Abstract
In this study, we have examined the potential of second-generation antisense chimeric 2′-O-(2-methoxy)ethyl/DNA phosphorothioate oligonucleotides (ONs) to affect cell growth through non-antisense mechanisms. Evaluation of a series of ONs demonstrated that only a small number were cytotoxic at concentrations close to those required for antisense activity. Toxicity of the ONs appeared to be sequence dependent and could be affected by base and backbone modifications. Caspase-3 activation occurs with some ONs and it is most likely secondary to necrosis rather than apoptosis, since cells treated with toxic ONs did not show chromatin condensation, but did exhibit high-extracellular lactate dehydrogenase activity. Caspase-3 activation does not correlate with and appears not to be required for the inhibition of cell proliferation. Toxicity was only observed when ONs were delivered intracellularly. The mechanism by which one of the most cytotoxic ON produces cytotoxicity was investigated in more detail. Treatment with the cytotoxic ON caused disruption of lysosomes and Pepstatin A, a specific inhibitor of aspartic proteases, reduced the cytotoxicity of the ON. Reduction of lysosomal aspartic protease cathepsin D by prior treatment with cathepsin D-specific antisense ON did not attenuate the cytotoxicity, suggesting that other aspartic proteases play a crucial role in the cellular proliferation inhibition by ONs.
INTRODUCTION
Inhibition of gene expression by antisense technology is widely used to determine function of genes in cell culture and in animals. In addition, there is much interest in the therapeutic potential of the technology with one approved antisense oligonucleotide (ON) on the market and multiple antisense ONs in clinical trials. The safety and tolerability of antisense ONs in rodents, primates and man is well established [reviewed in (1,2)]. Overall, both first- and second-generation antisense ONs are well tolerated at therapeutically relevant doses. In cell culture assays, one of the undesirable effects noted is the non-antisense mediated cytotoxicity at high doses of ONs [reviewed in (3)]. Although there is no evidence for similar cytotoxicity occurring in vivo, cellular toxicity complicates interpretation of gene functionalization experiments in cell culture. There are reports describing non-antisense effects of ONs that were added at high concentrations to the culture medium of cells. First-generation phosphorothioate-modified oligodeoxyribonucleotides have been shown to interact with extracellular proteins such as growth factor receptors and viral coat proteins (4,5). Other investigators have noted that phosphorothioate oligodeoxyribonucleotides are rather benign when added to cultured cells (6).
Most studies, currently using antisense ONs, transfect the ONs into cells by means of cationic lipids, cationic polymers or electroporation. There have been several studies characterizing non-antisense effects of ONs under these conditions of cellular delivery. Papucci and co-workers demonstrated that lipotransfection of Rat-1luc fibroblasts with single- or double-stranded ONs at concentrations >20–30 μM resulted in mitotic arrest and apoptosis independently of the sequence of ONs (7). These concentrations are ≫10–500 nM concentrations typically used for antisense experiments. Nur et al. (8) have shown that the treatment of Balb/c 3T3 cells with phosphorothioate oligodeoxyribonucleotides at concentrations of 200 nM, in the presence of cellfectin, induced apoptosis that could be attenuated by the caspase-3 inhibitor z-DEVD-FMK. More recently, Raffo et al. (9) have reported that a phosphorothioate ON designed to inhibit Bcl-2 expression inhibited cell growth and generated reactive oxygen species by a non-antisense mechanism. We have also found that many first-generation phosphorothioate ONs inhibit cell proliferation at high concentrations, by an apparent non-antisense mechanism.
The last decade has brought new discoveries to the field of antisense technology. Addition of new chemistries, such as 2′-O-(2-methoxy)ethyl (2′-MOE) modifications has resulted in ONs with more attractive properties, such as increased potency and longer duration of action (10). In general, optimized second-generation antisense ONs have been shown to reduce their target mRNA levels at concentrations <100 nM, when introduced into cells using cationic lipid-mediated transfection or <10 μM when electroporated. However, there is some variance in relative potencies based upon the cell line used. In most cases, such treatments have no effect on cell viability, thus appear to be better tolerated than first-generation phosphorothioate ONs. However, we have found that a few second-generation ONs, some designed as controls, thus, not being complementary to any known human gene, were observed to substantially affect cell proliferation rate at doses of 100–300 nM. In this report, we demonstrate that a limited number of second-generation antisense ONs may inhibit cell proliferation when transfected into cultured cells in a sequence-specific manner. To provide more information on the mechanism of antiproliferative potency of second-generation antisense ONs, we investigated sequence and chemistry effects on the ability of a particularly toxic ON to affect cell growth rate as well as caspase-3 activation. In addition, we used protease inhibitors to dissect the pathway of cytotoxicity. Our results suggest that the transfection of a cytotoxic ON into cell results in destabilization of lysosomes and release of lysosomal products that activate an aspartyl protease, resulting in the inhibition of cell proliferation. We were also able to identify several structural elements that contribute to the ability of ONs to activate caspase-3 and inhibit cellular growth.
MATERIALS AND METHODS
Materials
Tissue culture cells (A549 and HepG2) were obtained from American type culture collection (Rockville, MD). HTS Fluorometric caspase-3 activity assay, Lab-Tek chambered borosilicate cover glass system and protease inhibitors were purchased from VWR (West Chester, PA). Propidium iodide and staurosporine were products of Sigma-Aldrich (St Louis, MO) and donkey serum was purchased from Chemicon (Temecula, CA). CA-074 Me was obtained from Peptides international (Louisville, KY). CyQUANT cell proliferation kit was purchased from Molecular probes (Eugene, OR). Lipofectin, DMEM and FBS were purchased from Invitrogen (Carlsbad, CA). Donkey anti-rabbit IgG flourescein-linked whole secondary antibody was purchased from Amersham Biosciences (Piscataway, NJ), while antibiotic–antimycotic (AA) mixture and Opti-MEM were purchased from Gibco (Carlsbad, CA). Collagen type I from rat-tail was from BD Biosciences (San Diego, CA). Anti-Fas primary antibody was purchased from Oncogene (La Jolla, CA), while anti-cathepsin D antibody came from Zymed laboratories (So San Francisco, CA). GraphPad Prism was purchased from GraphPad software (San Diego, CA). CytoTox-ONE Homogenous membrane integrity assay kit was purchased from Promega (Madison, WI).
ON synthesis
All ONs were synthesized as described previously (10,11). After synthesis, each ON was purified by anion exchange chromatography and desalted by reverse phase liquid chromatography. The final product was analyzed using liquid chromatography mass spectroscopy on Agilent Series 1100 Spectrometer and capillary electrophoresis for homogeneity and purity. The ONs were at least 90% full length and contained not more than 10% phosphodiester. All other impurities were <2%.
Cell culture and transfection of ONs
For Lipofectin-mediated transfection, A549 and HepG2 cells were plated with a density of 105 cells/ml in DMEM containing 10% fetal bovine serum (FBS) and 1× AA mixture, at 37°C in the presence of 5% CO2 overnight. The following day the media was aspirated and replaced with prewarmed Opti-MEM containing ON–Lipofectin mixture (3 μg of Lipofectin per 100 pmol of ON). After 4 h, the transfection mixture was exchanged for fresh DMEM+FBS+AA and cells were left for additional 44 h at 37°C in the presence of 5% CO2. For electroporation, cells were resuspended in prewarmed Opti-MEM at the density 2.5 million cells/ml and aliquoted in 100 μl fractions. ONs were directly added to these fractions and cells were electroporated in 1 mm cuvettes with one 6 ms pulse at 80 V. Cell were diluted to 2 × 105 cells/ml in prewarmed DMEM+FBS+AA and incubated for 48 h at 37°C in the presence of 5% CO2.
Cell viability, caspase-3 and LDH activity determination
A549 or HepG2 cells were plated in collagen coated 96-well microtiter plates, and treated with ONs the next day. Forty-eight hours after transfection, cell viability was determined using CyQUANT cell proliferation kit; caspase-3 activation was measured with HTS caspase-3 activity assay and extracellular LDH activity was measured with CytoTox-ONE Homogenous membrane integrity assay kit according to the manufacturer's instructions.
Inhibition studies
Protease inhibitors and antioxidants were added to the transfection mixture at the specified concentrations. Where applicable DMSO (1% final concentration) was used as a negative control. To ensure that inhibitors did not affect transfection process, their effects on the activity of a PTEN antisense ON (12) to reduce PTEN expression were examined.
Immunofluorescence microscopy
A549 cells were plated on borosilicate cover glasses and treated the next day with either ON in the presence of Lipofectin or 250 nM staurosporine. Twenty-four hours after treatment, cells were washed twice with phosphate-buffered saline (PBS), fixed for 10 min in 4% paraformaldehyde, washed again twice with PBS and permeabilized for 5 min in 1:1 mixture of acetone and ethanol. After permeabilization, cells were washed twice with PBS and incubated overnight with a primary anti-cathepsin D antibody in 5% donkey serum/PBS. Next day, the cells were washed twice with PBS followed by a 1 h incubation with FITC-labeled secondary antibody in 5% donkey serum/PBS. Cells were washed again twice with PBS and incubated for 5 min with 5 μg/ml solution of propidium iodide in PBS, then the cells were washed twice with PBS and visualized in both FITC and TRITC channels on Nikon Eclipse TE300 microscope at the magnification of 60×.
RESULTS
Second-generation antisense ONs inhibit cell proliferation and activate caspase-3 in a sequence-dependent manner
To investigate the cytotoxicity of second-generation antisense ONs, we evaluated 43 ONs for effects on cell proliferation and caspase-3 activity (Table 1). These ONs were designed not to be complementary to any known human gene, thus all toxicity caused by them should, in principle, be non-antisense related. Human lung carcinoma cells, A549 were treated with 100–1000 nM of these ONs, in the presence of Lipofectin reagent. Forty-eight hours post-treatment, cell number was measured using CyQUANT cell proliferation kit and caspase-3 activity was measured with a fluorometric caspase-3 activity assay (zDEVD-AFC). This group of ONs exhibited variable effects on cell proliferation and caspase-3 activation (Table 1). It should be noted that the absolute value obtained for the inhibition of cell proliferation and caspase-3 activation varied in each experiment. However, the rank order potency remained the same. To allow a direct comparison of the effects of ONs, the data presented in table were derived from a single experiment. Most of the ONs had no significant effect on cell viability at 100 nM, but markedly inhibited cell proliferation at concentrations of 1 μM, which corresponded to 30 μg/ml of Lipofectin reagent. An ON containing a random mixture of bases at each position, ISIS 130358, had minimal effects on cell growth and caspase activation (Table 1). ISIS 126965 caused as much as 85% reduction in cell number and was able to induce caspase-3 activity ∼200 times relatively to untreated cell control (UTC) at a concentration of 300 nM. ISIS 129696 reduced cell number by 30% and induced caspase-3 activity approximately seven times relatively to UTC at the same concentration. Lipofectin alone, used as a negative control, had a minimal effect on cell proliferation and caspase-3 activation at concentrations <25 μg/ml. These results clearly indicate that the sequence of ON plays an important role in cytotoxicity. To test if activation of caspase-3 correlates with antiproliferative activity, we compared data obtained with CyQUANT cell proliferation and caspase-3 activity measurement kits using GraphPad Prizm software. Analysis of the data with linear regression failed to demonstrate a significant correlation [r2 = 0.3047 and P-value (runs test) 0.0468], indicating that caspase activation may not be directly linked to inhibition of proliferation by ONs and vice versa.
Table 1. Screening ONs for the toxicity in A549 cells.
| ISIS no. | Sequence | % Cell number | Fold caspase-3 activity | ||
|---|---|---|---|---|---|
| 100 nM | 300 nM | 1 μM | 300 nM | ||
| 126965 | CCAGGGCTGCCTCAGACACA | 39.0 ± 6.5 | 14.8 ± 0.6 | 7.3 ± 0.6 | 200.6 ± 15.3 |
| 128425 | CCTGTGGCAGCTTCTCCTGT | 56.8 ± 3.4 | 21.2 ± 1.6 | 8.9 ± 1.4 | 72.9 ± 6.5 |
| 147200 | ATCTTTTGCATAGCAGCACA | 65.9 ± 4.0 | 28.5 ± 1.7 | 10.9 ± 0.7 | 25.5 ± 3.9 |
| 129688 | TTCGCGGCTGGACGATTCAG | 82.0 ± 1.4 | 36.2 ± 5.0 | 9.3 ± 0.3 | 2.4 ± 1.6 |
| 131410 | CCTGGCCATCACTGAATCCA | 82.8 ± 4.4 | 39.1 ± 6.1 | 10.2 ± 3.2 | 89.7 ± 3.6 |
| 145649 | GCCCACTCCAGTACTTCTGA | 83.4 ± 8.4 | 41.6 ± 1.4 | 9.8 ± 1.0 | 8.6 ± 1.5 |
| 146003 | CGAGGTCTGAGTGATGTGGT | 89.1 ± 3.2 | 41.8 ± 1.8 | 14.2 ± 6.2 | 51.4 ± 5.5 |
| 113529 | CTCTTACTGTGCTGTGGACA | 65.1 ± 1.5 | 45.0 ± 4.8 | 11.9 ± 1.6 | 6.2 ± 0.8 |
| 185560 | TGTCTCTTGCCTCTTCAGCA | 79.1 ± 5.0 | 45.8 ± 4.7 | 10.5 ± 1.7 | 10.9 ± 5.0 |
| 147454 | GTCTGTCACCTCCAACACAT | 77.5 ± 2.8 | 46.9 ± 5.4 | 12.1 ± 1.6 | 4.6 ± 0.5 |
| 129686 | CGTTATTAACCTCCGTTGAA | 78.5 ± 17.8 | 49.9 ± 3.5 | 14.2 ± 3.8 | 14.9 ± 3.8 |
| 135075 | GGAGTCCATGAGGAGCTGTC | 78.2 ± 2.8 | 52.2 ± 4.0 | 24.2 ± 0.6 | 10.9 ± 1.1 |
| 185540 | CGGTGTCTGTAGTGGCTTGA | 79.9 ± 3.5 | 53.2 ± 7.4 | 21.6 ± 4.1 | 4.0 ± 0.5 |
| 120221 | CTCCAGCGCCTCCACCAGGC | 61.5 ± 2.9 | 53.4 ± 6.9 | 14.5 ± 2.1 | 37.6 ± 3.0 |
| 101763 | GCTTCGTCCACAGAGATCCG | 85.8 ± 2.5 | 54.7 ± 8.6 | 11.2 ± 4.3 | 8.1 ± 1.2 |
| 129700 | TAGTGCGGACCTACCCACGA | 94.7 ± 5.0 | 55.0 ± 3.0 | 13.1 ± 1.3 | 2.0 ± 0.5 |
| 129605 | CCTGCTCCCTCTAATGCTGC | 87.8 ± 2.4 | 55.5 ± 1.6 | 13.0 ± 1.9 | 18.0 ± 3.7 |
| 127505 | CTGCTCTCCGCATCACGCAT | 66.7 ± 3.9 | 55.7 ± 6.9 | 34.0 ± 1.5 | 4.9 ± 0.5 |
| 129689 | GAGGTCTCGACTTACCCGCT | 81.3 ± 9.8 | 58.0 ± 6.8 | 15.6 ± 2.6 | 2.9 ± 1.0 |
| 134696 | GAGGCCTCCGCCAGACTCAA | 88.3 ± 4.7 | 59.4 ± 5.5 | 31.0 ± 2.7 | 29.9 ± 1.6 |
| 129695 | TTCTACCTCGCGCGATTTAC | 90.8 ± 8.1 | 60.1 ± 3.9 | 10.9 ± 1.6 | 17.8 ± 2.1 |
| 129787 | CAAGGTCGCACACCACCTGC | 91.9 ± 11.1 | 62.0 ± 1.6 | 27.0 ± 4.8 | 4.8 ± 1.4 |
| 109197 | TGCCCTCCCGGCACTGAGTG | 85.6 ± 7.0 | 62.4 ± 2.2 | 13.1 ± 1.2 | 5.6 ± 0.8 |
| 144477 | CCCAGCACCTGGTTTGCCGT | 90.1 ± 11.7 | 63.6 ± 3.4 | 29.0 ± 0.8 | 5.7 ± 1.6 |
| 129698 | TTTGATCGAGGTTAGCCGTG | 95.2 ± 1.6 | 65.3 ± 2.3 | 17.9 ± 2.3 | 2.2 ± 0.6 |
| 122291 | TATTCCACGAACGTAGGCTG | 79.1 ± 9.1 | 66.4 ± 6.7 | 23.2 ± 4.3 | 8.4 ± 0.7 |
| 116948 | AGCGCAGACAAACCCATCAC | 81.6 ± 6.4 | 66.4 ± 8.1 | 13.8 ± 2.3 | 63.1 ± 2.7 |
| 134599 | ATGCAGCTTTTTGGTCAGCA | 89.3 ± 1.2 | 66.5 ± 5.3 | 26.1 ± 2.4 | 9.1 ± 1.7 |
| 101761 | TGTGGTGTGAACACATTTAA | 88.9 ± 3.0 | 69.1 ± 6.3 | 7.4 ± 1.8 | 19.6 ± 0.7 |
| 147418 | TTGAAGAGTCGCTCCCACTG | 87.7 ± 6.9 | 69.3 ± 2.2 | 33.0 ± 4.9 | 4.4 ± 0.5 |
| 129692 | ACATGGGCGCGCGACTAAGT | 91.9 ± 2.6 | 69.6 ± 4.3 | 30.3 ± 4.1 | 15.3 ± 6.4 |
| 129696 | ATTCGCCAGACAACACTGAC | 99.2 ± 6.3 | 71.2 ± 5.1 | 26.6 ± 2.8 | 6.6 ± 1.6 |
| 134785 | AGGACCACGGGCTCCCACTC | 82.9 ± 1.1 | 74.9 ± 1.4 | 31.6 ± 3.2 | 8.6 ± 1.9 |
| 219646 | GCTTCTCAATGGCATTGTGG | 84.9 ± 1.2 | 79.2 ± 7.7 | 54.1 ± 9.6 | 4.3 ± 1.0 |
| 135377 | TGTATCTTGGACTTCTGAGC | 84.2 ± 9.9 | 79.6 ± 2.2 | 36.2 ± 4.1 | 15.0 ± 4.8 |
| 199048 | CCTGCTCACTCTAATGCTGC | 91.8 ± 9.7 | 79.7 ± 12.9 | 50.7 ± 7.9 | 5.3 ± 0.5 |
| 124189 | GCCAGAAAGCTCAAACTTGA | 87.3 ± 1.6 | 82.6 ± 4.3 | 25.2 ± 4.1 | 6.1 ± 1.2 |
| 128426 | CCCGTCGTTCTAAGAGAGAC | 107.2 ± 12.9 | 83.6 ± 0.8 | 30.8 ± 2.0 | 10.5 ± 0.9 |
| 129694 | GTACAGTTATGCGCGGTAGA | 97.4 ± 2.4 | 85.3 ± 7.2 | 34.6 ± 4.6 | 7.4 ± 1.1 |
| 105390 | CTGATCATAGCGAGTAAGTA | 91.4 ± 2.1 | 86.4 ± 4.4 | 34.7 ± 8.2 | 12.8 ± 1.3 |
| 130358 | NNNNNNNNNNNNNNNNNNNN | 90.7 ± 4.8 | 89.2 ± 1.0 | 65.1 ± 3.8 | 1.4 ± 0.3 |
| 199047 | CCTGATCCCTCTAATGATGC | 89.2 ± 8.8 | 90.2 ± 6.3 | 73.6 ± 7.6 | 2.0 ± 0.8 |
| 129687 | ACAAGCGTCAACCGTATTAT | 93.0 ± 9.9 | 91.9 ± 3.4 | 45.4 ± 6.9 | 7.0 ± 2.7 |
| Lipofectin only treatment | 103.5 ± 9.0 | 97.4 ± 2.4 | 75.5 ± 3.5 | 1.2 ± 0.2 |
All ONs are second-generation gapmers: they contain phosphorothioate linkers and 5-methylcytosines (nucleotides in plain font are 2′-deoxyribo, underlined: 2′-MOE).Cells were treated with indicated doses of ONs for 44 h following 4-h transfection in the presence of Lipofectin (3 μg/ml per 100 nM ON). Cell number for each treatment was measured with CyQUANT cell proliferation kit and untreated cell control (UTC) was assigned a value of 100%. The caspase-3 activity was measured using HTS Fluorometric caspase-3 activity assay, the value for activity normalized for the cell number with UTC is designated as 1. All measurements have been performed in triplicate. Mean values and standard deviations were calculated using GraphPad software. ONs are distributed in the table according to their ability to inhibit cell proliferation at a dose of 300 nM.
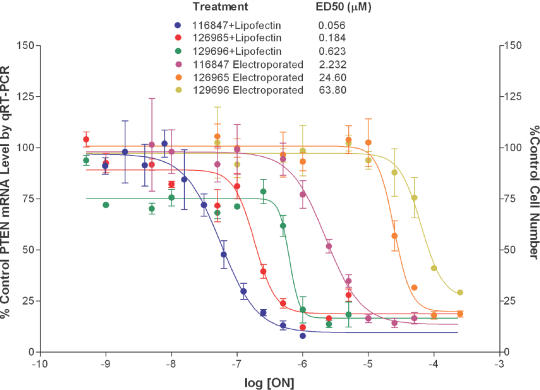
To help put these data in perspective with regard to antisense experiments, we performed a dose–response study to compare the concentrations required to inhibit gene expression with those that result in cytotoxicity. We selected two ONs: ISIS 126965, an ON that came up in our screen as a potent cell proliferation inhibitor and ISIS 129696, an ON which was less cytotoxic. For the antisense ON, we employed ISIS 116847, a second-generation antisense ON designed to target PTEN mRNA (12). The PTEN antisense ON failed to inhibit PTEN expression when added to the culture media of cells without transfection agent. Similarly, the ONs failed to produce toxicity when added to the culture media at concentrations as high as 50 μM (data not shown). ISIS 116847 inhibited PTEN expression in a dose-dependent manner with an ED50 of 56 nM when delivered to cells in the presence of a cationic lipid (Figure 1). The most toxic ON we identified, ISIS 129695 (Table 1), exhibited an ED50 of 180 nM for inhibition of cell proliferation, while ISIS 129696 exhibited an ED50 value of 600 nM for inhibition of cell proliferation. These values were 3- and 10-fold greater than that required to produce a downregulation of PTEN expression by ISIS 116847 (Figure 1). To determine if cytotoxicity is dependent on the transfection method, we electroporated the same ONs into A549 cells. Electroporation is less efficient in delivering ONs into the cytosol; thus, higher concentrations of ONs were used. The differences between doses required to inhibit PTEN expression and those that resulted in the inhibition of cell proliferation were better separated (Figure 1) following electroporation (10- and 30-fold), suggesting that the cationic lipid, in part contributes to the toxicity.
Figure 1.
Dose–response of gene downregulation versus cytotoxicity of ONs in A549 cells. Cells were transfected with various amounts of either ISIS 126965 or ISIS 129696 for toxicity studies or ISIS 116847 for PTEN downregulation analysis (Lipofectin-mediated transfection or electroporation). Forty-eight hours after transfection, the cell number was measured with CyQUANT cell proliferation kit and PTEN mRNA level was determined using Taqman quantitative RT–PCR. Values for UTC are designated as 100%.
Cytotoxicities of second-generation ONs are not cell line specific
To test if the antiproliferative effects produced by treatment with ONs are unique to A549 cells, we compared the toxicity profiles following transfection with 300 nM ONs in A549 and a human hepatocellular carcinoma cell line HepG2 (data not shown). Using GraphPad Prizm software, a strong correlation for the inhibition of proliferation was found [r2 = 0.45, P < 0.0001, P-value (runs test) 0.73]. We also demonstrated a good correlation between the rank order potency for producing cytotoxicity in HepG2 and a p53-null, hepatocellular carcinoma cell line Hep3B (data not shown). These results indicate that the observed cytotoxicity appears to be cell type-independent.
Multiple (5-MeCT)-repeats may contribute to the cytotoxicity of ONs
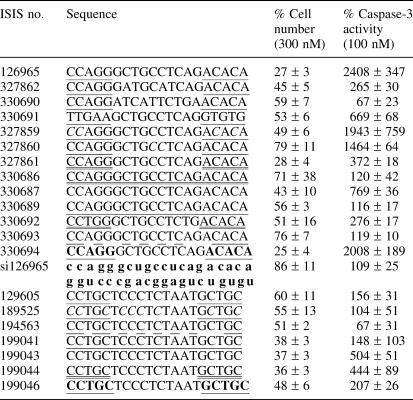
The results described above, suggest that ONs may inhibit cell proliferation and induce caspase-3 activity in a sequence-specific manner. Although there was no clearly defined motifs common to all highly cytotoxic ONs, our preliminary data analysis indicated a possible correlation between the presence of 5-methylcytosine-thymine (5-meCT) pairs in the sequence of ON and cytotoxicity (i.e. ISIS 129695, 128425, 185560 and 147454). To directly test this concept, a group of ONs, consisting of 5-meCT repeats was designed and synthesized. To help rule out the possibility that these ONs affect cell proliferation via inhibition of a gene expression by full or partial hybridization, the ONs had degenerated sequence in their ends or middle (Table 2). ISIS 269387, an ON containing deoxy-(5-meCT)5 gap with degenerated 2′-MOE wings proved to be highly toxic in A549 cells, inhibiting cell proliferation to ∼25% of control (at 300 nM) and increasing caspase-3 activity to >700% (at 100 nM) of untreated cell control (Table 2). ISIS 269386, a 20 nt-long ON that had a degenerated 2′-deoxy gap and flanking 2′-MOE-(5-meCT)2.5 had less pronounced effects with cell proliferation reduced by 35% and caspase-3 activity increased by 150%. ISIS 327027, a 10mer oligodeoxyribonucleotide with a sequence identical to the gap portion of ISIS 269387, failed to activate caspase-3 and inhibit cell proliferation. These results indicate that the presence of 2′-deoxy-(5-meCT) gap is important for proliferation inhibition and caspase-3 activation, although length of ON or presence of 2′-MOE wings may also play a role. To further define the role of 5-meCT in producing cytotoxicity, the 5-meCT gap in ISIS 269387 was shortened (ISIS 294151-294154, Table 2). The ONs were tested for caspase-3 activation and inhibition of cell proliferation in A549 cells (Table 2). Regression analysis of the data indicated a good exponential correlation between caspase-3 activation by ON and its oligodeoxyribonucleotide 5-meCT content (r2 = 0.98). No clear correlation between the number of 5-meCT pairs in the gap of ON and its ability to affect cellular proliferation was detected. Next, the gap in ISIS 269387 was ‘sequentially degenerated’ i.e. every 5-meC or T was replaced by N (G,A,T or 5-meC), resulting in ONs ISIS 294155-294164 (Table 2). Overall, there was no specific 5-meC or T within the gap that contributed to cytotoxicity, although replacement of the 5-meC at position 12 (ISIS 294161) did appear to reduce toxicity significantly (Table 2). To determine if methylation of cytosines contributed to cytotoxicity, the 5-meC in the gap were replaced with unmodified cytosine in the ISIS 269387 sequence (ISIS 327858). Replacement of 5-meC with C decreased caspase-3 activation and inhibition of cell proliferation of the treated cells (Table 2).
Table 2. Test of the involvement of (5-meCT)n motif in the cytotoxicity of ON.
| ISIS no. | Sequence | % Cell number (300 nM) | % Caspase-3 activity (100 nM) |
|---|---|---|---|
| 327027 | CTCTCTCTCT | 90.8 ± 5.7 | 94.9 ± 26.0 |
| 269386 | CTCTCNNNNNNNNNNCTCTC | 65.6 ± 1.6 | 162.8 ± 29.8 |
| 269387 | NNNNNCTCTCTCTCTNNNNN | 22.8 ± 3.2 | 734.7 ± 37.0 |
| 294151 | NNNNNNTCTCTCTCNNNNNN | 67.8 ± 5.8 | 384.6 ± 42.5 |
| 294152 | NNNNNNNCTCTCTNNNNNNN | 61.4 ± 3.8 | 234.9 ± 16.0 |
| 294153 | NNNNNNNNTCTCNNNNNNNN | 47.0 ± 3.0 | 175.3 ± 19.5 |
| 294154 | NNNNNNNNNCTNNNNNNNNN | 50.1 ± 2.9 | 123.6 ± 12.6 |
| 294155 | NNNNNNTCTCTCTCTNNNNN | 55.4 ± 6.3 | 467.5 ± 24.1 |
| 294156 | NNNNNCNCTCTCTCTNNNNN | 57.7 ± 10.6 | 408.0 ± 24.8 |
| 294157 | NNNNNCTNTCTCTCTNNNNN | 55.6 ± 11.5 | 239.1 ± 26.9 |
| 294158 | NNNNNCTCNCTCTCTNNNNN | 44.2 ± 1.2 | 423.3 ± 57.5 |
| 294159 | NNNNNCTCTNTCTCTNNNNN | 68.8 ± 12.6 | 721.8 ± 44.9 |
| 294160 | NNNNNCTCTCNCTCTNNNNN | 45.1 ± 3.3 | 766.6 ± 31.3 |
| 294161 | NNNNNCTCTCTNTCTNNNNN | 74.2 ± 9.7 | 290.2 ± 11.4 |
| 294162 | NNNNNCTCTCTCNCTNNNNN | 38.5 ± 2.6 | 492.8 ± 81.3 |
| 294163 | NNNNNCTCTCTCTNTNNNNN | 72.3 ± 4.5 | 476.0 ± 52.3 |
| 294164 | NNNNNCTCTCTCTCNNNNNN | 68.7 ± 9.8 | 598.1 ± 54.8 |
| 327858 | NNNNNCTCTCTCTCTNNNNN | 77.0 ± 10.0 | 151.0 ± 11.0 |
Nucleotides in plain font are 2′-deoxyribo, underlined: 2′-MOE; non-5-methylated cytosines are in italics. A549 cells were treated with indicated doses of ONs for 44 h following 4-h transfection in the presence of Lipofectin (3 μg/ml per 100 nM ON). Cell number for each treatment was measured with CyQUANT cell proliferation kit and caspase-3 activity was measured using HTS Fluorometric caspase-3 activity assay. Untreated cell control (UTC) was assigned a value of 100%. All measurements have been performed in triplicate. Mean values and standard deviations were calculated using GraphPad software.
Identification of chemical moieties involved in the cytotoxicity of ONs
To better understand the effect of chemical modifications on cytotoxicity of the ONs, we performed a structure–activity relationship (SAR) study of ONs derived from ISIS 126965, a cytotoxic ON. Derivatives of ISIS 129605, a mildly toxic ON, were used as controls (Table 3).
Table 3. Structure–activity relationship (SAR) study.
Nucleotides in plain font are 2′-deoxyribo, single underlined: 2′-MOE; double underlined are 2′-OMe; 2′-ribonucleotides are in small font. Non-5-methylated cytosines are in italics, nucleotides liked by phosphodiester are in boldface. A549 cells were treated with indicated doses of ON for 44 h following 4-h transfection in the presence of Lipofectin (3 μg/ml per 100 nM ON). Cell viability was assessed by CyQUANT cell proliferation kit, caspase-3 activity was measured using HTS Fluorometric caspase-3 activity assay. In both cases, values for UTC were designated as 100%. All measurements have been performed in triplicate. Mean values and standard deviations were calculated using GraphPad software.
Sequence changes
Sequence changes in the deoxyribo-gap of ISIS 126965 (ISIS 327862 and ISIS 330690) had dramatic effects on the caspase-3 induction and modest effects on inhibition for cell proliferation, while substitutions in the 2′-O-methoxyethyl flanking sequences had less pronounced effect, with ISIS 330691 retaining partial activation of caspase-3. Interestingly a version of ISIS 126965 with degenerated 2′-MOE wings had toxicity profile similar to its parent (data not shown), supporting that the fact that oligodeoxyribonucleotide portion of ON contributes to cytotoxicity. These results also suggest that the effects of ISIS 126965 are not antisense-mediated.
Base modifications
In agreement with results described above, substitution of 5-methylcytosines with unmethylated cytosine nucleotides in the ISIS 126965 sequence (ISIS 327859, ISIS 327860) led to a decrease in the anti-proliferative effects of the ON, which changes in a gap containing more pronounced effect.
Linker modifications
Replacement of the phosphorothioate modification with phosphodiesters in the 2′-MOE wings (ISIS 330694) did not decrease the anti-proliferative effect, but did decrease caspase activation. We did not modify the phosphorothioate linkers for the oligodeoxyribonucleotides in the middle of the ON due to the concerns that loss of stability would make interpretation of results difficult (13).
Ribose modifications
Replacement of the deoxy nucleosides with 2′-O-methyl (ISIS 330686) or 2′-MOE (ISIS 330689) nucleosides reduced both caspase activation and anti-proliferative effects, with caspase activation most dramatically affected. A uniform phosphorothioate oligodeoxyribonucleotide (ISIS 330687) was also less toxic than the chimeric parent. Distribution of the 2′-MOE throughout the sequence, while keeping the same number of 2′-MOE and 2′-deoxyribonucleotides (ISIS 330693) also reduced toxicity compared with ISIS 126965. Replacement of 2′-MOE wings with 2′-OMe (ISIS 327861) significantly decreased the potential of ON to activate caspase-3, with its antiproliferative potency staying the same. The fact that uniform 2′-deoxyribo (ISIS 199043) and 2′-OMe gapmer (ISIS 199044) versions of control ON ISIS 129605 were more toxic than their parent clearly indicates that 2′-MOE gapmers are not necessarily more toxic than their full 2′-deoxy or 2′-OMe gapmer counterparts. As a matter of fact, we found in several cases that a replacement of 2′-MOE wings with 2′-OMe rendered nontoxic ON quite toxic. For example, ISIS 322173, a 2′-OMe analog of ISIS 199047 (see Table 1), decreased cell viability down to 33% of UTC (versus 90% for ISIS 199047) at the dose of 300 nM plus Lipofectin, and was 10-fold more potent in caspase-3 activation than its 2′-MOE counterpart. The 2′-MOE modification is significantly more stable than the 2′-OMe modification; thus, the increased toxicity of ISIS 126965 compared with ISIS 327861 and ISIS 330687 may be, in part, explained by being more stable in cells.
To help support that the cytotoxicity observed for ISIS 126965 was not due to an antisense effect, we tested an siRNA variant (si126965) and found it to be not toxic at the doses tested in both assays (Table 3). The data presented demonstrates that the toxicity of ON depends not only on its sequence, but also on its chemistry.
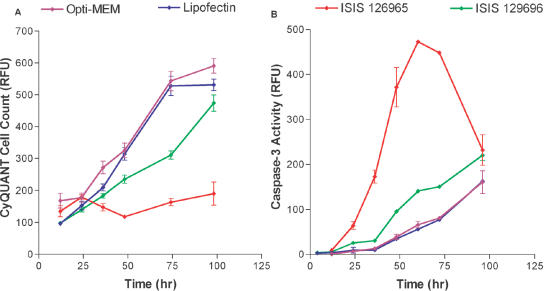
Time course of cytotoxicity and caspase-3 activation
The kinetics of caspase activation and antiproliferative effects were analyzed following treatment of A549 cells with ISIS 129695 and 129696. A549 cells were transfected with 300 nM of ONs in the presence of Lipofectin and cells were analyzed at various times after transfection; Lipofectin reagent alone and transfection media (Opti-Mem) were used as negative controls. Lipofectin alone did not affect proliferation rate, compared with a 4 h Opti-Mem treatment. Treatment with ISIS 129696 slows cell growth up to 3 days. After that time cells resume normal growth rate. Cells treated with ISIS 126965 exhibited dramatic reduction in proliferation rate at all tested time points (Figure 2A). Treatment of cells with ISIS 129696 slightly increased caspase-3 activity compared with controls. Caspase-3 activity sharply rose at 12 h post-treatment with ISIS 126965, peaked at 60 h and by the end of experiment decreased, almost down to the levels in cells transfected with ISIS 129696 (Figure 2B). This decrease in caspase activity could be due to the consumption of caspase-3.
Figure 2.
Time course of cell growth and caspase-3 activation after transfection with ONs. A549 cells were treated with Opti-Mem alone, Lipofectin (9 μg/ml) in Opti-MEM or with 0.3 μM of ISIS 126965 or ISIS 129696 in the presence of Lipofectin (3 μg/ml per 100 nM ON). Cells were collected at 12, 24, 36, 48, 74 and 98 h after the beginning of the transfection. (A) Cell number was measured with CyQUANT cell proliferation kit. (B) Caspase-3 activity was measured using HTS caspase-3 activity assay. The data are presented in relative fluorescence units (RFU).
Treatment with toxic ONs leads to necrosis rather than apoptosis
Although cytotoxic ONs induce an increase in caspase-3 activity in cells, it was not clear if this was a direct pro-apoptotic effect of the ON versus a secondary activation of caspases resulting from necrosis. We analyzed A549 cells treated with our most toxic ON ISIS 126965 for chromatin changes. No chromatin condensation was observed 30 or 60 h post-treatment (data not shown). On the other hand, treatment resulted in more than a 50-fold increase over untreated cell control in extracellular lactate dehydrogenase (LDH) activity (Table 4). In general, there was good correlation between the ability of ON to inhibit cell proliferation and their potency to increase the extracellular levels of LDH. The fact that toxic ONs cause release of LDH activity suggests that treatments induce necrosis in A549 cells.
Table 4. Treatment of A549 cells with toxic ONs leads to increase in extracellular LDH activity.
| Treatment | Fold LDH activity |
|---|---|
| Lipofectin | 1.4 ± 0.2 |
| ISIS 199047 | 1.5 ± 0.4 |
| ISIS 129696 | 1.8 ± 0.1 |
| ISIS 116948 | 6.9 ± 2.2 |
| ISIS 199044 | 26.2 ± 2.1 |
| ISIS 367861 | 52.1 ± 6.1 |
| ISIS 126965 | 52.3 ± 1.1 |
| ISIS 269387 | 118.7 ± 18.6 |
A549 cells were treated with 300 nM of various ONs in the presence of Lipofectin (3 μg/ml per 100 nM ON) or Lipofectin alone, 48 h after transfection, extracellular LDH activity was measured with CytoTox-ONE Homogenous membrane integrity assay kit. The value for activity normalized for the cell number with UTC is designated as 1. All measurements have been performed in triplicate. Mean values and standard deviations were calculated using GraphPad software. ONs are distributed in the table according to their ability to activate extracellular LDH at a dose of 300 nM.
Treatment with ISIS 126965 causes the disruption of lysosomes
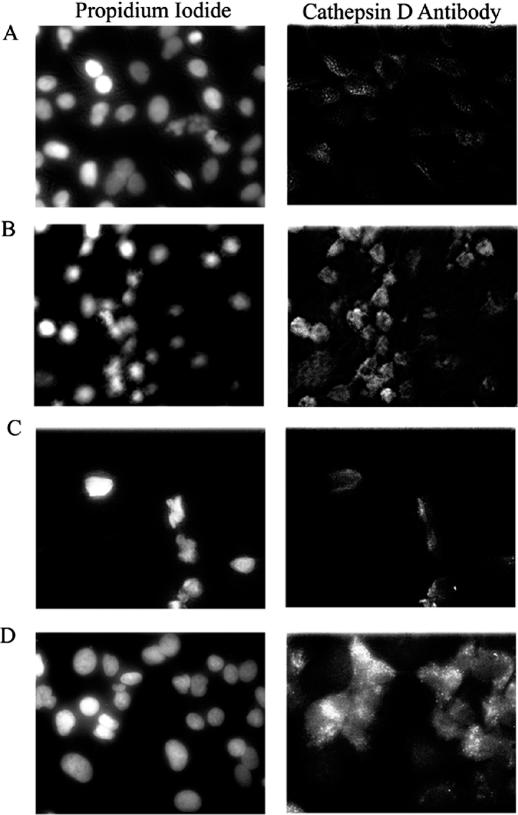
Although the molecular mechanisms by which cationic lipids or electroporation introduce ONs into the cytosol of cells is poorly understood, it has been suggested that cationic lipids/ON complexes may initially be endocytosed with the ON escaping endosomal/lysosomal structures (14,15). Several cytotoxic substances have been demonstrated to act through disruption of lysosomes (16). To test if transfection of cells with ISIS 129695 leads to the destabilization of lysosomes, we monitored the localization of cathepsin D, a lysosomal protease, in A549 cells treated with either 300 nM of ISIS 126965 or ISIS 130358 in the presence of Lipofectin. Staurosporine was used as a positive control (17). Twenty-four hours after transfection, the cellular localization of cathepsin D was detected using immunofluorescence microscopy. In untreated cells and cells treated with non-toxic ON, cathepsin D was localized in vesicular structures in the cytoplasm. Staining pattern for cathepsin D in cells treated with either staurosporine or ISIS 126965 was different from untreated cells. In both cases, there was an increase in staining intensity for cathepsin D in the cytosol with a diffuse cytosolic staining apparent, indicating that both treatments lead to the translocation of cathepsin D into cytosol (Figure 3). Both treatments also resulted in the release of FITC-dextran from vesicular cytosolic structures to cytosol in A549 cells that were preloaded prior to treatment (data not shown).
Figure 3.
Treatment of A549 cells with staurosporine or ISIS 126965 releases cathepsin D from lysosomes. A549 cells (A) untreated or treated with (B) 250 nM of staurosporine, (C) 0.3 μM of ISIS 130358 in the presence of Lipofectin (3 μg/ml per 100 nM ON) or (D) 0.3 μM of ISIS 126965 in the presence of Lipofectin (3 μg/ml per 100 nM ON), were simultaneously stained 24 h after transfection with propidium iodide and anti-cathepsin D FITC-labeled antibody and examined by fluorescent microscopy.
Role of cellular proteases in the proliferation inhibition by ONs
Although our data suggests that there was not a good correlation between inhibition of cell proliferation and activation of caspase-3, we wanted to determine if inhibition of this caspase had a protective effect. Treatment of cells with 100 nM z-DEVD-FMK completely inhibited caspase-3 activity (data not shown) and reduced the anti-proliferative effects of doxorubicin and anti-Fas monoclonal antibody, but failed to rescue A549 cells treated with 300 nM of toxic ON (Table 5). Other caspase inhibitors, (z-VAD-FMK, Boc-D-FMK, z-YVAD-FMK, z-VDVAD-FMK, z-WEHD-FMK, z-VEID-FMK, z-IEDT-FMK and z-LEHD-FMK) also failed to attenuate the anti-proliferative effects of ISIS 129695 (data not shown).
Table 5. Inhibition of caspase-3 cannot attenuate the toxicity of ONs in A549 cells.
| Treatment | % Control cell number | |
|---|---|---|
| No inhibitor | z-DEVD-FMK | |
| Anti-Fas Ab | 64.1 ± 2.7 | 112.4 ± 1.9 |
| Doxorubicin | 57.5 ± 2.3 | 89.8 ± 7.8 |
| ISIS 126965 | 20.5 ± 3.7 | 21.0 ± 3.4 |
Cells were treated for 4 h in the presence or absence of 100 μM z-DEVD-FMK with 50 ng/ml of mouse monoclonal anti-human Fas antibody, 0.5 μM doxorubicin or 300 nM ISIS 126965 in the presence of Lipofectin (3 μg/ml per 100 nM ON) in Opti-Mem for 4 h. Then, transfection mixture was replaced with fresh media and 48 h after the beginning of transfection, the cell number was measured with CyQUANT cell proliferation kit. All measurements have been performed in triplicate. Mean values and standard deviations were calculated using GraphPad software. The value for UTC is designated as 100%.
Lysosomal proteases can also play an important role in the cell death process (18). To assess the involvement of two such proteases, cathepsins B and D, in the toxicity of ONs, we treated A549 cells with a group of ONs in the presence of CA-074 and Pepstatin A, inhibitors of cathepsins B and D, respectively. CA-074 failed to demonstrate any protective effects at 100 μM, as a matter of fact, it decreased cell viability. Pepstatin A, on the other hand, attenuated the toxicity of ONs (Table 6). To determine if cathepsin D plays a role in cellular proliferation inhibition by ONs, we identified a specific antisense ON to cathepsin D. This antisense ON inhibited cathepsin D expression by >80% at a dose of 100 nM in the presence of Lipofectin, as measured by western blot and qRT–PCR (data not shown). Our results indicated that the reduction in cellular cathepsin D levels had no effect on the ability of ISIS 129695 to affect the growth rate (data not shown). There are two possible explanations for this result. First, it is possible that 80% reduction in cathepsin D may not be adequate or second, since Pepstatin A was demonstrated to inhibit other aspartic proteases besides cathepsin D (19–22), another aspartic protease may be responsible for the toxicity of ISIS 126965.
Table 6. Pepstatin A, but not CA-074 attenuates the toxicity of ONs in A549 cells.
| Sample | No inhibitor | CA-074 | Pepstatin A |
|---|---|---|---|
| UTC | 100 ± 9.3 | 89.9 ± 14.9 | 106.1 ± 14.3 |
| ISIS 126965 | 20.8 ± 3.1 | 12.8 ± 3.8 | 74.0 ± 4.7 |
| ISIS 199044 | 39.0 ± 10.5 | 26.0 ± 6.1 | 67.0 ± 20.8 |
| ISIS 199046 | 82.5 ± 18.6 | 77.3 ± 17.8 | 99.9 ± 11.5 |
Cells were treated for 4 h in the presence or absence of either 100 μM CA-074 Me or Pepstatin A Me with 300 nM of various ONs in the presence of Lipofectin (3 μg/ml per 100 nM ON) in Opti-Mem for 4 h. Then, transfection mixture was replaced with fresh media and 48 h after the beginning of transfection, the cell number was measured with CyQUANT cell proliferation kit. All measurements have been performed at least in triplicate. Mean values and standard deviations were calculated using GraphPad software. The value for UTC is designated as 100%.
DISCUSSION
While using various second-generation antisense ONs in cell culture for in vitro gene functionalization experiments, we noted that occasionally an ON would exhibit effects on cell viability that appeared to be independent of an antisense mechanism of action. We did not observe a correlation between cellular toxicity of ONs and toxicities in mice (data not shown), suggesting that the effects may be limited to cell culture. Nevertheless, antisense ONs are widely used in cell culture experiments and recognition that these effects occur is important in interpreting results, even if limited to a few sequences. Phosphorothioate oligodeoxyribonucleotides inhibit cell proliferation at concentrations 2- to 3-fold higher than required to inhibit target gene expression. In this paper, we present a study of in vitro toxicity of second-generation antisense ONs, 2′-MOE phosphorothioate gapmers. These ONs have several advantages over first-generation phosphorothioate oligodeoxyribonucleotides, including increased potency, longer duration of action and decreased immune stimulation in animals (10,23,24). In addition, we have found that they tend to produce less non-specific cytotoxic effects on cultured cells than first-generation ONs.
Our results suggest that only a small number of 2′-MOE modified ONs that we tested inhibit cell proliferation and activate caspase-3, at the concentration of 100 nM, which is at the high end of concentrations used for antisense experiments. These effects were not due to synthetic impurities in tested ONs, since different preparations of the same ON behaved similarly in our assays. Although, we cannot unequivocally rule out that the effects of the cytotoxic ONs are not due to the inhibition of a target gene by antisense and antisense mechanism, our results suggest the effects are not antisense mediated. First, the ONs used in the study were designed so that they were not complementary to known human mRNAs, although, several ONs had three mismatches to human genes. Second, concentrations required to produce effects on cell proliferation are 3- to 30-fold higher than those typically required to inhibit target gene expression. Third, the observed toxicity of degenerate ONs supports a non-antisense mechanism. Fourth, an siRNA consisting of the same sequence as ISIS 129695 failed to exhibit cytotoxicity. We have published previously that there is a good correlation between sites on a target RNA that work for RNase H-dependent ONs and siRNA ONs (25). Finally, some of the ONs were tested and found to be cytotoxic in mouse cells in addition to human cells. It is feasible that the sequence to which the ON potentially binds, to produce the observed toxicity, is conserved between human and mouse. However, in aggregate, we suspect that the cytotoxic effects are not antisense mediated.
The observed toxicity was sequence dependent, but the only sequence motif that could be identified, which contributed to cellular cytotoxicity, was the presence of (5-meCT) repeats. Redistribution or uniform replacement of deoxyribonucleotides with 2′-modified nucleosides in most cases negated the toxicity. The 5-methyl deoxycytosine modification also appeared to contribute to cytotoxicity, as replacement with deoxycytosine decreased the anti-proliferative effects and caspase activation. However, many ONs containing 5-methyl deoxycytosine exhibited minimal toxicity, suggesting that it is not just the presence of the modification but perhaps its sequence context that contributes.
There was not a good correlation between ONs that inhibited cell proliferation and activation of caspase-3, suggesting that there may be more than one mechanism by which ONs produce cytotoxicity. Alternatively, the kinetics for activation of caspase-3 may vary from ON to ON; thus, we failed to sample for caspase-3 activity at the optimal time for each ON. However, we did examine kinetics of caspase-3 activation for several ONs that markedly inhibited cell proliferation and only marginally activated caspase-3, and failed to identify a time when caspase-3 was markedly activated. The fact that various caspase inhibitors failed to protect cells from antiproliferative effects of highly toxic ON, suggests that the inhibition of cell proliferation does not depend on caspase-3 activation.
We used one of the more cytotoxic ONs, ISIS 129695, to investigate the mechanism of toxicity. It has been published previously that cultured cells readily accumulate ONs intracellularly when added to the culture media (26,27). However, for most cultured cells, the ON appears to reside in cytoplasmic vesicular structures and fails to produce specific antisense effects. Cationic lipid mediated transfection and/or electroporation is required for antisense ONs to gain access to the target RNA in the cytosol or nucleus for most cultured cells, such as A549 (27). Similarly, we demonstrate that the ONs used in this study only produce cellular toxicity when introduced into cells by methods that allow antisense ONs to gain access to cellular RNA's, such as cationic lipids or electroporation. These results suggest that the ON interacts with intracellular molecules rather than on the cell surface to produce cytotoxicity. Delivery of macromolecules into cells is not a benign process. Often, one must carefully optimize between efficient delivery and cytotoxicity. Our results suggest that the cationic lipid mixture, Lipofectin, enhanced the toxicity of ISIS 126965. There was a 3-fold improvement between the ED50 to inhibit PTEN expression by an antisense ON, and toxicity when the ONs were delivered by electroporation compared with Lipofectin. We have also observed that several other cationic lipids and dendrimers also enhance toxicity of ONs (data not shown), suggesting that the effects were not limited to Lipofectin reagent.
To obtain some insights in the mechanism of ON cellular toxicity, we decided to identify proteases, other than caspases, that can serve as mediators of this toxicity. Several cytotoxic substances, such as staurosporine (16), affect cell viability through the destabilization of lysosomes and release of executionary proteases. To test if modified ONs act the same way, we employed immunofluorescent microscopy to monitor the release of cathepsin D, a lysosomal protease, as a marker of lysosomal disruption. We showed that treatment with toxic ONs, but not with the non-toxic ones, leads to the redistribution of cathepsin D in the cells. Using lysosomal protease inhibitors, we demonstrated that decrease in cell number triggered by toxic ON can be attenuated by Pepstatin A, an inhibitor for cathepsin D and other aspartic proteases (21,22), but not by a cathepsin B inhibitor CA-074. Since inhibition of cathepsin D expression with siRNA had no effect on the toxicity of ON and Pepstatin A was demonstrated to inhibit other proteases (20–23), we conclude that these ONs affect cell viability through an aspartyl protease other than cathepsin D.
The present report demonstrates that while in general second-generation ONs are benign in cell culture at concentrations required for antisense activity, in some rare instances these ONs can affect cell proliferation in an antisense-independent manner. The obtained data provides insight into the mechanism of this cytotoxicity and helps us to eliminate the potentially undesirable features in the design of antisense ONs, such as 5-methyldeoxycytosine-deoxythmidine repeats. Further analysis is warranted to better define the pathways and to facilitate the design of ONs that have less cytotoxicity in cell-based assays.
Acknowledgments
ACKNOWLEDGEMENTS
We would like to thank Mr Neeraj Rao for designing ASO to inhibit the expression of cathepsin D. We also extend our gratitude to Dr Brenda Baker and Dr Eric Marcusson for their helpful comments on the manuscript. Special thanks go to Ms Jamie Bacey and Mrs Wanda Sullivan for their help in preparation of the manuscript.
REFERENCES
- 1.Levin A.A., Henry,S.P., Monteith,D. and Templin,M.V. (2001) Toxicity of antisense oligonucleotides. In Crooke,S.T. (ed.), Antisense Drug Technology: Principles, Strategies and Applications. Marcel Dekker, Inc., New York, NY, pp. 201–269. [Google Scholar]
- 2.Dorr F.A., Glover,J.G. and Kwoh,T.J. (2001) Clinical safety of phosphorothioate oligonucleotides. In Crooke,S.T. (ed.), Antisense Drug Technology: Principles, Strategies, and Applications. Marcel Dekker, Inc., New York, NY, pp. 269–318. [Google Scholar]
- 3.Stein C.A. (2001) The experimental use of antisense oligonucleotides: a guide for the perplexed. J. Clin. Invest., 108, 641–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rockwell P., O'Connor,W.J., King,K., Goldstein,N.I., Zhang,L.M. and Stein,C.A. (1997) Cell-surface perturbations of the epidermal growth factor and vascular endothelial growth factor receptors by phosphorothioate oligodeoxynucleotides. Proc. Natl Acad. Sci. USA, 94, 6523–6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balakireva L.A., Levashova,Z.B., Chroboczek,J. and Vlassov,V.V. (1997) Rapid sequence-independent cellular response to oligodeoxynucleotides. FEBS Lett., 400, 267–270. [DOI] [PubMed] [Google Scholar]
- 6.Crooke R.M., Crooke,S.T., Graham,M.J. and Cooke,M.E. (1996) Effect of antisense oligonucleotides on cytokine release from human keratinocytes in an in vitro model of skin. Toxicol. Appl. Pharmacol., 140, 85–93. [DOI] [PubMed] [Google Scholar]
- 7.Papucci L., Schiavone,N., Donnini,M., Lapucci,A., Luzi,E., Tempestini,A., Witort,E. and Capaccioli,S. (2002) Phosphodiester oligonucleotides inhibit mitosis and trigger apoptosis by a non-antisense, p53-mediated mechanism. Antisense Nucleic Acid Drug Dev., 12, 21–31. [DOI] [PubMed] [Google Scholar]
- 8.Nur E.K.A., Li,T.K., Zhang,A., Qi,H., Hars,E.S. and Liu,L.F. (2003) Single-stranded DNA induces ataxia telangiectasia mutant (ATM)/p53-dependent DNA damage and apoptotic signals. J. Biol. Chem., 278, 12475–12481. [DOI] [PubMed] [Google Scholar]
- 9.Raffo A., Lai,J.C., Stein,C.A., Miller,P., Scaringe,S., Khvorova,A. and Benimetskaya,L. (2004) Antisense RNA down-regulation of bcl-2 expression in DU145 prostate cancer cells does not diminish the cytostatic effects of G3139 (Oblimersen). Clin. Cancer Res., 10, 3195–3206. [DOI] [PubMed] [Google Scholar]
- 10.McKay R.A., Miraglia,L.J., Cummins,L.L., Owens,S.R., Sasmor,H. and Dean,N.M. (1999) Characterization of a potent and specific class of antisense oligonucleotide inhibitor of human protein kinase C-alpha expression. J. Biol. Chem., 274, 1715–1722. [DOI] [PubMed] [Google Scholar]
- 11.Monia B.P., Lesnik,E.A., Gonzalez,C., Lima,W.F., McGee,D., Guinosso,C.J., Kawasaki,A.M., Cook,P.D. and Freier,S.M. (1993) Evaluation of 2′-modified oligonucleotides containing 2′-deoxy gaps as antisense inhibitors of gene expression. J. Biol. Chem., 268, 14514–14522. [PubMed] [Google Scholar]
- 12.Butler M., McKay,R.A., Popoff,I.J., Gaarde,W.A., Witchell,D., Murray,S.F., Dean,N.M., Bhanot,S. and Monia,B.P. (2002) Specific inhibition of PTEN expression reverses hyperglycemia in diabetic mice. Diabetes, 51, 1028–1034. [DOI] [PubMed] [Google Scholar]
- 13.Monia B.P., Johnston,J.F., Sasmor,H. and Cummins,L.L. (1996) Nuclease resistance and antisense activity of modified oligonucleotides targeted to Ha-ras. J. Biol. Chem., 271, 14533–14540. [DOI] [PubMed] [Google Scholar]
- 14.Marcusson E.G., Bhat,B., Manoharan,M., Bennett,C.F. and Dean,N.M. (1998) Phosphorothioate oligodeoxyribonucleotides dissociate from cationic lipids before entering the nucleus. Nucleic Acids Res., 26, 2016–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zelphati O. and Szoka,F.C.,Jr (1996) Mechanism of oligonucleotide release from cationic liposomes. Proc. Natl Acad. Sci. USA, 93, 11493–11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bidere N., Lorenzo,H.K., Carmona,S., Laforge,M., Harper,F., Dumont,C. and Senik,A. (2003) Cathepsin D triggers Bax activation, resulting in selective apoptosis-inducing factor (AIF) relocation in T lymphocytes entering the early commitment phase to apoptosis. J. Biol. Chem., 278, 31401–31411. [DOI] [PubMed] [Google Scholar]
- 17.Johnson D.E. (2003) Noncaspase proteases in apoptosis. Leukemia, 10, 1253–1259. [DOI] [PubMed] [Google Scholar]
- 18.Johnson D.E. (2000) Noncaspase proteases in apoptosis. Leukemia, 14, 1695–1703. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki M., Komori,T., Okunishi,H. and Toda,N. (1979) Inhibition of dog renin activity by pepstatin A. Jpn Circ. J., 43, 818–823. [DOI] [PubMed] [Google Scholar]
- 20.Tian G., Sobotka-Briner,C.D., Zysk,J., Liu,X., Birr,C., Sylvester,M.A., Edwards,P.D., Scott,C.D. and Greenberg,B.D. (2002) Linear non-competitive inhibition of solubilized human gamma-secretase by pepstatin A methylester, L685458, sulfonamides, and benzodiazepines. J. Biol. Chem., 277, 31499–31505. [DOI] [PubMed] [Google Scholar]
- 21.Umezawa H., Aoyagi,T., Morishima,H., Matsuzaki,M. and Hamada,M. (1970) Pepstatin, a new pepsin inhibitor produced by Actinomycetes. J. Antibiot. (Tokyo), 23, 259–262. [DOI] [PubMed] [Google Scholar]
- 22.Tatnell P.J., Lees,W.E. and Kay,J. (1997) Cloning, expression and characterisation of murine procathepsin E. FEBS Lett., 408, 62–66. [DOI] [PubMed] [Google Scholar]
- 23.Geary R.S., Watanabe,T.A., Truong,L., Freier,S., Lesnik,E.A., Sioufi,N.B., Sasmor,H., Manoharan,M. and Levin,A.A. (2001) Pharmacokinetic properties of 2′-O-(2-methoxyethyl)-modified oligonucleotide analogs in rats. J. Pharmacol. Exp. Ther., 296, 890–897. [PubMed] [Google Scholar]
- 24.Henry S., Stecker,K., Brooks,D., Monteith,D., Conklin,B. and Bennett,C.F. (2000) Chemically modified oligonucleotides exhibit decreased immune stimulation in mice. J. Pharmacol. Exp. Ther., 292, 468–479. [PubMed] [Google Scholar]
- 25.Vickers T.A., Koo,S., Bennett,C.F., Crooke,S.T., Dean,N.M. and Baker,B.F. (2003) Efficient reduction of target RNAs by small interfering RNA and RNase H-dependent antisense agents. A comparative analysis. J. Biol. Chem., 278, 7108–7118. [DOI] [PubMed] [Google Scholar]
- 26.Tonkinson J.L. and Stein,C.A. (1994) Patterns of intracellular compartmentalization, trafficking and acidification of 5′-fluorescein labeled phosphodiester and phosphorothioate oligodeoxynucleotides in HL60 cells. Nucleic Acids Res., 22, 4268–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett C.F., Chiang,M.Y., Chan,H., Shoemaker,J.E. and Mirabelli,C.K. (1992) Cationic lipids enhance cellular uptake and activity of phosphorothioate antisense oligonucleotides. Mol. Pharmacol., 41, 1023–1033. [PubMed] [Google Scholar]