Abstract
Background and Objective:
Any role of human papillomavirus (HPV) in the development of breast cancer is conjectural. The aim of this study was to investigate possible links between HPV and breast cancer in women, Sanandaj, Iran.
Methods:
In this case-control study, 70 formalin fixed and paraffin embedded blocks of breast malignant tumors as a case group and 70 blocks of lesions without malignancy were selected as controls. Sections about 10 µm thick were prepared. After removing the paraffin, DNA was extracted. Samples were tested by PCR using general and high-risk specific HPV primers.
Results:
All 70 malignant breast tumors (cases) were invasive ductal carcinomas, and of the 70 controls, 17 (24.3%) were fibrocystic tumors and 53 (75.7%) fibroadenomas. The age range of women in the case group was 25-72 years old and in the control group It was13-66 years. Using HPV general primers two samples were positive in the case group, confirmed to be HPV-18 using high-risk specific primers.
Conclusion:
No statistically significant association was found between breast cancer and HPV. It is necessary to confirm this result by further investigations in other populations.
Keywords: Human papillomavirus (HPV), breast cancer, women, benign and malignant tumors
Introduction
Breast cancer is the most frequent malignancy in women around the world. Its incidence and mortality rates have been rising in Asian countries (Zhou et al., 2015). A number of risk factors have been associated for breast cancer, including age, dense breast and familial history. However, recognized risk factors have been absent in some percent of breast cancers, which has caused more interest in identifying new risk factors for the pathogenesis of breast cancer (Wang et al., 2012).
Recently, investigators have linked breast cancer to viruses including Epstein–Barr virus, mouse mammary tumor virus, and human papillomavirus (HPV). Association between high risk HPVs and other cancers, such as cervical, anogenital, upper respiratory, gastrointestinal tract, and skin cancers, has been established. For example, 99.7% of cervical cancer has been related to HPV infection. Theory of HPV infection is important in the prevention of cancer because HPV vaccines are available (Wang et al., 2012; Bae and Kim, 2016).
The association between HPV infection and breast cancer was first proposed in 1992. A number of studies have identified HPV DNA in 0 to 86% of breast cancer specimens. This wide range indicates an inconsistent association between HPV and breast cancer (Zhou et al., 2015). However, a meta-analysis reported that HPV infection increased the risk of breast cancer (Bae and Kim, 2016). Also, HPV type 16, 18, 31, 33, 45, 51 and 58 were more prevalent in breast cancer in China (Fu et al., 2015).
Studies demonstrated the presence of HPV DNA in both malignant and benign tumors in women with breast lesions using polymerase chain reaction in Isfahan, Iran (Manzouri et al., 2014), in the north part of Iran (Sigaroodi et al., 2012) and in Iraq using in situ hybridization (Ali et al., 2014). Some studies reported that HPV was not present in any of the breast specimens irrespective of histology, hormonal status and stage of disease (Vernet-Tomas et al., 2015).
Thus, controversy exists about the association between HPV and breast cancer. In particular, there is debate over the use of formalin fixed and paraffin embedded (FFPE) tissue to test for HPV DNA, because DNA can be destroyed during the DNA extraction procedure, it means that FFPE will have more false negative errors than fresh frozen (FF) tissue. Although HPV was detected in some studies and not in others, it is suggested that these differences can be attributed to differences in the DNA extraction procedures and HPV detection methods. In addition, the detection rate of HPV infection can be influenced by HPV DNA source, heterogeneity of primers used in HPV detection, contamination and geographic region (Bae and Kim, 2016). The aim of this study was to evaluate the association between HPV and breast cancer among two groups of women (women with breast cancer and women with noncancerous breast tissues) in Sanandaj, Iran.
Material and Methods
Study population and Specimens
In this case-control study, 70 formalin fixed and paraffin embedded (FFPE) blocks from breast tumors in women with malignant tumor as the case group and 70 blocks from breast tumors without malignancy were selected as controls. These samples were collected from patients who were referred to Besat and Tohid hospitals and Noor pathology laboratory (west of Iran, Sanandaj) from 2009 to 2014. The study was approved by ethic committee at Kurdistan University of Medical Scientist (ethic code: MUK.REC.1392.32). Telephone base agreement was prepared from the patients to participate their samples in the study. Sections with 5 to10 µm thick were prepared from blocks using sterile microtome blade and collected in sterile microtubes.
Tissue Deparaffinization
Breast tissue sections were deparaffinized (Genetbio, Korea), according to manufacturer’s instruction. Briefly, breast tissue sections were thrown into 1 ml xylene at 50-60º C for 15 minutes. Then supernatant was separated by centrifugation at 13,000 rpm and this step repeated 3-4 times and washed in 100%, 90%, 70%, 50% ethanol for 5 minutes.
DNA extraction
DNA was extracted using PrimePrepTM genomic DNA isolation kit (GenetBio, Korea) from deparaffinated breast tissue sections according to manufacturer’s instruction. Nucleic acid concentrations were determined by spectrophotometry. To verify the integrity of extracted DNA, PCR test was performed using primers for human beta-globin gene on all samples. To reduce the exposure of patient samples to potential sources of DNA contamination, standard laboratory procedures were practiced when handling and processing of specimens.
PCR tests for detection of beta-globin and HPV
PCR reaction was done in a total volume of 25µl reaction mixture including 10µl of PCR 2×master mix (SinaClon, Iran), 1µl forward and reverse primers each, 5µl DNA template, and 8µl distilled water.
PCR amplification program for beta-globin was as: Initial denaturation at 94ºC 5 min, followed by 30 cycles of denaturation at 94ºC, 40s; annealing at 59ºC 30s, extension at 72ºC, 40s; and final extension at 72ºC for 5 min.
All beta-globin positive samples were tested for the detection of HPV DNA using HPV general primers (GP5+ and GP6+) designed for L1 region of HPV genome. Again, PCR test was performed on all samples using high-risk HPV specific primers designed for E7 gene of viral genome. Primers sequences are shown in Table 1.
Table 1.
Name and Sequences of Human Beta-Globin, Human Papillomavirus (HPV) General and HPV Type Specific Primers, the Target and Length of PCR Products are Shown
| Primer name | Primer sequence | Target | Length of PCR product (base pairs) | Reference |
|---|---|---|---|---|
| PCO4 PCO3 | 5’-CAACTTCATCCACGTTCACC-3’ 5’-ACACAACTGTGTTCACTAGC-3’ |
Human Beta-globin | 110 | (Aguayo et al., 2011) |
| GP5+ GP6+ | 5’-TTTGTTACTGTGGTAGATACTAC-3’ 5’-GAAAAATAAACTGTAAATCATATTC-3’ |
L1 | 150 | (de Roda Husman et al., 1995; Mohamadian Roshan et al., 2014) |
| HPV-16 | 5’-TTATGAGCAATTAAATGACAGCTCAG-3’ 5’-TGAGAACAGATGGGGCACACAAT-3’ |
E7 | 215 | (Gheit and Tommasino, 2011) |
| HPV-18 | 5’-GACCTTCTATGTCACGAGCAATTA-3’ 5’-TGCACACCACGGACACACAAAG-3’ |
E7 | 236 | (Gheit and Tommasino, 2011) |
| HPV-31 | 5’-AGCAATTACCCGACAGCTCAGAT-3’ 5’-GTAGAACAGTTGGGGCACACGA-3’ |
E7 | 210 | (Gheit and Tommasino, 2011) |
| HPV-33 | 5’-CTACAGTGCGTGGAATGCAAAAAACC-3’ 5’-CGGGACCTCCAACACGCCGCAC-3’ |
E6 | 347 | (Cuzick et al., 1994) |
PCR amplification program for HPV using general primers was as: Initial denaturation 94ºC, 5 min; followed by 30 cycles of denaturation at 94ºC, 30s; annealing at 50ºC, 30s; extension at 72ºC, 40s; and final extension at 72ºC for 5 min.
PCR amplification program for HPV using genotype specific primers was as: Initial denaturation 94ºC for 5 min, followed by 30 cycles of denaturation at 94ºC, 30s; annealing at 58ºC, 30s; extension at 72ºC, 40s; and final extension at 72ºC for 5 min.
HPV-18 positive HeLa cell line was used as positive control (national cell bank, Iran Pasteur institute, NCBI Code: C115). PCR products were separated by electrophoresis on 2% agarose gel, stained by SYBR Green and visualized by UV light and photographed.
Sequencing
In order to confirm the results, positive PCR products (having DNA band with expected molecular length, Table 1) were sequenced (ABI 3730.1 DNA analyzer, 96-capilary sequencer, Macrogen, Korea). The results of sequencing were compared with the sequences stored in GenBank database using NCBI BLAST software.
Statistical analysis: The data were entered into SPSS statistical software, version 20, and analyzed. For comparison of qualitative variables Chi-square and Fischer tests were used in different groups.
Results
All 70 malignant breast tumors (cases) had invasive ductal carcinoma, but in 70 nonmalignant breast tissues (controls), 17 patients (24.3%) had fibrocystic tumors and 53 patients (75.7%) had fibroadenomas. In malignant group 2 (1.4%) had malignancy grade II, 34 (24.3%) grade III, 32 (22.9%) grade II/III and 2 (1.4%) grade III/IV. In the case group, 45 (64.3%) had a tumor in her right breast and 25 (35.7%) in their left breast. But, in the control group, 35 (50%) had tumor in her right breast and 35 (50%) in her left breast (Table 2).
Table 2.
Age Range, Tumor Type and Location, Degree of Malignancy, and Frequency of Human Papillomavirus (HPV) Infection in Women with Malignant Breast Tumors (cases) and Women with Nonmalignant Breast Tissues (Controls)
| Variables | Cases (malignant breast tumors) n=70 | Controls (nonmalignant breast tumors) n=70 | p-value | |
|---|---|---|---|---|
| Age range (years) | 25-72 (mean: 47.8, SD: 10.1) | 13-66 (mean: 31.5, SD: 10.4) | 0.001 | |
| Invasive Ductal Carcinoma | 70 | 0 | ||
| Fibrocystic tumor | 0 | 17 (24.3%) | ||
| Fibroadenoma | 0 | 53 patients (75.7%) | ||
| Tumor location | Left breast: 25 (35.7%) | Left breast: 35 (50%) | 0.088 | |
| Right breast: 45 (64.3%) | Right breast: 35 (50%) | |||
| Degree of malignancy | II | 2 (1.4%) | ||
| III | 34 (24.3%) | |||
| II/III | 32 (22.9%) | |||
| III/IV | 2 (1.4%) | |||
| HPV-16 | 0 | 0 | ||
| HPV-18 | 2 (2.56%) | 0 | 0.496 | |
| HPV-31 | 0 | 0 | ||
| HPV-33 | 0 | 0 | ||
The age of women in the case group was 25-72 (mean 47.8) years and in the control group 13-66 (mean 31.5) years, respectively (Table 2).
In the case group by using HPV general primers two samples had positive PCR result. Also by using specific primers, HPV-18 was found in the same two samples that were positive with general primers. The results were verified by sequencing.
Other high risk human papillomavirus types such as HPV-16 and HPV-31 and HPV-33 were not found in malignant and nonmalignant tumor tissues. The prevalence of HPV infection was 2 out of 70 (2.56%) in the case group, and 0 in control group. The difference of the prevalence rate was not statistically significant among two groups (p=0.496). Age range, tumor type, tumor location, degree of malignancy, frequency of HPV infection in women with malignant breast tumors (cases) and women with nonmalignant breast tissues (controls) are shown in Table 2.
A representative stained electrophoresis gels of PCR assays are shown in Figures 1 and 2.
Figure 1.
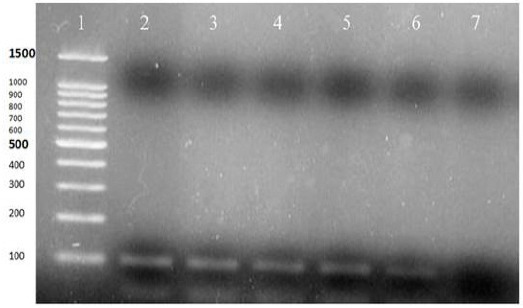
PCR Test for Human Beta-Globin Gene: Lane 1) 100bp DNA Ladder (CinnaClon, Iran, Cat no PR9015644), Lanes 2-6) PCR Positive (110bp), Lane 7) Negative Control
Figure 2.

PCR Test for Human Papillomavirus Using Type Specific Primers; Lane 1) 100bp DNA Ladder (CinnaClon, Iran), Lane 2) Positive Control (236 bp), Lane 3) HPV Positive Result (236 bp), Lane 4) Negative Control, Lanes 5 and 6) Negative Results
Discussion
In our study, HPV-18 was found in 2.6% of breast cancer samples and there was no association between HPV and breast cancer.
In a previous systematic review twenty-nine studies, including 2211 samples, overall HPV prevalence in patients with breast cancer was 23.0%. The prevalence of HPV in controls was 12.9%. The prevalence of HPV ranged from 13.4% in Europe to 42.9% in North America and Australia (Simoes et al., 2012).
Recently, a meta-analysis was conducted among publications with data from case-control studies on HPV DNA in breast tissues. In twenty-two case-control studies the total number of individuals in the case and control group was 1897 and 948, respectively. According to the meta-analysis among 22 publications, HPV infection increased the risk of breast cancer. No statistical significance was found in meta-regression conducted on nationality, type of tissue, type of HPV, and publication year, although some researchers think they are sources of heterogeneity. However, there are disagreements regarding this association. Particularly, the risk differs depending on nationality of patients, type of tissue tested, type of HPV, and publication year (Bae and Kim, 2016).
In a previous study conducted in Isfahan, Iran, out of 55 malignant and 51 benign breast specimens, 18.2% and 13.7% were positive to HPV DNA, respectively. 70% malignant and 43% benign breast specimens were positive to high-risk HPV genotypes (Manzouri et al., 2014). In another study in north part of Iran among women with breast cancer, 25.9% were positive for HPV DNA in breast tumor sample in contrast to 2.4% of women with noncancerous breast tumors. The high risk HPV genotypes, 16 and 18 were the predominant types (53.34%) in breast cancers. Other genotypes were 6, 11, 15, 23, and 124 (Sigaroodi et al., 2012). Among 67 frozen samples from breast cancers in Tehran 30% were positive for HPV. The prevalence of HPV 16, 11 ⁄ 6, and 31 ⁄ 33 were 14.9%, 11.94%, and 2.99%, respectively (Ghaffari et al., 2011). Also, HPV DNA was detected in 48% samples of breast carcinoma, which 26% of them had high-risk and 16% had low risk HPV. Three breast cancer tissues were infected by both high and low risk HPV genotypes. All of nonmalignant mammary tissues were negative for HPV. There was no association between the HPV and prognostic factors of breast cancer such as age, grade of tumor (Alavi et al., 2009).
In our neighboring country, Iraq, a study used in situ hybridization (ISH) to detect the frequency and genotypes of HPV in tissues from 129 patients with malignant breast cancer, 24 with benign breast tumors and 20 healthy controls. In the breast cancer group HPV genotypes were detected in 60 (46.5%) of archived tissue blocks. Of these, genotypes 16, 18, 31 and 33 was 55.5%, 58.4%, 65.0% and 26.6%, respectively. Also mixed HPV genotypes 16/18, 16/18/31, 16/18/33, 18/33, 16/31 and 18/31 were found in 5%, 25%, 8.3%, 7.7%, 10% and 13.3% of cancer cases, respectively. Only three benign breast tumor tissues (12.5%) and none of the healthy breast tissues were HPV positive (Ali et al., 2014). HPV was found in 4 of 70 specimens (5.7%) using ISH and in 2 of 70 specimens (2.9%) of specimens using IS-PCR in USA (Baltzell et al., 2012). In Argentina the HPV DNA prevalence in the breast cancers was 26%. Clinical parameters were not statistically associated with HPV presence. Sequence analysis in a subgroup of cases indicates the prevalence of low risk HPV-11, followed by high risk HPV-16. Also, they found no HPV transcriptional activity (Pereira Suarez et al., 2013). In comparison, prevalence of HPV in our population is very low or we had possibility of detection failure.
In some studies researchers did not find HPV DNA in any of the breast cancer tissue specimens they tested, for example in China (Chang et al., 2012; Zhou et al., 2015), in Mexican female population (Herrera-Romano et al., 2012) and Brazil (Silva and da Silva, 2011). Also in our neighboring country, Turkey, all tested samples (84 breast carcinoma) were negative for HPV DNA (Yavuzer et al., 2010). These results are somewhat compatible with our result.
Studies have shown that the early viral proteins (E6 and E7) are important in carcinogenesis. Since in cancerous tissues the HPV genome integrates into the host chromosome to express more early viral proteins, thus other genes, including L1 gene may be removed. Thus, in studies in which identification of HPV is based only on detection of L1 gene, there is possibility of false negative results. In our study, HPV identifying PCR primers were designed to detect the presence of viral early gene (E7) in order to have no false negative results.
Although both type-specific primers and general primers have been used for detection of HPV in previous studies, most of these primers were designed for mucosal HPV types (10, 11, 13, 16, 20, and 21). Thus, coetaneous HPV types might escape detection (Zhou et al., 2015).
Studies in immunocompromised patients showed that, there is a substantial increase in the prevalence of HPV associated cancers, such as cervical, head and neck cancers. But, there is no increase in breast cancer prevalence in immunocompromised patients who have AIDS or organ transplantations and immunosuppression therapies. Thus, HPVs may act early in some breast oncogenesis by “hit-and-run” phenomena. A study conducted immunohistochemistry for the identification of HPV E7 oncoprotein expression in sets of benign and subsequent breast cancer specimens from the same Australian patients. There was no HPV E7 protein expression in 30% of the breast cancer specimens that had prior HPV E7 protein-positive benign breast tissues in the same patients. Therefore early influence of HPVs on breast may be the reason why there is no increase in the HPV associated breast cancer in immunocompromised patients as compared to HPV associated cervical cancer (Ngan et al., 2015) or breast cancers in some populations.
Limitation of our study was that, we did not have access to fresh tissues. Thus, archiving of formalin fixed and paraffin embedded tissue blocks for long time, sectioning and deparaffinization of sections with xylol may cause DNA damage thus possibility of detection failure. However, we did the PCR test for detection of HPV in betaglobin positive samples. In addition, we could not conduct cohort study and immunohistochemistry for the identification of HPV E7 oncoprotein expression to evaluate “hit-and-run” phenomena.
No statistically significant association was found between HPV and breast cancer in this study. So there may be other factors involved in tomurogenesis of breast cancer in our population or HPVs may act early in some breast oncogenesis by “hit-and-run” phenomena. However, there are possibility of detection failure, thus it is necessary to confirm this result by further investigations by different detection methods and in other populations.
Acknowledgements
We would like to thank Kurdistan University of Medical Sciences for financial support and Pathology laboratory, Tohid hospital, Sanandaj, Iran, for preparing specimens and tissue sectioning. This study was a medical microbiology MSc thesis.
Conflict of Interest
No conflict of interest was declared by the Authors.
References
- Aguayo F, Khan N, Koriyama C, et al. Human papillomavirus and Epstein-Barr virus infections in breast cancer from chile. Infect Agent Cancer. 2011;6:7. doi: 10.1186/1750-9378-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi SG, Sharifi N, Sadeghian A, et al. (in Persian) Presence of Human Papilloma Virus sequences in breast cancer tissues and association with histopathological features. Iran J Obstet Gynecol Infertil. 2009;12:1–4. [Google Scholar]
- Ali SH, Al-Alwan NA, Al-Alwany SH. Detection and genotyping of human papillomavirus in breast cancer tissues from Iraqi patients. East Mediterr Health J. 2014;20:372–7. [PubMed] [Google Scholar]
- Bae JM, Kim EH. Human papillomavirus infection and risk of breast cancer: a meta-analysis of case-control studies. Infect Agent Cancer. 2016;11:14. doi: 10.1186/s13027-016-0058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltzell K, Buehring GC, Krishnamurthy S, et al. Limited evidence of human papillomavirus in breast tissue using molecular in situ methods. Cancer. 2012;118:1212–20. doi: 10.1002/cncr.26389. [DOI] [PubMed] [Google Scholar]
- Chang P, Wang T, Yao Q, et al. Absence of human papillomavirus in patients with breast cancer in north-west China. Med Oncol. 2012;29:521–5. doi: 10.1007/s12032-011-9945-5. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Terry G, Ho L, et al. Type-specific human papillomavirus DNA in abnormal smears as a predictor of high-grade cervical intraepithelial neoplasia. Br J Cancer. 1994;69:167–71. doi: 10.1038/bjc.1994.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roda Husman AM, Walboomers JM, Van den Brule AJ, et al. The use of general primers GP5 and GP6 elongated at their 3’ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995;76:1057–62. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]
- Fu L, Wang D, Shah W, et al. Association of human papillomavirus type 58 with breast cancer in Shaanxi province of China. J Med Virol. 2015;87:1034–40. doi: 10.1002/jmv.24142. [DOI] [PubMed] [Google Scholar]
- Ghaffari SR, Sabokbar T, Meshkat Z, et al. Tracing human papilloma virus in breast tumors of Iranian breast cancer patients. Breast J. 2011;17:218–9. doi: 10.1111/j.1524-4741.2010.01053.x. [DOI] [PubMed] [Google Scholar]
- Gheit T, Tommasino M. Detection of high-risk mucosal human papillomavirus DNA in human specimens by a novel and sensitive multiplex PCR method combined with DNA microarray. Methods Mol Biol. 2011;665:195–212. doi: 10.1007/978-1-60761-817-1_12. [DOI] [PubMed] [Google Scholar]
- Herrera-Romano L, Fernandez-Tamayo N, Gomez-Conde E, et al. Absence of human papillomavirus sequences in epithelial breast cancer in a Mexican female population. Med Oncol. 2012;29:1515–7. doi: 10.1007/s12032-011-0059-x. [DOI] [PubMed] [Google Scholar]
- Manzouri L, Salehi R, Shariatpanahi S, et al. Prevalence of human papilloma virus among women with breast cancer since 2005-2009 in Isfahan. Adv Biomed Res. 2014;3:75. doi: 10.4103/2277-9175.125873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamadian Roshan N, Jafarian A, Ayatollahi H, et al. Correlation of laryngeal squamous cell carcinoma and infections with either HHV-8 or HPV-16/18. Pathol Res Pract. 2014;210:205–9. doi: 10.1016/j.prp.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Ngan C, Lawson JS, Clay R, et al. Early Human Papilloma Virus (HPV) Oncogenic Influences in Breast Cancer. Breast Cancer. 2015;9:93–7. doi: 10.4137/BCBCR.S35692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira Suarez AL, Lorenzetti MA, Gonzalez Lucano R, et al. Presence of human papilloma virus in a series of breast carcinoma from Argentina. PLoS One. 2013;8:e61613. doi: 10.1371/journal.pone.0061613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigaroodi A, Nadji SA, Naghshvar F, et al. Human papillomavirus is associated with breast cancer in the north part of Iran. Scientific World J. 2012;2012:837191. doi: 10.1100/2012/837191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RG, Jr, Da Silva BB. No evidence for an association of human papillomavirus and breast carcinoma. Breast Cancer Res Treat. 2011;125:261–4. doi: 10.1007/s10549-010-1129-z. [DOI] [PubMed] [Google Scholar]
- Simoes PW, Medeiros LR, Simoes Pires PD, et al. Prevalence of human papillomavirus in breast cancer: a systematic review. Int J Gynecol Cancer. 2012;22:343–7. doi: 10.1097/IGC.0b013e31823c712e. [DOI] [PubMed] [Google Scholar]
- Vernet-Tomas M, Mena M, Alemany L, et al. Human papillomavirus and breast cancer: no evidence of association in a Spanish set of cases. Anticancer Res. 2015;35:851–6. [PubMed] [Google Scholar]
- Wang T, Chang P, Wang L, et al. The role of human papillomavirus infection in breast cancer. Med Oncol. 2012;29:48–55. doi: 10.1007/s12032-010-9812-9. [DOI] [PubMed] [Google Scholar]
- Yavuzer D, Salepci T, Karadayi N, et al. Human papillomavirus is not associated with breast carcinoma. Breast Cancer Res Treat. 2010;122:899–900. doi: 10.1007/s10549-010-0963-3. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Li J, Ji Y, et al. Inconclusive role of human papillomavirus infection in breast cancer. Infect Agent Cancer. 2015;10:36. doi: 10.1186/s13027-015-0029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


