Abstract
Background:
Intrahepatic cholangiocarcinoma (IHCCA) is an aggressive tumor for which surgical resection is a mainstay of treatment. However, recurrence after resection is common associated with a poor prognosis. Studies regarding recurrence of mass-forming IHCCA are rare; therefore, we investigated the pattern with our dataset.
Methods:
We retrospectively reviewed the medical and pathological records of 50 mass-forming IHCCA patients who underwent hepatic resection between January 2004 and December 2009 in order to determine the patterns of recurrence and prognosis. All demographic and operative parameters were analyzed for their effects on recurrence-free survival.
Results:
The median recurrence-free survival time was 188 days (95%CI: 149-299). The respective 1-, 2-, and 3-year recurrence-free survival rates were 16.2% (95% CI: 6.6-29.4), 5.4% (95% CI: 1.0-15.8) and 2.7% (95% CI: 0.2-12.0). There was an equal distribution of recurrence at solitary and multiple sites. Univariate analysis revealed no factors related to recurrence-free survival.
Conclusion:
The overall survival and recurrence-free survival after surgery for mass-forming IHCCA were found to be very poor. Almost all recurrences were detected within 2 years after surgery. Adjuvant chemotherapy after surgery may add benefit in the affected patients.
Keywords: Mass forming intrahepatic cholangiocarcinoma, recurrence, survival, curative resection
Introduction
Intrahepatic cholangiocarcinoma is the second most common primary malignancy of the liver (Khan SA et al., 2002). Most patients have advanced disease at the time of presentation and die within 1 year, only subset of patients are candidates for curative treatment (Tan JCC et al., 2008; Luvira V et al., 2002). The mainstay of treatment of intrahepatic cholangiocarcinoma is surgical resection (Bridgewater J et al., 2014; Konstantinidis IT et al., 2016); however, most patients experience recurrence despite completely removing the tumor (Konstantinidis IT et al., 2016; Spolverato G et al., 2016) and the subsequent prognosis is very poor (Spolverato G et al., 2016). The data on the pattern of recurrence of cholangiocarcinoma would be useful for determining the best operative procedure and further treatment strategies.
Several studies have been conducted to investigate the pattern of recurrence of all types of intrahepatic cholangiocarcinoma (Jung SJ et al., 2012; Weber SM et al., 2001; Yamamoto M and Ariizumi S, 2006; Hyder O et al., 2013). Recurrence after hepatic resection for intrahepatic cholangiocarcinoma is common (Hyder O et al., 2013; De jong MC et al., 2011), and the recurrence rate is significantly higher in patients with the mass-forming type. Recurrences occur equally at the intrahepatic and extrahepatic sites. The authors suggest adjuvant treatment is likely to have an impact on patient outcomes (Hyder O et al., 2013).
According to their gross features, intrahepatic cholangiocarcinoma includes the mass-forming, periductal-infiltrating, and intraductal-growth (IG) types (Yamamoto J et al., 1993). Recent evidence shows that there are differences in nature among the three type of intrahepatic cholangiocarcinoma; particularly the mass-forming type, which is believed to originate from progenitor cells at the canals of Hering (Aishima S and Oda Y, 2015; Nakanuma Y et al., 2010). The mass-forming cholangiocarcinoma might have a specific pattern of recurrence.
To achieve a better understanding of the pattern of recurrence of mass-forming intrahepatic cholangiocarcinoma (MF-IHCCA), we investigated the rate and location of recurrences of mass-forming intrahepatic cholangiocarcinoma. We additionally analyzed the association between recurrence-free and peri-operative parameters.
Materials and methods
This retrospective study ran between January 2004 and December 2009. We reviewed the medical records of all the patients who underwent curative-intent hepatic resection for hepatobiliary neoplasm at Srinagarind Hospital, Khon Kaen University. The definition of mass-forming intrahepatic cholangiocarcinoma in this study included pathologically-confirmed adenocarcinoma of the biliary tree and the presence of a visible mass lesion without any signs of periductal infiltration or intraductal neoplasm. After reviewing the records, 50 cases of mass-forming intrahepatic cholangiocarcinoma were found.
Regarding the treatment protocol at our center, all of the patients were evaluated for resectability using crossectional imaging (i.e., computed tomography, or magnetic resonance imaging). The intent of all of the operations was to at least remove all visible tumors. All surgical specimens were sent to the Department of Pathology for pathological diagnosis and staging. All patients were followed- up for tumor recurrence and death.
Ethical consideration
The Institutional Review Board (IRB), Office of Human Research Ethics, Khon Kaen University, reviewed and approved the present study (HE571293).
Outcome variables
The primary outcome of this study was the recurrence rate and sites of recurrence of tumors. We also investigated recurrence-free survival and the factors effecting it, including tumor number, stage of the tumor, tumor grade, types of operation, and surgical margin status.
Statistical analysis
The data are presented as medians (min: max), or as counts and percentages. A survival analysis was performed using the Kaplan-Meier analysis; patients who died before recurrence of a tumor was detected were censored. Comparisons among groups were analyzed using a log-rank test. All statistical analyses were performed using STATA version 10.
Results
Clinical and pathological characteristics
Between January 2004 and December 2009, fifty patients underwent curative-intent hepatectomy for mass-forming intrahepatic cholangiocarcinoma. The median age at diagnosis was 57.2 years (range, 32-72). The male to female ratio was 1 (male=26, female=24).
Upper abdominal pain (82%) was the most common presentation. According to number of tumors, the majority of patients (86%) had a single tumor; the mean tumor size was 6.49 cm (95%CI: 5.17-7.80).
According to AJCC staging, most of the patients had advanced disease, stage 4, according to lymph node involvement, at the time of surgery. Most of the tumors were well-differentiated.
Regarding the surgery, right-sided hepatectomy outnumbered left-sided hepatectomy 3 to 1 (78% vs. 22%). The margin-free surgical status was achieved in about half of the patients. Lymph node dissection was performed in 26 (52%) patients.
Patients’ survival
The respective 1-, 2- and 3-year survival rate was 32% (95%CI: 19.7-45), 16% (95%CI: 7.5-27.4), and 6% (95%CI: 0.2-12.0). The median survival time was 188 days (95%CI: 1.60-14.9) (Figure 1a).
Figure 1.
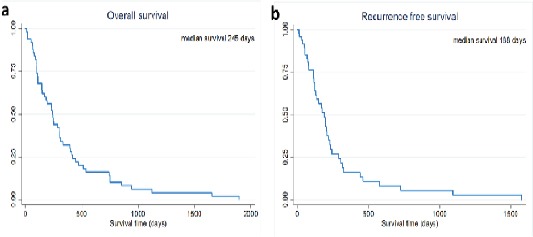
(a) Overall Survival and (b) Recurrence-Free Survival of Mass-Forming Intrahepatic Cholangiocarcinoma Patients Treated by Hepatic resection
Recurrence-free survival and recurrence pattern
During the study period, recurrence was detected in 40 patients. The rate of recurrence was 80% (40/50). The remaining patients died without evidence of recurrence. The respective 1-, 2-, and 3-year recurrence-free survival rate was 16.2% (95%CI: 6.6-29.4), 5.4% (95%CI: 1.0-15.8), and 2.7% (95%CI: 0.2-12.0). The median recurrence-free survival time was 188 days (95%CI: 149-299) (Figure 1b).
Table 1 lists the clinicopathological characteristics and univariate analyses for recurrence-free survival. Several factors seemed to be related with recurrence-free survival but the differences were not statistically significant (viz., AJCC staging, surgical margin status (Figure 2) and postoperative chemotherapy (Figure 3)).
Table 1.
Characteristics of 50 Patients and Univariate Analyses for Recurrence-Free Survival
| Variable | No. of patients (%) | Median RFS, day. | 1 yrs. RFS (%) | P value |
|---|---|---|---|---|
| Age (yrs.) | 0.568 | |||
| <60 | 31 (62.0%) | 153 | 13.8% | |
| >60 | 19 (38.0%) | 201 | 19.5% | |
| Gender | 0.244 | |||
| Male | 26 (52.0%) | 195 | 11.2% | |
| Female | 24 (48.0%) | 188 | 20.9% | |
| Tumor number | 0.268 | |||
| Solitary | 43 (86.0%) | 170 | 13.0% | |
| Multiple | 7 (14.0%) | 235 | 33.3% | |
| T Staging | 0.895 | |||
| T1 | 11 (22.0%) | 153 | 27.3% | |
| T2 | 9 (18.0%) | 231 | 29.6% | |
| T3 | 30 (60.0%) | 195 | 5.1% | |
| T4 | 0 | - | - | |
| N Staging | 0.419 | |||
| N0 | 18 (36.0%) | 195 | 19.2% | |
| N1 | 32 (64.0%) | 170 | 14.1% | |
| AJCC 7th Staging | 0.19 | |||
| 1 | 3 (6.0%) | 575 | 66.7% | |
| 2 | 3 (6.0%) | 112 | 0.0% | |
| 3 | 12 (24.0%) | 195 | 10.4% | |
| 4 | 32 (64.0%) | 170 | 14.1% | |
| Tumor grade | 0.796 | |||
| Well diff. | 24 (48.0%) | 127 | 11.2% | |
| Mod diff. | 7 (14.0%) | 195 | 0.0% | |
| Poor diff. | 5 (10.0%) | 318 | 0.0% | |
| Not specified | 14 (28.0%) | 205 | 31.4% | |
| Type of liver resection | 0.295 | |||
| Right sided hepatectomy | 39 (78.0%) | 170 | 10.4% | |
| Left sided hepatectomy | 11 (22.0%) | 195 | 35.8% | |
| Surgical margin status | 0.127 | |||
| Free | 23 (46.0%) | 205 | 26.7% | |
| Not free | 27 (54.0%) | 133 | 4.9% | |
| Postoperative chemotherapy | 0.137 | |||
| Yes | 18 (32.0%) | 219 | 20.6% | |
| No | 32 (64.0%) | 127 | 13.5% |
Figure 2.
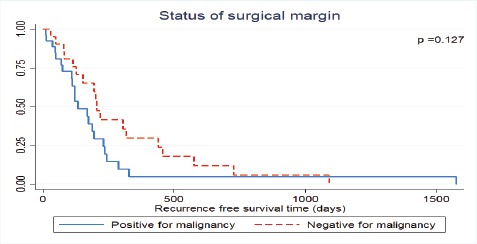
Recurrence-Free Survival of Mass-Forming Intrahepatic Cholangiocarcinoma Patients Treated by Hepatic Resection Stratified by Status of Resection Margin
Figure 3.
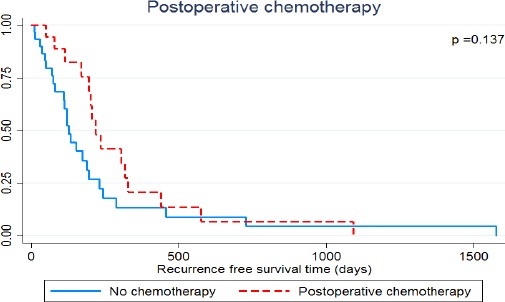
Recurrence-Free Survival of Mass-Forming Intrahepatic Cholangiocarcinoma Patients Treated by Hepatic Resection Stratified by Postoperative Treatment
About half of the patients experienced a solitary recurrence (n =22; 55.0%). The most frequent site of recurrence was the surgical margin (n=18; 45.0%), followed by regional lymph nodes (n =17; 42.5%), and the liver remnant (n =15; 37.5%). Among the patients with multiple sites of recurrence, only three did not have an intrahepatic recurrence. The pattern of recurrence is summarized in Table 2.
Table 2.
Site of initial recurrences of mass-forming cholangiocarcinoma
| Site of recurrences | No. of patients (%) |
|---|---|
| Solitary recurrence | |
| Surgical margin | 4 (9.8%) |
| Liver remnant | 4 (9.8%) |
| Regional lymph node | 4 (9.8%) |
| Aortocaval lymph node | 4 (9.8%) |
| Peritoneum | 1 (2.4%) |
| Lung | 2 (4.9%) |
| Bone | 3 (7.3%) |
| Multiple recurrences | |
| Surgical margin and liver remnant | 2 (4.9%) |
| Surgical margin and regional lymph node | 3 (7.3%) |
| Surgical margin and aortocaval lymph node | 1(2.4%) |
| Surgical margin, liver remnant and regional lymph node | 3(7.3%) |
| Surgical margin, liver remnant and aortocaval lymph node | 1(2.4%) |
| Surgical margin, regional lymph node and peritoneum | 1(2.4%) |
| Surgical margin, liver remnant, regional lymph node and peritoneum | 1(2.4%) |
| Surgical margin, liver remnant, regional lymph node and bone | 1(2.4%) |
| Surgical margin, liver remnant, regional lymph node and aortocaval | 1(2.4%) |
| lymph node | |
| Liver remnant and aortocaval lymph node | 1(2.4%) |
| Regional lymph node and aortocaval lymph node | 1(2.4%) |
| Regional lymph node and bone | 1(2.4%) |
| Regional lymph node and peritoneum | 1(2.4%) |
According to the side of the hepatectomy, the patients who underwent left-sided hepatectomy had more loco-regional recurrences (i.e., surgical margin, liver remnant, and regional lymph node). The distant organ recurrences were observed only in the patients who underwent right-sided hepatectomy (Figure 4).
Figure 4.
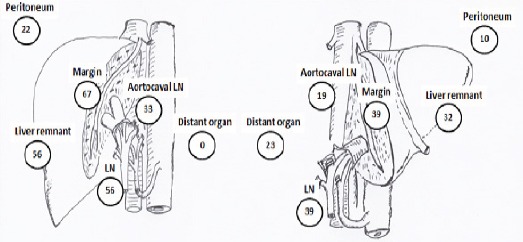
Pattern of Recurrences (%) of Mass-Forming According to Side of Hepatic Resection: (a) Left-Sided Hepatectomy, and (b) Right-Sided Hepatectomy
Among the 18 patients who experienced surgical margin recurrences, 14 (77.8%) had microscopic tumors on the surgical margin after the first operation. Of the 27 patients (29.6%) with microscopic tumors on the surgical margin, eight had no surgical margin recurrence. There was no relationship between lymph node dissection in the first operation and regional lymph node recurrence.
Discussion
Our study shows that mass-forming cholangiocarcinoma has a demonstrably poor prognosis. The overall and disease-free survival in our study was poor compared with other series (De jong MC et al., 2011; Suzuki S et al., 2002; Endo I et al., 2008), but comparable to a previous study at our center (Bhudhisawasdi V et al., 2012).
We aimed to determine the pattern of initial recurrence of mass-forming intrahepatic cholangiocarcinoma. We found that after hepatectomy, the pattern of recurrence of cholangiocarcinoma was disrupted, which is to say unpredictable. Although intrahepatic recurrences were observed in most of cases with multiple sites of recurrences, but solitary extrahepatic recurrences were also observe. Several researchers have concluded that lymph node involvement of intrahepatic cholangiocarcinoma represents systemic disease (Morine Y et al., 2012; Morine Y and Shimada M, 2015). The current study had very high (64%) prevalence of lymph node involvement compare with previous reports (Konstantinidis IT et al., 2016; Ribero D et al., 2012), which may explain why our study had a higher rate of systemic recurrence than previous reports. The present study confirmed that regional lymphadenectomy may not improve survival, as reported by others (Morine Y and Shimada M, 2015; Wu ZF et al., 2015; Kim DH et al., 2015). We found no relationship between lymph node dissection and the rate of regional lymph node recurrences.
As for making changes in treatment policy, extension of surgical procedures may decrease the recurrence of surgical margins and regional lymph node recurrences. The other sites of recurrence can only be treated using systemic therapy. Others demonstrated that intrahepatic metastasis (liver remnant recurrence) was the salient feature after surgery (Spolverato G et al., 2016; Suzuki S et al., 2002), and this could not be prevented by extension of surgery; so, adjuvant chemotherapy may add some benefit regarding recurrence of tumors (Suzuki S et al., 2002). Our study failed to demonstrate any relationship between routine lymph node dissection and regional lymph node recurrence, but we found that patients who had received post-operative chemotherapy trended to have better recurrence-free survival, albeit not statistically significant-perhaps owing to the small sample size. Our findings were, however, consistent with previous studies that found that mass-forming cholangiocarcinoma represents an advance of the disease, such that there is a higher chance of systemic recurrence, so adjuvant chemotherapy would be needed (Konstantinidis IT et al., 2016; Hyder O et al., 2013; Suzuki S et al., 2002; Wirasorn K et al., 2013).
Although our study focused on mass-forming cholangiocarcinoma and had quite a high number compared with previous reports (Weber SM et al., 2001; Suzuki S et al., 2002), this study had limitations. First, the retrospective nature may introduce a bias vis-à-vis the pattern of recurrence. Second, during the current study, there was no uniformity of treatment for intrahepatic cholangiocarcinoma; whether it was width of surgical margin, routine lymph node dissection, or postoperative chemotherapy. Finally, although we attempted to identify only mass-forming cholangiocarcinoma by excluding cases with evidence of periductal infiltration and intraductal tumor, the mass-forming type was not confirmed immunohistochemically.
In conclusion, overall and recurrence-free survival after surgery for mass-forming intrahepatic cholangiocarcinoma were very poor as recurrence was common. Almost all recurrences were detected within 2 years after surgery but the pattern of recurrence was disrupted. Consequently, adjuvant chemotherapy after surgery nominally improve both recurrence-free and overall survival.
Conflicts of interest
All authors declare that they have no competing of interests.
Acknowledgements
This study was supported by the Anti-cancer Foundation, Faculty of Medicine, Khon Kaen University, Thailand (KKU). The authors thank (a) the KKU Cancer Unit staff for data collection and follow-up of the patients, and (b) Mr. Bryan Roderick Hamman for assistance with the English-language presentation of the manuscript under the aegis of the Publication Clinic KKU, Thailand.
References
- Aishima S, Oda Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: different characters of perihilar large duct type versus peripheral small duct type. J Hepatobiliary Pancreat Sci. 2015;22:94–100. doi: 10.1002/jhbp.154. [DOI] [PubMed] [Google Scholar]
- Bhudhisawasdi V, Khuntikeo N, Chur-in S, et al. Cholangiocarcinoma: Experience of Srinagaraind Hospital. Srinagarind medical Journal. 2012;27:331–9. [Google Scholar]
- Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268–89. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- De Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140–5. doi: 10.1200/JCO.2011.35.6519. [DOI] [PubMed] [Google Scholar]
- Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery. 2013;153:811–8. doi: 10.1016/j.surg.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SJ, Woo SM, Park HK, et al. Patterns of initial disease recurrence after resection of biliary tract cancer. Oncology. 2012;83:83–90. doi: 10.1159/000339695. [DOI] [PubMed] [Google Scholar]
- Khan SA, Taylor-Robinson SD, Toledano MB, et al. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806–13. doi: 10.1016/s0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- Kim DH, Choi DW, Choi SH, et al. Is there a role for systematic hepatic pedicle lymphadenectomy in intrahepatic cholangiocarcinoma? A review of 17 years of experience in a tertiary institution. Surgery. 2015;157:666–75. doi: 10.1016/j.surg.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Konstantinidis IT, Arkadopoulos N, Ferrone CR. Surgical management of intrahepatic cholangiocarcinoma in the modern era: advances and challenges. Chin Clin Oncol. 2016;5:9–15. doi: 10.3978/j.issn.2304-3865.2016.02.06. [DOI] [PubMed] [Google Scholar]
- Luvira V, Nilprapha K, Bhudhisawasdi V, et al. Cholangiocarcinoma Patient Outcome in Northeastern Thailand: Single-Center Prospective Study. Asian Pac J Cancer Prev. 2002;17:401–6. doi: 10.7314/apjcp.2016.17.1.401. [DOI] [PubMed] [Google Scholar]
- Morine Y, Shimada M, Utsunomiya T, et al. Clinical impact of lymph node dissection in surgery for peripheral-type intrahepatic cholangiocarcinoma. Surg Today. 2012;42:147–51. doi: 10.1007/s00595-011-0057-9. [DOI] [PubMed] [Google Scholar]
- Morine Y, Shimada M. The value of systematic lymph node dissection for intrahepatic cholangiocarcinoma from the viewpoint of liver lymphatics. J Gastroenterol. 2015;50:913–27. doi: 10.1007/s00535-015-1071-2. [DOI] [PubMed] [Google Scholar]
- Nakanuma Y, Sato Y, Harada K, et al. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol. 2010;2:419–27. doi: 10.4254/wjh.v2.i12.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribero D, Pinna AD, Guglielmi A, et al. Surgical Approach for Long-term Survival of Patients With Intrahepatic Cholangiocarcinoma: A Multi-institutional Analysis of 434 Patients. Arch Surg. 2012;147:1107–13. doi: 10.1001/archsurg.2012.1962. [DOI] [PubMed] [Google Scholar]
- Spolverato G, Kim Y, Alexandrescu S, et al. Management and Outcomes of Patients with Recurrent Intrahepatic Cholangiocarcinoma Following Previous Curative-Intent Surgical Resection. Ann Surg Oncol. 2016;23:235–43. doi: 10.1245/s10434-015-4642-9. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Sakaguchi T, Yokoi Y, et al. Clinicopathological prognostic factors and impact of surgical treatment of mass-forming intrahepatic cholangiocarcinoma. World J Surg. 2002;26:687–93. doi: 10.1007/s00268-001-0291-1. [DOI] [PubMed] [Google Scholar]
- Tan JCC, Coburn NG, Baxter NN, et al. Surgical management of intrahepatic cholangiocarcinoma-a population-based study. Ann Surg Oncol. 2008;15:600–8. doi: 10.1245/s10434-007-9627-x. [DOI] [PubMed] [Google Scholar]
- Weber SM, Jarnagin WR, Klimstra D, et al. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193:384–91. doi: 10.1016/s1072-7515(01)01016-x. [DOI] [PubMed] [Google Scholar]
- Wirasorn K, Ngamprasertchai T, Khuntikeo N, et al. Adjuvant chemotherapy in resectable cholangiocarcinoma patients. J Gastroenterol Hepatol. 2013;28:1885–91. doi: 10.1111/jgh.12321. [DOI] [PubMed] [Google Scholar]
- Wu ZF, Wu XY, Zhu N, et al. Prognosis after resection for hepatitis B virus-associated intrahepatic cholangiocarcinoma. World J Gastroenterol. 2015;21:935–43. doi: 10.3748/wjg.v21.i3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J, Kosuge T, Shimada K, et al. Intrahepatic cholangiocarcinoma: proposal of new macroscopic classification. Nihon Geka Gakkai Zasshi. 1993;94:1194–200. [PubMed] [Google Scholar]
- Yamamoto M, Ariizumi S. Intrahepatic recurrence after surgery in patients with intrahepatic cholangiocarcinoma. J Gastroenterol. 2006;41:925–6. doi: 10.1007/s00535-006-1901-3. [DOI] [PubMed] [Google Scholar]


