-cardia stomach cancer cases in the Netherlands, where the screening programme was not adopted, supported the link between the programme and early detection (Dassen and Dikken, 2014). In the period from 2004 to 2008, the total number of stage I, II and III cardia and non-cardia stomach cancer cases, classified according to the International Union Against Cancer TNM classification, accounted for less than 45% of the total number of stomach cancer cases. Stage I, II and III cases included those spread to regional lymph nodes and/or immediately adjacent tissues; thus, the proportion of localised cancer in the Netherlands was lower than in Japan. Screening is less likely to provide an advantage to countries with low incidence rates such as European countries and the USA, but countries with high incidence rates should strongly consider the adoption of screening programmes for stomach cancer.
There are other potential causes of delays in diagnosis for the residents of Aomori prefecture. A long waiting period for detailed evaluation after screening results become available can contribute to the deterioration of the disease. The number of specialists certified by the Japan Gastroenterological Endoscopy Society were 16,170 and 118, or 12.7 and 9.8 per 100,000 in whole of Japan and Aomori prefecture, respectively, in 2015 (Japan Gastroenterological Endoscopy Society, 2015). While the magnitude of poor quality control of the screening programme in Aomori prefecture might be overestimated, the influence of specialists on early detection could not be overemphasised. Thus, a lower number of specialists could also contribute to the low sensitivity of detailed evaluations caused by increased numbers of patients per specialist and longer waiting times for treatment after diagnosis in Aomori prefecture.
There were other limitations in this study. The percentages of cases with unknown stage in Aomori prefecture were higher than those in whole of Japan. Although the proportions of DCO cases in Aomori prefecture was lower than MCIJ, data quality of Aomori cancer registry could be poorer than that of MCIJ. In spite of it, the results of this study would not change because cases with unknown stage were presumed to consist of cases with severe stage mainly.
The screening programme for stomach cancer using double-contrast barium X-ray radiography is effective for improving early detection in Japan. However, proper implementation of the screening tools, including good quality control, is critical for reducing mortality rates. Screening providers and researchers should inform residents of ‘what we know, what we don’t know, and what we believe’ (Brawley and Goldberg, 2012) for the screening of not only prostate cancer but also other cancers. Reduction of stomach cancer mortality rates through early detection requires efficient quality control of the screening programme, and performance indicators such as the sensitivity and positive predictive value should be disclosed for accurate evaluation of its progress.
References
- Abnet CC, Corley DA, Freedman ND, Kamangar F. Diet and upper gastrointestinal malignancies. Gastroenterology. 2015;148:1234–43. doi: 10.1053/j.gastro.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawley O, Goldberg P. How We Do Harm: A Doctor Breaks Ranks About Being Sick in America. New York: St. Martin’s Press; 2012. [Google Scholar]
- Cancer Registry and Statistics. Cancer information service, national cancer center of Japan. Attendance rate of cancer screening by 47 prefectures in Japan. 2016. Retrieved 4 July 2016, from http://ganjoho.jp/reg_stat/statistics/dl/index.html .
- Dassen AE, Dikken JL, Bosscha K, et al. Gastric cancer: decreasing incidence but stable survival in the Netherlands. Acta Oncol. 2014;53:138–42. doi: 10.3109/0284186X.2013.789139. [DOI] [PubMed] [Google Scholar]
- Forman DBF, Brewster DH, Gombe Mbalawa C, et al. Cancer Incidence in Five Continents. X. Lyon: IARC Scientific Publication No. 164. International Agency for Research on Cancer; 2014. pp. 126–908. [Google Scholar]
- Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015;148:719–31. doi: 10.1053/j.gastro.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba S, Hirayama H, Nagata C, et al. Evaluation of a screening program on reduction of gastric cancer mortality in Japan: preliminary results from a cohort study. Prev med. 1999;29:102–6. doi: 10.1006/pmed.1999.0507. [DOI] [PubMed] [Google Scholar]
- Kamineni A, Williams MA, Schwartz SM, et al. The incidence of gastric carcinoma in Asian migrants to the United States and their descendants. Cancer Causes Control. 1999;10:77–83. doi: 10.1023/a:1008849014992. [DOI] [PubMed] [Google Scholar]
- Lambert R, Guilloux A, Oshima A, et al. Incidence and mortality from stomach cancer in Japan, Slovenia and the USA. Int J Cancer. 2002;97:811–8. doi: 10.1002/ijc.10150. [DOI] [PubMed] [Google Scholar]
- Locke FB, King H. Cancer mortality risk among Japanese in the United States. J Natl Cancer Inst. 1980;65:1149–56. [PubMed] [Google Scholar]
- Mizoue T, Yoshimura T, Tokui N, et al. Prospective study of screening for stomach cancer in Japan. Int J Cancer. 2003;106:103–7. doi: 10.1002/ijc.11183. [DOI] [PubMed] [Google Scholar]
- Rabeneck L, Lansdorp-Vogelaar I. Assessment of a cancer screening program. Best practice & research. Clin Gastroenterol. 2015;29:979–85. doi: 10.1016/j.bpg.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Japan Gastroenterological Endoscopy Society. List of specialists certified by Japan Gastroenterological Endoscopy Society. 2016. Retrieved 4 July 2016, from http://www.jges.net/index.php/member_submenu/archives/115 .
- Verdecchia A, Mariotto A, Gatta G, et al. Comparison of stomach cancer incidence and survival in four continents. Eur J Cancer. 2003;39:1603–9. doi: 10.1016/s0959-8049(03)00360-5. [DOI] [PubMed] [Google Scholar]
- Vital, Health and Social Statistics Division, Ministry of Health, Labour and Welfare (2014). Vital Statistics of Japan. Retrieved 4 July 2016, from http://www.e-stat.go.jp/SG1/estat/GL08020103.do?_toGL08020103_&list ID=000001137965&disp=Other&requestSender=dsearch .
- Zheng L, Wu C, Xi P, et al. The survival and the long-term trends of patients with gastric cancer in Shanghai, China. BMC cancer. 2014;14:300. doi: 10.1186/1471-2407-14-300. [DOI] [PMC free article] [PubMed] [Google Scholar]


