Abstract
Objective:
Dorema glabrum Fisch. & C.A. Mey is a perennial plant that has several curative properties. Anti-proliferative activity of seeds of this plant has been demonstrated in a mouse fibrosarcoma cell line. The aim of the present study was to evaluate cytotoxicity of D. glabrum root extracts in a human gastric adenocarcinoma (AGS) cell line and explore mechanisms of apoptosis induction, cell cycle arrest and altered gene expression in cancer cells.
Materials and Methods:
The MTT assay was used to evaluate IC50 values, EB/AO staining to analyze the mode of cell death, and flow cytometry to assess the cell cycle. Quantitative real-time polymerase chain reaction (qRT-PCR) amplification was performed with apoptosis and cell cycle-related gene primers, for cyclin D1, c-myc, survivin, VEGF, Bcl-2, Bax, and caspase-3 to determine alteration of gene expression.
Results:
Our results showed that n-hexane and chloroform extracts had greatest toxic effects on gastric cancer cells with IC50 values of 6.4 µg/ml and 4.6 µg/ml, respectively, after 72 h. Cell cycle analysis revealed that the population of treated cells in the G1 phase was increased in comparison to controls. Cellular morphological changes indicated induction of apoptosis. In addition, mRNA expression levels of Bax and caspase-3 were increased, and of bcl-2 survivin, VEGF, c-myc and cyclin D1 were decreased.
Conclusion:
Our study results suggest that D. glabrum has cytotoxic effects on AGS cells, characterized by enhanced apoptosis, reduced cell viability and arrest of cell cycling.
Keywords: Dorema glabrum, cytotoxic, gastric cancer, apoptosis, cell cycle
Introduction
Dorema glabrum Fisch. & C.A. Mey is a perennial plant that grows in loamy or rocky slopes of southern Caucasus especially, Azerbaijan, Armenia and Iran. Even though, already it has been reported that the distribution of D. glabrum is restricted to Transcaucasia region (Nakhichevan and Armenia zone), recent studies have shown that this plant is growing in some locations in North-West of Iran (Ajani et al., 2008; Asnaashari et al., 2011). This plant belongs to Apiacea and the gum-resin of this species is used for treating diarrhoea and as a diuretic (Delnavazi et al.). Herbs of this group have also antispasmodic, expectorant, carminative, diaphoretic, emmenagogue, stimulant, vasodilator (Mood, 2008; Yousefzadi et al., 2011), antioxidant (Delnavazi et al., 2015), antimicrobial and antifungal (Kumar et al., 2006), and hepatoprotector (Govind, 2011) activities. The plants of this group are widely used as a green vegetable or as a folk medicine for treatment of many disorders (Ibadullayeva et al., 2011).
Based on the folk beliefs of Azeri and Armenian people, Dorema species can remedy many abnormalities especially catarrh, bronchitis and also for treating diarrhoea and as a diuretic (Mir-Babayev et al., 1993). It appears that widespread use of the plant for medicinal and local purposes is the main reason of extreme reduction of the natural resources of D. glabrum (Gabrielian, 1981; Ibadullayeva et al., 2011). It has been shown that methanol extract of D. glabrum seed has anti-proliferative effect on WEHI-164 mouse fibrosarcoma cell line and could induce apoptosis is this cell line (Amirkhiz et al., 2013; Bannazadeh Amirkhiz et al., 2013). Moreover, cytotoxic activity of Dorema ammoniacum another member of this group has been reported (Yousefzadi et al., 2011).
Gastric cancer is the fourth most common cancer and second leading cause of cancer death worldwide (Crew and Neugut, 2006). The gastric adenocarcinoma is the most prevalent type of gastric cancer (Alberts et al., 2003). Gastric adenocarcinoma (AGS) cell line is one of the widely studied cell line that is proper for apoptosis and cell cycle experiments (Bohlooli et al., 2012; Jafari et al., 2012).
The current study was conducted to evaluate cytotoxic effects of Dorema glabrum Fisch. & C.A. Mey root extracts (n-hexane, ethyl acetate, chloroform, and methanol) on AGS (human gastric adenocarcinoma) cell line.
Materials and Methods
The human gastric adenocarcinoma (AGS) cell line was provided from Pasteur Institute of Iran. All reagents, chemicals and media were used and prepared freshly.
Plant preparation
The roots of D. glabrum were collected from “Ghaflankuh” mountains located in East-Azerbaijan (northwest of Iran) during its flowering stage in June 2012. The plant was authenticated by a botanist Dr. Yousef Ajani and its voucher specimen (No. 2120 MPIH) was deposited at the herbarium of Institute of Medicinal Plants, ACECR, Karaj, Iran.
Extraction
The air-dried and comminuted roots (2.4 kg) were undergone extraction by using maceration method, sequentially, with n-hexane, chloroform, ethyl acetate and methanol (3×5 L each) at the room temperature. The attained extracts were concentrated using a rotary evaporator under reduced pressure at 45 °C and then dried in a vacuum oven at 40 °C for 24 h.
Cell Culture and Treatment
Cancer cells were grown in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), penicillin 100 unit/ml and streptomycin 100 μg/ml. Cells were cultured at 37 °C in a moistened atmosphere of 5% CO2 and 95% air. Then, cells trypsinizd and plated in 96-well plates at a density of 1×104 cells per well in 150 μl medium and incubated overnight; next, cells were treated with a FBS-free medium containing 1 mg/ml of each compound by 1/4 serial dilutions. Then, plates were incubated for 24, 48, and 72 h. The cytotoxicity of D. glabrum extracts was determined by MTT assay.
MTT Assay
Culture media were removed 4 h before completion of the incubation time, then 200 μl of 0.25 mg/ml MTT (Merck, Germany) was added to each well. Plates were incubated again for an additional 4 h in order to complete the incubation time. The supernatants were removed and 200 μl DMSO was added to wells and plates were shaken for 10 min. The absorbance was measured at 540 nm by a plate reader (Synergy HT, BioTek).
Apoptotic Cell detection by EB/AO Staining
Cells were cultured in 96-well plates at a confluence of 1×103 cells/well and incubated overnight and then treated with extracts in their IC50 doses. Then plates were centrifuged for 5 min (129 g, 1,000 rpm) at 4 °C. The EB/AO dye mix (100 μg/ml ethidium bromide and 100 μg/ml acridine orange) was dissolved in PBS and 20 μl of the dye mix was added to wells. Cells were analyzed and counted by an inverted fluorescence microscope (IX 71, OLYMPUS). Live cells were determined by normal green color which were resulted by up-taking of acridine orange (green fluorescence) and repelling of ethidium bromide (red fluorescence).
Apoptotic cells display apoptotic bodies, and perinuclear condensation of chromatin stained by ethidium bromide. While live cells were identified as normal nuclear chromatin stained by acridine orange. Necrotic cells were detected by uniform ethidium bromide staining of the cells (Ribble et al., 2005). Images were captured with a digital camera (DP 71, OLYMPUS) equipped microscope. Experiments were done in triplicate, quantifying a minimum of 100 cells each time.
Flow Cytometric analysis of the Cell Cycle
Cells were cultured in 6-well plates at a confluence of 1×106 cells/well. Cells were treated with extracts for 24 h with respective IC50 values. Then, cell cycle phases and DNA content were analyzed by flow cytometry. Briefly, cells were collected and fixed with ice ethanol 70% for 2 h. Fixed cells were centrifuged (300 g, 4 °C, 5 min) and washed with cold PBS, and then stained with diamidino-2-phenylindole (DAPI, 10 μg/mL, Triton X-100 0.1% v/v in PBS). Then cells were filtered by a nylon mesh with a pore size of 30 μm. Cell cycle analysis was done using a flow cytometer (Partec CyFlow space, Germany). The distribution of cells in different cell cycle phases was assessed by means of Partec FloMax software.
mRNA expression analysis by qPCR
Cells were cultured in a 6-well plates at a confluence of 2×105 cells/well and kept at 37 °C in a moistened air of 5% CO2 for an overnight. Cells were then treated for 48h with chloroform, ethyl acetate, n-hexane and methanol extracts at 8.2, 48.7, 9.4 and 256.1 µg/ml concentrations respectively.
For RNA extraction from cells Trizol reagent (Cat. No: 15596-026, Invitrogen, CA, USA) was used according to manufacturer’s protocol. First-strand cDNA was generated from the cells’ extracted RNA by RevertAid First Strand cDNA Synthesis Kit, Fermentas (Cat No: #K1621, Maryland, USA) based on the kit’s procedure.
Primers (Bax, cyclin D1, VEGFA, Bcl-2, Caspase 3, c-myc, survivin and the homo sapiens ribosomal protein L38 (RPL38) as a housekeeping gene were designed using Primer Express 3.0 (PE Applied Biosystems, Foster City, CA, USA). See Supplementary Table 1 for the details of primers used in quantitative real-time PCR. For accuracy and specificity all primers were blasted in NCBI website: http://www.ncbi.nlm.nih.gov/tools/primer-blast/. Primers were synthesized by the custom oligonucleotide synthesis service, Metabion (Martinsried, Germany).
Table 1.
Primers Used in Quantitative PCR and the Amplicon Sizes (bp: Base Pair)
| Target | Forward primer | Reverse primer | Amplicon Size (bp) |
|---|---|---|---|
| Bax | 5’-GCCCTTTTGCTTCAGGGTTTC | 5’-CATCCTCTGCAGCTCCATGT | 168 |
| Cyclin D1 | 5’-GGCGGAGGAGAACAAACAGA | 5’-TGTGAGGCGGTAGTAGGACA | 181 |
| VEGFA | 5’- TGTCTAATGCCCTGGAGCCT | 5’- GCTTGTCACATCTGCAAGTACG | 175 |
| Bcl-2 | 5’- CAGGATAACGGAGGCTGGGATG | 5’- AGAAATCAAACAGAGGCCGCA | 70 |
| Caspase 3 | 5’- GCGGTTGTAGAAGAGTTTCGTG | 5’- CTCACGGCCTGGGATTTCAA | 101 |
| c-myc | 5’- CCCTCCACTCGGAAGGACTA | 5’- GCTGGTGCATTTTCGGTTGT | 96 |
| Survivin | 5’-TTCAAGGAGCTGGAAGGCTG | 5’-AGCAATGAGGGTGGAAAGCA | 151 |
| RPL38 | 5’-TCACTGACAAAGAGAAGGCAGAG | 5’- TCAGTGTGTCTGGTTCATTTCAGTT | 88 |
Quantitative analysis was done by StepOne Real-Time PCR System (Applied Biosystems 7500, Foster City, CA, USA) by using the PowerSYBR Green PCR Master Mix (Cat. No: 4309155, Applied Biosystems, Foster City, CA, USA).
Individual reaction mix contained an overall volume of 25 μl (master mix 12.5 μl, cDNA 3 μl, primer 3 μl, and H2O 6.5 μl). The quantitative RT-PCR settings were: 50 °C for 2 minutes, 95 °C for 10 minutes, then 60 cycles of 95 °C for 30 seconds, and 60 °C 30 seconds and 72 °C for 30 seconds. Relative quantities of target mRNA in test sample was measured and standardized to the housekeeping gene, RPL38 mRNA transcript level. The comparative Ct method was used to assess expression as previously described by Livak and Schmittgen (Livak and Schmittgen, 2001).
Statistical Analysis
IC50 values were calculated using Sigma Plot 12 software. Data values for the growth inhibition study are presented as mean±SD, except in figures where error bars represent standard error of mean (SEM).
Results
Cytotoxic effect of the D. glabrum has been analyzed with MTT assay. n-hexane and chloroform extracts had most toxic effect on gastric cancer cells with IC50 values of 6.4 µg/ml and 4.6 µg/ml after 72 h respectively (Figure 1 and Table 2). Chloroform extract was the most potent with least IC50 of 14.3 µg/ml after 24 h (Table 2).
Figure 1.
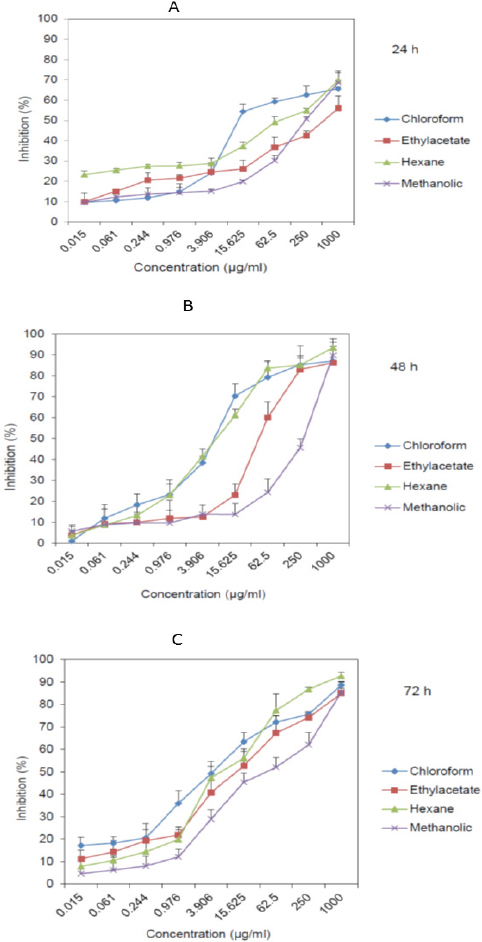
Cytotoxic Effect of D. Glabrum Root Extracts (N-Hexane, Ethyl Acetate, Chloroform, and Methanol) on AGS Cells Analyzed by MTT Assay after 24 h (A), 48 h (B), and 72 h (C)
Table 2.
IC50 Values of Dorema Glabrum Fisch. and C.A. Mey Root Extracts Analyzed by MTT Assay after 24 h, 48 h, and 72 h. Values are in µg/ml.
| Extract | 24 h | 48 h | 72 h |
|---|---|---|---|
| Chloroform | 14.3 ± 3.2 | 8.2 ± 1.2 | 4.6 ± 1.6 |
| Ethyl acetate | 124.6 ± 15.6 | 48.7 ± 3.5 | 14.7 ± 2.8 |
| n-Hexane | 65.3 ± 7.4 | 9.4 ± 1.4 | 6.4 ± 1.5 |
| Methanol | 261.6 ±21.7 | 256.1 ± 18.2 | 59.8 ± 8.4 |
In order to determine the apoptosis induction of the D. glabrum, EB/AO staining was conducted. Quantification of the cells showed that after incubation of the cells with D. glabrum extracts, they displayed a series of morphological changes including condensation and fragmentation of chromatin and nucleus, and formation of apoptotic bodies which were designated as typical evidence of apoptotic bodies. In contrast, control cells displayed a normal appearance (Figure 2 and Table 3).
Figure 2.
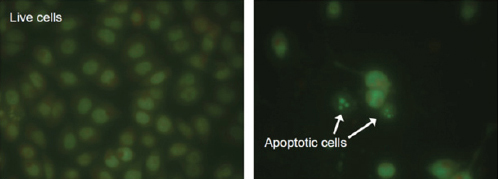
Representative Micrograph of Cells after Treatment with Ethylacetate Extract of the D. Glabrum Root in IC50 Concentration. Apoptotic Cells are Condensed and Fragmented in Comparison to the Live Untreated Cells
Table 3.
Apoptosis Induction of Dorema Glabrum Fisch. and C.A. Mey Root Extracts on AGS Cell Line. Cells WEre were Treated with IC50 Values of Extracts. Data are Presented as Percentage of Cells
| Column1 | Normal live cells | Apoptotic cells | Necrotic cells |
|---|---|---|---|
| Untreated cells | 96.4 ± 1.4 | 2.4 ± 0.9 | 0.8 ± 0.2 |
| Chloroform | 45.6 ± 2.8 | 53.4 ± 1.5 | 2.1 ± 0.8 |
| Ethylacetate | 48.4 ± 2.7 | 50.1 ± 2.8 | 1.8 ± 0.6 |
| Hexane | 46.8 ± 5.3 | 53.6 ± 4.2 | 1.2 ± 0.7 |
| Methanolic | 52.6 ± 3.1 | 47.6 ± 1.8 | 1.2 ± 0.4 |
In this study, the DNA content of D. glabrum treated cells during cell cycle phases were measured in order to obtain information about the cell cycle progression. The percentage of cells in G1, S, and G2/M phases were calculated using Partec FloMax software and is shown in Table 4. After 24 h of D. glabrum treatment, population of cells in the G1 phase increased in comparison to controls. The increase of cell population at the G1 phase was accompanied by a decrease of cell population in the G2/M phase of cell cycle (Table 3).
Table 4.
Population of the Cells was Analyzed by Flow Cytometry. Cells were Treated with Extracts of Dorema Glabrum in Respective IC50 Values. Cell Cycle Analysis of the Cells Demonstrates that All Extracts Induce Cell Cycle arrest in G1 Phase
| Extract (µg/ml) | G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|
| Untreated cells | 42.1± 5.7 | 18.7±6.4 | 38.4±4.2 |
| Chloroform (14.3) | 62.3±3.8 | 15.3±5.1 | 22±1.7 |
| Ethyl acetate (124.6) | 58.6±3.4 | 17.5±2.7 | 24.6±5.3 |
| n-Hexane (65.3) | 60.7±3.8 | 16.3±2.5 | 23.2±2.4 |
| Methanol (261.6) | 53.6±4.6 | 17.8±1.8 | 29.4±3.6 |
Q-PCR results showed that all extracts reduced mRNA expression of intracellular Bcl-2, survivin, VEGF, cyclin D1 and c-myc genes and increased Bax, and caspase-3 genes expression more distinctly than the untreated control cells. Chloroform extract had the most potent effect on the down-regulation of the Bcl-2, survivin, VEGF, cyclin D1 and c-myc genes, and up-regulation of the Bax, and caspase-3 genes.
Discussion
Gastric cancer is one of the most common cancers worldwide. Because of high incidence rate in some areas, finding an effective treatment is the challenge of researchers. Introducing natural plants with curative features would be proper replacement for chemical anti-cancer drugs. Hence, natural plants are less toxic to healthy body; finding new plants with cytotoxic effects could be promising. We found that D. glabrum could inhibit cell proliferation, induce apoptosis and arrest cell cycle in G1 phase among via four extracts of D. glabrum. n-hexane and chloroform extracts showed most cytotoxic effects with low IC50 values 6.4 µg/ml and 4.6 µg/ml after 72 h (Figure 1) respectively. After verifying cytotoxicity, we aimed to determine the mode of cell death. EB/AO staining was conducted to quantify number of live, necrotic and apoptotic cells. By analysis of morphological changes including condensation and fragmentation of chromatin and nucleus, and also formation of apoptotic bodies it revealed that cell underwent apoptosis pathway. These results prompted us to evaluate the mRNA expression of relevant genes in apoptosis process.
Bcl-2 (B-cell lymphoma 2) is considered as an important anti-apoptotic protein and is therefore classified as an oncogene. Targeting Bcl-2 by therapeutic agents, block its inhibitory effects on apoptosis and therefor malignant cells could undergo apoptosis. Our results showed that all extracts could down-regulate Bcl-2 expression, nevertheless, chloroform and n-hexane were more potent in reducing its expression.
Survivin is a member of the inhibitor of apoptosis (IAP) family. Survivin by inhibiting caspase activation could suppress apoptosis. It has been found by inhibition of survivin activation pathways, apoptosis increases and tumor growth reduces. We showed that chloroform extract decreased survivin expression much more than the other extracts. However, methanol extract was not more effective.
Second pathway responsible for caspase activation is the release of cytochrome c from mitochondria (Wong, 2011). The release of cytochrome c to cytoplasm activates capase-3 via the formation of apoptosome complex. This complex includes cytochrome c, APAF-1 and caspase-9 (Jackson and Combs Jr, 2008). Pro-apoptotic agent Bax is also affect this pathway. Bax promotes apoptosis by binding to and antagonizing the Bcl-2 protein (Kumar et al., 2013). Our experiments showed that D. glabrum extracts could up-regulate caspase-3 and Bax expression and thereby promote apoptosis induction (Figure 3).
Figure 3.
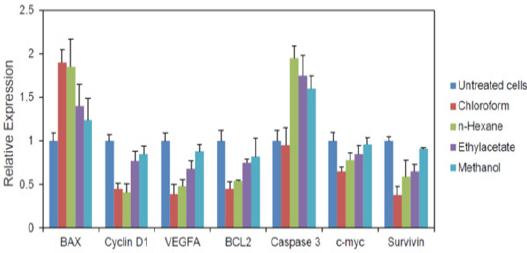
Relative mRNA Expression Levels of Genes Involved in Apoptosis and Cell Cycle which Performed Using qPCR. The Data are Expressed as the Means ± SEM of a Representative Experiment Performed in Triplicate (n=3)
In order to validate our data, we decided to analyze the effect of extracts on cell cycle distribution. The flow cytomentric results revealed that 24 h incubation of cells with extracts, increase the population of cell in G1 phase. These findings encouraged us to evaluate expression levels of cyclin D which is one of important regulators in cell cycle G1/S transition.
Cyclin D1 regulates CDK4 or CDK6, which are required for cell cycle G1/S transition. Overexpression of this gene modifies cell cycle progression and triggers tumorigenesis (Nakamura et al., 2013). All extracts down-regulated the expression of cyclin D1, especially, n-hexane extract effect was higher than the other extracts.
Myc (c-Myc) functions as a regulator in cell cycle progression, apoptosis and cellular transformation (Petrich et al., 2014). Myc enhances unregulated expression of many genes that are involved in cell division, therefore it is considered as a promising target for anti-cancer drugs. D. glabrum extracts reduced mRNA levels of Myc, nevertheless, methanol extract did not have significant effect on its expression.
Vascular endothelial growth factor (VEGF) stimulates vasculogenesis and angiogenesis and therefore, provides oxygen to tissues when blood circulation is insufficient. Tumors that can express VEGF could grow and metastasize. Our experiments demonstrated that D. glabrum extracts could down-regulate mRNA expression of VEGFA in AGS cells. Chloroform and n-hexane extracts had the most effect and methanol had the least effect in decreasing VEGFA expression levels.
In conclusion, these findings may suggest that D. glabrum has some cytotoxic effects on gastric cancer cells and could induce apoptosis and cell cycle arrest in G1 phase. It may be used as adjuvant chemotherapy agent in gastric originated cancers. Nevertheless, the concentrations used in the current study are achieved by an in vitro study and the possibility to extrapolate these concentrations to clinical practice needs more investigations. D. glabrum needs to be investigated further, especially with animal tumor models to confirm its anticancer and chemotherapeutic activity in vivo.
Acknowledgments
This study was supported by a thesis grant for Ph. D. in Cell and Molecular Biology from the University of Tehran.
Conflict of interest
We declare that there is no conflict of interest.
References
- Ajani Y, Ajani A, Cordes JM, et al. Phylogenetic analysis of nrDNA ITS sequences reveals relationships within five groups of Iranian Apiaceae subfamily Apioideae. Taxon. 2008;57:383–401. [Google Scholar]
- Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14:31–6. doi: 10.1093/annonc/mdg726. [DOI] [PubMed] [Google Scholar]
- Amirkhiz MB, Rashtchizadeh N, Nazemieh H, et al. Cytotoxic effects of alcoholic extract of dorema glabrum seed on cancerous cells viability. Adv Pharm Bull. 2013;3:403. doi: 10.5681/apb.2013.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnaashari S, Dadizadeh E, Talebpour AH, et al. Free radical scavenging potential and essential oil composition of the Dorema glabrum Fisch. CA mey roots from Iran. Bioimpacts. 2011;1:241. doi: 10.5681/bi.2011.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannazadeh Amirkhiz M, Rashtchizadeh N, Nazemiyeh H, et al. Investigating apoptotic effects of methanolic extract of Dorema glabrum seed on WEHI-164 Cells. ISRN pharmacology. 2013:2013. doi: 10.1155/2013/949871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlooli S, Jafari N, Jahed S. Cytotoxic effect of freeze-dried extract of Ecballium elaterium fruit on gastric adenocarcinoma (AGS) and esophageal squamous cell carcinoma (KYSE30) cell lines. J Gastrointest Cancer. 2012;43:579–83. doi: 10.1007/s12029-012-9383-4. [DOI] [PubMed] [Google Scholar]
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delnavazi M-r, Hadjiakhoondi A, Delazar A, et al. Phytochemical and Antioxidant Investigation of the Aerial Parts of Dorema glabrum Fisch & CA Mey (2015 summer) Iran J Pharm Res. 2015;14:925–31. [PMC free article] [PubMed] [Google Scholar]
- Delnavazi M-R, Hadjiakhoondi A, Delazar A, et al. Azerosides A and B: Two new phloroacetophenone glycosides from the roots of Dorema glabrum Fisch & CA Mey. Med Chem Res. 2015;24:787–96. [Google Scholar]
- Gabrielian E. The conservation of rare, threatened species and types of vegetation in Armenia. Anales Jard Bot Madrid. 1981;81:773–8. [Google Scholar]
- Govind P. Medicinal plants against liver diseases. IJPR. 2011;2:115–21. [Google Scholar]
- Ibadullayeva S, Movsumova N, Gasymov H, et al. Protection of some rare and endangered vegetable plants in the flora of the Nakhichevan AR. Int J Biodivers Conserv. 2011;3:224–9. [Google Scholar]
- Jackson MI, Combs GF., Jr Selenium and anticarcinogenesis: underlying mechanisms. Curr Opin Clin Nutr Metab Care. 2008;11:718–26. doi: 10.1097/MCO.0b013e3283139674. [DOI] [PubMed] [Google Scholar]
- Jafari N, Bohlooli S, Mohammadi S, et al. Cytotoxicity of methylsulfonylmethane on gastrointestinal (AGS, HepG2, and KEYSE-30) cancer cell lines. J Gastrointest Cancer. 2012;43:420–5. doi: 10.1007/s12029-011-9291-z. [DOI] [PubMed] [Google Scholar]
- Kumar P, Chatterjee S, Acharya S, et al. Significant modulation of macrophages associated cytokines TNF-α, VEGF and apoptotoic protein Bax, Bcl2 abrogates tumor cells. Cell Immunol. 2013;284:172–81. doi: 10.1016/j.cellimm.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Kumar VP, Chauhan NS, Padh H, et al. Search for antibacterial and antifungal agents from selected Indian medicinal plants. J Ethnopharmacol. 2006;107:182–8. doi: 10.1016/j.jep.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mir-Babayev NF, Gasanov GG, Knight DW. Plants of the Republic of Azerbaijan with potential medicinal applications. Pharm Biol. 1993;31:47–54. [Google Scholar]
- Mood SG. A contribution to some ethnobotanical aspects of Birjand flora (Iran) Pak J Bot. 2008;40:1783–91. [Google Scholar]
- Nakamura Y, Felizola SJ, Kurotaki Y, et al. Cyclin D1 (CCND1) expression is involved in estrogen receptor beta (ERbeta) in human prostate cancer. Prostate. 2013;73:590–5. doi: 10.1002/pros.22599. [DOI] [PubMed] [Google Scholar]
- Petrich AM, Nabhan C, Smith SM. MYC-associated and double-hit lymphomas: a review of pathobiology, prognosis, and therapeutic approaches. Cancer. 2014;120:3884–95. doi: 10.1002/cncr.28899. [DOI] [PubMed] [Google Scholar]
- Ribble D, Goldstein NB, Norris DA, et al. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005;5:12. doi: 10.1186/1472-6750-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:1. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadi M, Heidari M, Akbarpour M, et al. In vitro cytotoxic activity of the essential oil of Dorema ammoniacum D. Don. Middle East J Sci Res. 2011;7:511–4. [Google Scholar]


