Abstract
Introduction:
Lung cancer is one of the most common cancers worldwide. In certain countries such as United States of America, it is the leading cause of related cancer mortality among both men and women. Natural products play an important role in overcoming the limitations of chemotherapy and radiotherapy.
Objectives:
In this study, we investigated the antiproliferative and apoptotic activities of Kanahia laniflora methanolic extract against human non-small cell lung cancer cells (A549).
Methods:
Sulforhodamine B colorimetric assays were used to determine the inhibitory effects of a leaf methanolic extract against A549 cells.
Results:
The extract showed strong cytotoxic activity against A549 cells with an IC50 value of 0.13 µg/ml compared to 0.21 µg/ml for doxorubicin. The extract also significantly increased the percentage of apoptotic cells to 49.7% as compared to 1.4% and 47.4% for control and doxorubicin respectively.
Conclusion:
These results showed, for the first time, that a methanolic extract of Kanahia laniflora leaves can inhibit the proliferation of human non-small cell lung cancer cells (A549). Further attention to its potential as a new effective anticancer agent is warranted.
Keywords: Lung cancer, A549, kanahia laniflora, apoptosis, herbal medicine
Introduction
Lung cancer is the most common cancer worldwide. It is responsible for more than 1.4 million death per year (Wood et al., 2015). In the United States alone, an estimation of 224,210.0 new cases and 159,260.0 deaths for lung cancer were predicted during 2014 (Lemjabbar-Alaoui et al., 2015). The highest incidence occurs in North America and Europe. In the United Kingdome, it is the second most common cancer in men and third most common cancer and leading cause of death in women (Teh and Belcher, 2014). According to the last cancer incidence report 2011 by Saudi Cancer Registry, the lung cancer is the most seventh cancer among Saudis with 452.0 registered cases accounting for 4.2% of all diagnosed cases in year 2011. Lung cancer ranked fourth among male and twelfth among female. It affected 343 (75.9%) males and 109.0 (24.1%) females (Saudi Cancer Registry, 2011). The lung cancer can be divided into two main types: non-small cell-lung cancer (NSCLC) and small cell-lung cancer (SCLC). NSCLC is the more common type accounting for approximately 85% of all lung cancer cases (Langevin et al., 2015).
Although radiotherapy and chemotherapy are effective against cancer, they also have serious and severe side effects and complications. Effectiveness of Chemotherapy and radiotherapy at improving patient survival is limited due to their highly cytotoxicity (Qi et al., 2010). Development of resistance in cancer cells toward many chemotherapies is another factor limiting their benefits (Safarzadeh et al., 2014). New therapeutic options for cancer treatment are a high priority for many research institutions and pharmaceutical companies around the world. These efforts are devoted to the discovery of more potent drugs, while reducing their toxic side effects. (Khazir et al., 2014).
Natural Products, especially plants, have been used for the treatment of various ailments for thousands of years. According to The World Health Organization estimations, approximately 80.0% of the world’s population depends on traditional medicine for their basic health care (Shoeb, 2006).
Kanahia laniflora (Forssk.) R. Br. (Apocynacea-Asclepidoideae) (Rapini et al., 2003) is a shrub populating lakeshores, seasonal streams and lakeshores of tropical and subtropical Africa (Clarkson et al., 2006). The plant has been recorded also in southwest of Saudi Arabia (Al Wadie, 2007; KAMEL et al., 2014) and Yemen (Mothana et al., 2009). It has been reported that the plant is used against epilepsy in Kenya (Clarkson et al., 2006). In Ethiopia, it is used as a treatment for anthrax, Render pest, dingetegna, TB, arthritis and headache. The latex of the plant is used for ear infection (Seifu et al., 2006; Giday et al., 2007; Gemechu et al., 2013). Other reported ethnomedicinal uses of Kanahia laniflora in Saudi Arabia and Yemen includes tumors and skin diseases, scabies and itching (Mothana et al., 2009; Al-Musayeib et al., 2012). To our best of knowledge, antiproliferative activity of Kanahia laniflora against human lung cancer cells has not been investigated so far. Therefore, the present study aimed to explore the antiproliferative activity of the methanolic extract of Kanahia laniflora leaves against human non-small cell lung cancer cells (A549).
Materials and Methods
Reagents
SulphoRhodamine-B (SRB), methanol and ethanol were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Culture media and growth supplements were purchased from Gibco-Life Technologies Co, (Carlsbad, CA, USA).
Preparation of plant materials
Kanahia laniflora fresh leaves were collected from Abha, southwest region, Saudi Arabia, on November 2015. Leaves were washed with distilled water and dried in the air. Dried leaves were grounded into fine powder using laboratory grinder and soaked in methanol for 24 hours at room temperature. The extract was filtered and the solvent was removed under reduced pressure. The dry extract was stored as aliquots at -20 until further use (Paydar et al., 2013; Alhazmi et al., 2014).
Cell culture
Human non-small cell lung cancer cells (A549) were obtained from Vacsera (Giza, Egypt). Cells were grown in RPMI media supplemented with 100 µg/mL streptomycin, 100 units/ml penicillin and 10% heat-inactivated fetal bovine serum in a humidified, 5% (v/v) CO2 atmosphere at 37 ºC.
In vitro cytotoxicity screening
The Sulforhodamine B colorimetric assay (SRB assay) (Skehan et al., 1990; Vichai and Kirtikara, 2006) was used to determine the inhibitory effect of Kanahia laniflora methanolic extract against A549 cells. Exponentially growing cells were detached from dishes using 0.25% trypsin–EDTA and plated in 96-well plates at 1,000.0 cells/well. After 24 hours of incubation, cells were exposed to various concentrations of extract or doxorubicin for 72 h. At the end of treatment time, cells were fixed with TCA (10%) for 1h at 4°C, washed several times with distilled water, stained with 0.4% SRB solution for 10 min in a dark place and washed with 1% glacial acetic acid. After drying overnight, Tris–HCl was used to dissolve the SRB-stained cells and the color intensity was measured at 450 nm using microplate reader (Anthos Zenyth-200RT, Cambridge, England). The half maximal inhibitory concentration (IC50) was determined by the trend line equation using SigmaPlot version 12.
Apoptosis assessment with Acridine orange/Ethidium bromide staining
The Acridine orange/Ethidium bromide staining (AO/EB) method was used to identify live, early apoptotic, late apoptotic and necrotic cells. A549 cells were seeded in 6-well tissue culture plates (1x106 cells/well). After 24 h of incubation, cells were treated with IC50 of methanolic extracts of Kanahia laniflora or with DEMSO and incubated for 48 h. After incubation, cells were detached with 0.25% trypsin-EDTA and washed once with phosphate buffer saline (PBS). 20 microliters of the cells were then transferred to a glass slide and mixed with 2 μl of AO/EB mixture (100 μg/ml of AO and 100 μg/ml of EB in phosphate buffer saline). The apoptotic, necrotic and live cells of at least 100.0 cells were examined immediately under a fluorescence microscope (Nikon Eclipse E400). Viable cells stained only by AO were bright green with an intact structure; early apoptotic cells stained by AO had a bright green area in the nucleus. Late apoptotic cells stained by AO and EB were red–orange with condensation of chromatin visible as dense orange areas; these cells had a reduced size (Squier and Cohen, 2001; Ribble et al., 2005; Lou et al., 2009). The experiment was performed in triplicate and the percentages of the apoptotic cells were calculated.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). The Statistical significance of differences between means was determined one-way analysis of variance (one-way ANOVA) followed by Tukey’s or Dunnett’s multiple comparisons test. P-values less than 0.05 was considered to be statistically significant. All statistical analysis was performed using GraphPad Prism 6 software.
Results
In vitro cytotoxicity
SRB assay was used to assess the anti-proliferative effect of Kanahia laniflora methanolic extract against human non-small cell lung cancer cells (A549). The extract decreased cells proliferation and exerted high cytotoxic effect against A594 cells with IC50 = 0.13 µg/ml. On the other hand, doxorubicin which was used as a positive control, also suppressed proliferation of A549 cells with IC50 = 0.21 µg/ml. Table 1 and figure 1 summarized cytotoxic effects of Kanahia laniflora extract comparing to doxorubicin. The extract and doxorubicin reduced A549 cell viability in concentration-dependent manner. The concentration 0.1 µg/ml reduced cell viability in both treatments by nearly 40%, and the 100 µg/ml of extract reduced cell viability by 96.5% while 100 µg/ml of doxorubicin reduced cell viability by 79%.
Table 1.
Percentages of Viable A549 Cells after 73 h of Treatment with Different Concentrations of Kanahia Laniflora Methanolic Extract or Doxorubicin
| Concentration | % of viable cells | |
|---|---|---|
| µg/ml | K. laniflora | Doxorubicin |
| 0.0 | 100.0 ± 0.7 | 100.0 ± 11.4 |
| 0.01 | 87.3 ±2.0 | 75.4 ± 4.0 |
| 0.1 | 61.3 ± 1.7 | 58.5 ± 0.80 |
| 1.0 | 5.8 ± 0.2 | 42.7 ± 1.75 |
| 10.0 | 4.6 ± 0.6 | 30.9 ± 0.9 |
| 100.0 | 3.5 ± 0.2 | 19.1 ± 0.8 |
Figure 1.
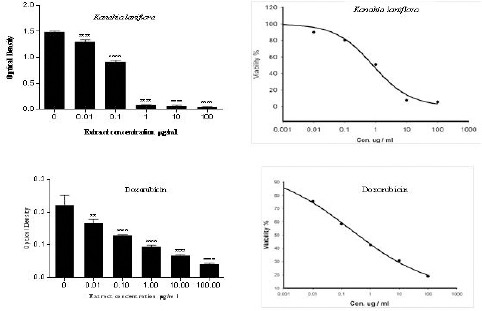
Dose Response Curve of Kanahia Laniflora Leaves methanolic Extract and Doxorubicin Against A549 Cell Line. Cells were Incubated with Different Concentrations of the Extract or Doxorubicin for 72 Hours
Apoptosis assessment with AO/EB staining
We used acridine orange/ethidium bromide double staining to investigate the apoptotic effects of Kanahia laniflora leaves methanolic extract against A549 cell line. Figures 2 and 3 and table 2 shows the effects of the extract on the treated cells comparing to that induced by doxorubicin. The cells treated with IC50 of the methanolic plant extract (0.13 µg/ml) showed significant increase in early apoptotic (8.1%) and apoptotic cells (49.7%) comparing to (0.7%) and (1.4%) found in control cells. The percentage of early apoptotic cells (8.3%) and apoptotic cells (47.4%) was comparable to those of the methanolic extract.
Figure 2.
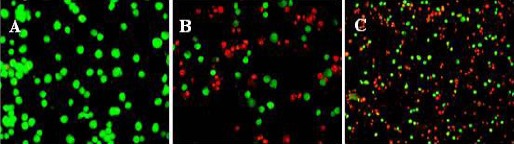
Apoptosis Assessment in A549 Cells by AO/EB Double Staining. Cells were Treated with IC50 of Kanahia laniflora Leaves Methanolic Extract or Doxorubicin and Incubated for 48 h. A: Control, B: Kanhia Laniflora and C: Doxorubicin
Figure 3.
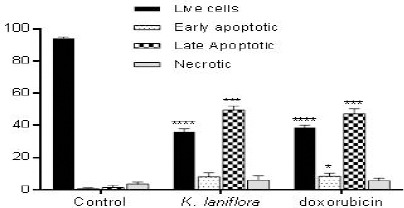
Percentage of Live, Early Apoptotic, Late Apoptotic and Necrotic Cells. A549 Cells were Stained with AO/EB After Treatment
Table 2.
Percentage of Live, Early Apoptotic, Late Apoptotic and Necrotic Cells. A549 Cells were Stained with AO/EB After Treatment
| Live cells | Early apoptotic | Late apoptotic | Necrotic | |
|---|---|---|---|---|
| Control | 94.0 ± 1.01 | 0.7 ± 0.58 | 1.4 ± 1.53 | 3.7 ± 1.16 |
| K. laniflora | 36.4 ± 1.53 | 8.1 ± 2.65 | 49.7 ± 2.52 | 6.0 ± 1.98 |
| Doxorubicin | 38.7 ± 1.54 | 8.3 ± 2.08 | 47.4 ± 1.06 | 5.7 ± 1.53 |
Discussion
Lung cancer is one of the most common cancers worldwide. In certain countries such as United States of America, it is the lead cause of related cancer mortality among both men and women (Lemjabbar-Alaoui et al., 2015). Lung cancer treatment includes surgery, radiotherapy, chemotherapy and immunotherapy (Monteiro et al., 2014; Ravinder Singh and Kathiresan, 2014). Regardless of advancement achieved in conventional cancer therapy, the 5-years survival rate for non-small cell lung cancer is only about 15.0%, which considered one of the lowest rates among cancers. Additionally, the conventional cancer chemotherapy can cause serious side effects that diminish the quality of life of the lung cancer patients. (Lee et al., 2015). Development of multidrug resistance toward many chemotherapies is another problem limiting their efficacy (Safarzadeh et al., 2014). There is a pressing need for developing more efficient and less toxic new drugs for cancer treatment (Khazir et al., 2014).
Plants have been used for a long time in the treatment of many diseases including cancer and continue to be an important source of therapeutic agents. It has been reported that approximately 77% of cancer patients used herbal medicines in combination with conventional chemotherapy (Monteiro et al., 2014). Kanahia laniflora (Forssk.) R. Br. is a medicinal plant distributed in several countries including Ethiopia, Saudi Arabia, Yemen and Kenya (Al Wadie, 2007; Mothana et al., 2009; Gemechu et al., 2013; Kamel et al., 2014).
In this study, for the first time, we investigated the potential antiproliferative effect of the methanolic extract of Kanahia laniflora against human non-small cell lung cancer (A549). Kanahia laniflora methanolic extract remarkably suppressed proliferation of A549 cells with IC50 of 0.13 µg/ml comparing to 0.2 µg/ml for the standard anticancer drug doxorubicin. The observed reduction of cell viability was in concentration dependent manner.
Acridine orange/ethidium bromide fluorescent double staining is one of the most reliable and fast techniques used to detect apoptosis (Baskić et al., 2006). Acridine orange can penetrate the plasma membrane of viable and nonviable cells and emits green fluorescence when bound to DNA, while ethidium bromide can only penetrate to nonviable cells that lost membrane integrity and emits red fluorescence when bound to DNA (Baskić et al., 2006; Elkady et al., 2014). In this study, treatment of A549 cells with the IC50 of Kanahia laniflora methanolic extract significantly increased the percentage of apoptotic cells. Apoptosis, a form of programmed cell death, is an important mechanism used by the body to eliminate damaged or unwanted cells (Khan et al., 2006). Many anticancer drugs are developed to induce apoptosis in cancer cells and finally kill them (Brunelle and Zhang, 2010). The antiproliferative activity and apoptosis induction capacity of Kanahia laniflora methanolic extract shown in this study suggested that this plant could contain active compounds that may be represent a good anticancer drugs for non-small cells lung cancer treatment.
Little is known about the chemical ingredients of Kanahia laniflora. The chemical tests of the plant’s parts (stem, fruits, roots and leaves) found positive for flavonoids, phenolic compounds and cardiac glycosides (Kruger and van der Vijver, 1986; Clarkson et al., 2005; Clarkson et al., 2006). Cardiac glycosides, phenolic compounds and flavonoids are natural products and many of these compounds have been reported to have anticancer activity (Kumar, 2013; Ravishankar et al., 2013; Calderón-Montaño et al., 2014; Roleira et al., 2015).
In conclusion, our results showed, for the first time, that the methanolic extract of Kanahia laniflora could remarkably inhibits the growth of human non-small cells lung cancer (A549) in vitro. Therefore, we suggest that this plant is worthy of further studies to assess its active chemical ingredients and their potential as anticancer drugs.
References
- Al-Musayeib NM, Mothana RA, Matheeussen A, et al. In vitro antiplasmodial, antileishmanial and antitrypanosomal activities of selected medicinal plants used in the traditional Arabian Peninsular region. BMC Complement Altern Med. 2012;12:49. doi: 10.1186/1472-6882-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Wadie HM. Plant communites in Wadi Ayaa southwestern Saudi Arabia. J Exp Biol. 2007;3:1–8. [Google Scholar]
- Alhazmi MI, Hasan TN, Shafi G, et al. Roles of p53 and caspases in induction of apoptosis in MCF- 7 breast cancer cells treated with a methanolic extract of Nigella sativa seeds. Asian Pac J Cancer Prev. 2014;15:9655–60. doi: 10.7314/apjcp.2014.15.22.9655. [DOI] [PubMed] [Google Scholar]
- Baskić D, Popović S, Ristić P, et al. Analysis of cycloheximide-induced apoptosis in human leukocytes: fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol Int. 2006;30:924–32. doi: 10.1016/j.cellbi.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Brunelle JK, Zhang B. Apoptosis assays for quantifying the bioactivity of anticancer drug products. Drug Resist Updat. 2010;13:172–9. doi: 10.1016/j.drup.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Calderón-Montaño JM, Burgos-Morón E, Orta ML, et al. Evaluating the cancer therapeutic potential of cardiac glycosides. Biomed Res Int. 2014:2014. doi: 10.1155/2014/794930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson C, Mellor IR, Lambert M, et al. 5alpha-cardenolides from Kanahia laniflora inhibit ionotropic acetylcholine receptors. Planta Medica. 2006;72:1418–20. doi: 10.1055/s-2006-951704. [DOI] [PubMed] [Google Scholar]
- Clarkson C, Stærk D, Hansen SH, et al. Hyphenation of Solid-Phase Extraction with Liquid Chromatography and Nuclear Magnetic Resonance: Application of HPLC-DAD-SPE-NMR to Identification of Constituents of Kanahia laniflora. Anal Chem. 2005;77:3547–53. doi: 10.1021/ac050212k. [DOI] [PubMed] [Google Scholar]
- Elkady A, Hussein R, El-Assouli S. Mechanism of Action of Nigella sativa on Human Colon Cancer Cells: the Suppression of AP-1 and NF-κB Transcription Factors and the Induction of Cytoprotective Genes. Asian Pac J Cancer Prev. 2014;16:7943–57. doi: 10.7314/apjcp.2015.16.17.7943. [DOI] [PubMed] [Google Scholar]
- Gemechu A, Giday M, Worku A, et al. In vitro Anti-mycobacterial activity of selected medicinal plants against Mycobacterium tuberculosis and Mycobacterium bovis Strains. BMC Complement Altern Med. 2013;13:291. doi: 10.1186/1472-6882-13-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giday M, Teklehaymanot T, Animut A, et al. Medicinal plants of the Shinasha, Agew-awi and Amhara peoples in northwest Ethiopia. J Ethnopharmacol. 2007;110:516–25. doi: 10.1016/j.jep.2006.10.011. [DOI] [PubMed] [Google Scholar]
- KAMEL EA-R, Sharawy SM, Karakish EAK. Cytotaxonomical investigations of the tribes asclepiadeae and ceropegieae of the subfamily asclepiadoideae-apocynaceae. Pak J Bot. 2014;46:1351–61. [Google Scholar]
- Khan N, Afaq F, Mukhtar H. Apoptosis by dietary factors: the suicide solution for delaying cancer growth. Carcinogenesis. 2006;28:233–9. doi: 10.1093/carcin/bgl243. [DOI] [PubMed] [Google Scholar]
- Khazir J, Mir BA, Pilcher L, et al. Role of plants in anticancer drug discovery. Phytochem Lett. 2014;7:173–81. [Google Scholar]
- Kruger A, van der Vijver L. A chemical and biological evaluation of Kanahia laniflora (Asclepiadaceae) Suid-Afrikaanse Tydskrif vir Natuurwetenskap. 1986;5:46–52. [Google Scholar]
- Kumar S. Cardiac glycosides as anticancer agent. J Pharm Biomed Sci. 2013;4:1371–8. [Google Scholar]
- Langevin SM, Kratzke RA, Kelsey KT. Epigenetics of lung cancer. Transl Res. 2015;165:74–90. doi: 10.1016/j.trsl.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-W, Kim W, Min B-I, et al. Traditional herbal medicine as an adjuvant treatment for non-small-cell lung cancer: A systematic review and meta-analysis. Eur J Integr Med. 2015;7:577–85. [Google Scholar]
- Lemjabbar-Alaoui H, Hassan OUI, Yang Y-W, et al. Lung cancer: Biology and treatment options. Biochimica et Biophysica Acta (BBA) Nat Rev Cancer. 2015;1856:189–210. doi: 10.1016/j.bbcan.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou C, Wang M, Yang G, et al. Preliminary studies on anti-tumor activity of 2′,4′-dihydroxychalcone isolated from Herba Oxytropis in human gastric cancer MGC-803 cells. Toxicol In Vitro. 2009;23:906–10. doi: 10.1016/j.tiv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Monteiro LdS, Bastos KX, Barbosa-Filho JM, et al. Medicinal plants and other living organisms with antitumor potential against lung cancer. Evid Based Complement Alternat Med. 2014:2014. doi: 10.1155/2014/604152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothana R, Gruenert R, Bednarski P, et al. Evaluation of the in vitro anticancer, antimicrobial and antioxidant activities of some Yemeni plants used in folk medicine. Pharmazie. 2009;64:260–8. [PubMed] [Google Scholar]
- Paydar M, Wong YL, Moharam BA, et al. In vitro anti-oxidant and anti-cancer activity of methanolic extract from Sanchezia speciosa leaves. Pak J Biol Sci. 2013;16:1212–5. doi: 10.3923/pjbs.2013.1212.1215. [DOI] [PubMed] [Google Scholar]
- Qi F, Li A, Inagaki Y, et al. Chinese herbal medicines as adjuvant treatment during chemo-or radio-therapy for cancer. Biosci Trends. 2010;4:297–307. [PubMed] [Google Scholar]
- Rapini A, Chase MW, Goyder DJ, et al. Asclepiadeae classification: evaluating the phylogenetic relationships of New World Asclepiadoideae (Apocynaceae) Taxon. 2003;36:33–50. [Google Scholar]
- Ravinder Singh C, Kathiresan K. Molecular understanding of lung cancers–A review. Asian Pac J Trop Biomed. 2014;4:35–41. doi: 10.12980/APJTB.4.2014C597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravishankar D, Rajora AK, Greco F, et al. Flavonoids as prospective compounds for anti-cancer therapy. Int J Biochem Cell Biol. 2013;45:2821–31. doi: 10.1016/j.biocel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Ribble D, Goldstein NB, Norris DA, et al. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005;5:12–20. doi: 10.1186/1472-6750-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roleira FM, Tavares-da-Silva EJ, Varela CL, et al. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015;183:235–58. doi: 10.1016/j.foodchem.2015.03.039. [DOI] [PubMed] [Google Scholar]
- Safarzadeh E, Sandoghchian Shotorbani S, Baradaran B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv Pharm Bull. 2014;4:421–7. doi: 10.5681/apb.2014.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifu T, Asres K, Gebre-Mariam T. Ethnobotanical and ethnopharmaceutical studies on medicinal plants of Chifra District, Afar Region, Northeastern Ethiopia. Ethiopian J Pharm. 2006;24:41–58. [Google Scholar]
- Shoeb M. Anticancer agents from medicinal plants. Bangladesh J Pharm. 2006;1:35–41. [Google Scholar]
- Skehan P, Storeng R, Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–12. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Squier MK, Cohen JJ. Standard quantitative assays for apoptosis. Mol Biotechnol. 2001;19:305–12. doi: 10.1385/MB:19:3:305. [DOI] [PubMed] [Google Scholar]
- Teh E, Belcher E. Lung cancer: diagnosis, staging and treatment. Surgery. 2014;32:242–8. [Google Scholar]
- Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–6. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- Wood SL, Pernemalm M, Crosbie PA, et al. Molecular histology of lung cancer: From targets to treatments. Cancer Treat Rev. 2015;41:361–75. doi: 10.1016/j.ctrv.2015.02.008. [DOI] [PubMed] [Google Scholar]


