Abstract
Background:
Brachytherapy is the most commonly used conservative treatment for the uveal melanoma. The aim of this study was to evaluate therapeutic results of Ruthenium-106 plaque brachytherapy in the management of localized uveal melanoma cases.
Methods:
We reviewed retrospectively the clinical records of all patients treated in our department for an uveal melanoma, undergoing Ruthenium-106 plaque brachytherapy, from January 1996 to December 2015. We focused on clinical features, therapeutic characteristics, local and distant tumor control and side effects.
Results:
Nineteen patients were enrolled in our study. Mean age was 56.2 years (28-79) and the sex ratio was 1.37:1 males to females. Diagnosis was made on the basis of ophthalmological clinical examination, angiography, ultrasound and/or magnetic resonance. Median tumor diameter was 9.7 mm (6-13) and median thickness 4.4 mm (2.5-8). The dose of Ruthenium-106 plaque brachytherapy prescribed to the apex of each tumor was 70 Gy in all cases. The median radiation dose to the sclera surface was 226.4 Gy (range: 179.6–342.3) and the median total application time 115.2 hours (range: 27 to 237). After a median follow-up of 61.5 months, local control was achieved in 17 patients (89%): 16 demonstrated a partial tumor response and 1 tumor stabilization. Two patients suffered local progression leading to enucleation, one dying of hepatic metastasis. Radiation-induced complications were cataracts in 3 cases and vitreal hemorrhage in 2.
Conclusion:
Ruthenium-106 plaque brachytherapy is an efficient treatment for localized uveal melanoma, offering good local control with low toxicity.
Keywords: Melanoma, uvea, ruthenium, plaque, brachytherapy
Introduction
Uveal melanoma (UM) is the most common primary intraocular malignant tumor in adults. It arises from melanocytes situated in the uveal tract of the eye. It can affect any part of the uveal tract, but choroidal melanoma is predominant (85-90%), while iris and ciliary body melanomas are far less frequent (Jovanovic et al., 2013).
Since the publication of the Collaborative Ocular Melanoma Group study, eye preservation therapy using brachytherapy (BT) has become the standard of care for most patients (Collaborative Ocular Melanoma Study Group, 2006). Various types of low-energy radioactive sources such as Iodine-125 (I-125) or beta plaques such as Ruthenium-106 (Figure 1) are used to treat UM, but there isn’t any prospective randomized clinical trial that compared their clinical results and side effects.
Figure 1.

Ruthenium Ophthalmic Plaque CCB Type
The aim of our study was to evaluate therapeutic results of Ruthenium-106 (Ru-106) plaque BT in the management of localized UM, with emphasis on clinical effectiveness and side effects of this treatment.
Materials and Methods
We reviewed retrospectively the clinical records of 19 consecutive patients referred to our department for an UM and treated with Ru-106 plaque BT, from January 1996 to December 2015. In our center, patients were eligible for plaque BT if the tumor’s size and depth were less than 20 mm and 9 mm respectively. Two types of ruthenium plaques (CCA or CCB) with an external diameter of 15.5 mm and 20 mm respectively could be used depending on the size of the tumor. The tumor’s edge has to be at least 1 cm distant from the optic nerve and the fovea. Patients with retinal detachment or neovascular glaucoma are not suitable for BT.
Only patients with a post-therapeutic follow up of 12 months or more were included in our study. We focused on patient’s age at diagnosis, gender, laterality and clinical symptoms at presentation. Evaluated tumor clinical characteristics included tumor location, thickness, largest basal diameter and TNM staging according to the 7th edition of the American Joint Committee on Cancer staging system (AJCC). BT features included plaque size, dose to the tumor apex, dose to the sclera surface and treatment duration. Treatment outcome included local and distant tumor control, salvage treatment and side effects of plaque BT.
Results
Nineteen eyes with localizedUM from 19 patientstreated with Ru-106 plaque BT in the radiation oncology department of The Salah Azaiez Institute between January 1996 and December 2015, were enrolled in our study. The mean age at diagnosis was 56.2 years (median 54 years, range: 28 to 79 years). There was a slight male predominance with a sex ratio of 1.37. All patients had single tumor. Seven patients were symptomatic at diagnosis and complained of an impaired vision. The tumor was discovered on a routine ophthalmological examination in 12 cases.
Diagnosis was made on ophthalmological clinical examination, angiography, ultrasound and/or magnetic resonance. The melanoma was located in the choroid in 17 eyes and in the ciliary body in 2 eyes. No patient had iris melanoma in our series. Median tumorlargest base diameter was 9.7 mm (range: 6 to 13) and median thickness 4.4 mm (range: 2.5 to 8). All tumors wereat least 1 cm distant from the optic nerve. According to the 7th edition of the American Joint Committee on Cancer staging system (AJCC), 11 tumors were staged T1a, 4 tumors T1b and 4 tumors T2a. No patients had clinical evidence of nodal involvement at diagnosis. Chest and abdominal imaging was performed for all patients and didn’t show any distant metastasis. None of the patients of our series had a pathologic confirmation of melanoma.
All patients were treated with Ru-106 plaque BT as their first treatment for uveal melanoma. Patients had tumor localization by transillumination under general anesthesia in the ophthalmologic oncology unit. Once the tumor was located and its dimensions and distance to the optic nerve and fovea checked to be suitable for plaque BT, the Ru-106 plaque was sewn onto the sclera to cover the tumor’s base with a minimal margin of 1 mm around the tumor borders (Figure 2). Thereafter, the patient was transferred to the BT unit of the radiation oncology department. Dosimetric planification was made by a radiophysicist and a radiation oncologist, upon the tumor features provided by the ophthalmologic oncologist. The plaque diameter was 15.5 mm in 14 cases and 20 mm in 5 cases. The dose prescribed to the apex of the tumor was 70 Gy in all cases. The median radiation dose to the sclera surface was 226.4 Gy (range: 179.6 - 342.3) andthe median total application time 115.2 hours (range: 27 - 237).
Figure 2.
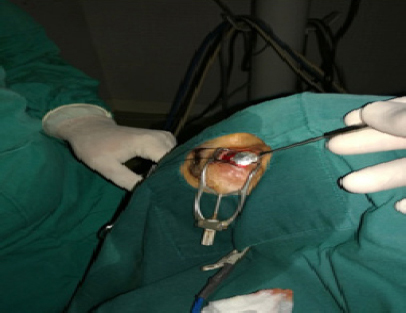
Ruthenium Plaque Application Under General Anesthesia
After a median follow-up of 61.5months, local control was achieved in 17 patients (89%): 16 partial tumor response and 1 tumor stabilization. Two patients had local progression leading to enucleation. One of these two patients died of hepatic metastasis. The main complications were cataract in 3 cases and vitreal hemorrhage in 2 cases.
Discussion
Unlike conjunctival melanoma that is showing continuous increase in incidence, uveal melanoma’s incidence has remainedstable over last three decades (Singh et al., 2011). Many risk factors such as light eye color, exposure to solar UV radiation or occupational cooking were incriminated in UM but evidences on their role in the development of this disease are still inconclusive (Ge et al., 2012; Jovanovic et al., 2013).
The majority of UM cases are sporadic but a few percentage of the cases occurs in families with an inherited predisposition for this malignancy. By next-generation sequencing efforts on UM tumors, several driver genes have been detected. The most frequent ones are BAP1, EIF1AX, GNA11, GNAQ, and SF3B1 (Helgadottir and Höiom, 2016).
The most common presenting symptoms are blurred vision, visual field defect, photopsia, irritation and pain (Damato and Damato, 2012). Diagnosis of uveal melanoma is mostly established by ophthalmic examination including slit lamp biomicroscopy, indirect ophthalmoscopy, and ancillary diagnostic testing such as ultrasonography, fluorescein angiography and optical coherence tomography (Jovanovic et al., 2013). Due to lack of lymphatic drainage in uvea, uveal melanoma does not spread to regional lymph nodes, except in rare cases of direct invasion of conjunctiva and then through conjunctival lymphatics to regional lymph nodes (Dithmar et al., 2000).
At present, the most commonly used classification for UM is the 7th edition of the American Joint Committee on Cancer eye cancer staging system for UM (AJCC Ophthalmic Oncology Task Force, 2015). Tumor size is the main prognostic factor in UM. Tumor thickness and largest basal tumor diameter are correlated to the risk for metastases (Shields et al., 2009).
Among small choroidal melanomas (≤ 3 mm thickness) those with diffuse growth configuration (thickness/base ≤ 20%) carry higher risk for metastases than small non-diffuse tumors (thickness/base >20 %). Factors predictive of metastasis from diffuse melanoma include larger tumor basal dimension and flat tumor configuration (Shields et al., 2013).
Ciliary body location, extraocularextension, patient age, presence of subretinal fluid or intraocular hemorrhage and presence of brown tumor are also associated with increased risk for metastases (Shields et al., 2009).
Management of uveal melanoma depends mostly on the site, the size and the local extension of the tumor. Clinical diagnosis of uveal melanoma is adequate for treatment and histopathologic verification is not required (American Brachytherapy Society - Ophthalmic Oncology Task Force, 2014).
Conservative treatments of UM include plaque brachytherapy, external beam radiotherapy, transpupillary thermotherapy and laser photocoagulation. Surgical options vary from local surgical resection to enucleation.
Small and medium-sized choroidal tumors are mostly treated by brachytherapy, while large tumors, especially if locally advanced, are still mostly treated by enucleation or orbital exenteration (Jovanovic et al., 2013). The COMS Group trial for medium-sized tumors did not show any difference in survival rates between patients managed by brachytherapy compared to those managed by enucleation, and concluded that a conservative approach is more appropriate in these cases (Collaborative Ocular Melanoma Study Group, 2006).
External beam radiation therapy (EBRT) techniques such as stereotactic RT or proton beam RT have also been used in the treatment of choroidal melanoma (Caujolle et al., 2010; Wackernagel et al., 2014). In aSurveillance, Epidemiology, and End Results database analysis comparing BT and EBRT in the management of UM, there was no difference in the 5-year overall survival (83.3% EBRT vs. 82.5% BT, p = 0.69) and 5-year cause-specific survival (88.3% EBRT vs. 88.3% BT, p = 0.92). In the survival analysis, older age and advanced tumor stage were predictors of increased risk of death. In the patterns-of-care analysis, later year of diagnosis and smaller tumor stage were predictors of BT use (Abrams et al., 2016). One reason that brachytherapy use may be increasing is due to its perceived normal tissue sparing capabilities of some ocular structures (Abrams et al., 2016). In a retrospective study comparing outcomes of patients with choroidal melanoma treated with I-125, Ru-106 BT, or proton beam radiation therapy (PBRT) (Wilson and Hungerford, 1999), patients treated with PBRT had a more rapid and substantial loss of vision than those treated with BT. A higherrate of enucleation in the PBRT group was also noticed and thought to bedue to a higher incidence of refractory neovascular glaucoma.
Brachytherapy is becoming the most commonly used primary treatment for the majority uveal melanomas. This includes iris, ciliary body and choroidal melanomas. Tumors with gross extra-ocular extension (> 5 mm), patients withblind painful eyes and those with no light perception vision are not suitable for brachytherapy. Patients with peripapillary and subfoveal UM and those with exsudative retinal detachments typically have poorer resultant vision and local control outcomes(American Brachytherapy Society - Ophthalmic Oncology Task Force, 2014).
The most commonly used radioactive sources in the management of UM with brachytherapy are beta Ru-106 or Strontium-90 (Sr-90) plaques and low-energy gamma I-125 or Palladium-103(Pd-103) plaques.Table 1 shows the relative dose distributions forRu-106, I-125 and Pd-103 sources.
Table 1.
Relative Dose Distributions for Three Radiation Sources (for Uveal Tumors Measuring 10 x 10mm in Base and 5mm in Height) (Finger, 1997)
| Ruthenium-106 | Iodine-125 | Palladium-103 | |
|---|---|---|---|
| Percentage of prescribed dose (%) | Percentage of prescribed dose (%) | Percentage of prescribed dose (%) | |
| Posterior tumors | |||
| Apex | 100 | 100.0 | 100.0 |
| Base | 946 | 384.0 | 463.0 |
| Lens | 0 | 14.0 | 9.0 |
| Fovea | 216 | 124.0 | 136.0 |
| Optic nerve | 140 | 103.0 | 106.0 |
| Equatorial tumors | |||
| Apex | 100 | 100.0 | 100.0 |
| Base | 946 | 384.0 | 463.0 |
| Lens | 0.04 | 25.0 | 18.8 |
| Fovea | 0.01 | 21.6 | 16.3 |
| Optic nerve | 0.01 | 19.8 | 14.1 |
| Anterior tumors | |||
| Apex | 100 | 100.0 | 100.0 |
| Base | 946 | 384.0 | 463.0 |
| Lens | 15 | 59.0 | 55.0 |
| Fovea | 0 | 11.0 | 6.7 |
| Optic nerve | 0 | 10.5 | 6.3 |
At present, there aren’t any prospective randomized or case-matched studies comparing the efficacy or side effects of available plaque radionuclide techniques. In patients treated with Ru-106 plaques (as compared to I-125 or Pd-103), the dose deposition close to the plaque can be more than nine times the apex dose (table 3). These high radiation doses to the sclera, choroid and retina are more likely to cause secondary retinal detachments and relatively rapid chorioretinal scar formation with visual loss (Lommatzsch and Lommatzsch, 1991; Finger, 1997).
Centers using Ru-106 plaques usually restrict tumor apical height less than a mean of 6 mm and rarely use commercially available Ru-106 plaques larger than 20 mm in diameter. In contrast, centers using I-125 or Pd-103 plaques do not as closely restrict their treatments based on tumor thickness (American Brachytherapy Society - Ophthalmic Oncology Task Force, 2014). The penumbra at the edge of beta (Ru-106 andSr-90) plaques is relatively sharp compared with the low-energy gamma of I-125 andPd-103 plaques, which has to be strongly considered in the treatment planning to insure optimal coverage of the target volume. Dose prescriptions for UM typically range from 70 to 100 Gy to the tumors apex. The treatment duration ranges usually between 3 and 7 days.
After brachytherapy, patients are followed for local control, complications, and systemic disease every 3 to 6 months. The most common ophthalmic BT complications are radiation cataract and intraocular radiation vasculopathy. BT can also affect the eyelids, eyelashes, conjunctiva, tear production, corneal surface integrity, sclera, and ocular muscles. These radiation-induced complications have to be treated to prevent loss of vision and quality of life. In a recent retrospective study including 143 eyes with UM treated with RU-106 plaque BT, the estimated local tumor recurrence rate at 12, 24 and 48 months after irradiation was 3%, 8.4% and 14.7%, respectively (Tarmann et al., 2015). Five-year survival rates for uveal melanoma ranges from 69% to 81.6% and ten-year survival rates from 57% to 62% (Mallone et al., 2012; Jovanovic et al., 2013). After detection of metastases, 80% of patients die within 1 year, and 92% within 2 years (Diener et al., 2005).
Ruthenium-106 plaque brachytherapy is an effective eye and vision-sparing treatment for early stage uveal melanoma. A straight collaboration between the radiation oncologist, the ophthalmic oncologist, and the medical physicist is compulsory for a successful treatment. However, metastatic disease remains the major cause of death;therefore, efforts have to be done in order to developand to improve effectiveness of systemic treatments such as targeted therapies and immunotherapy.
References
- Abrams MJ, Gagne NL, Melhus CS, Mignano JE. Brachytherapy vs. external beam radiotherapy for choroidal melanoma: Survival and patterns-of-care analyses. Brachytherapy. 2016;15:216–23. doi: 10.1016/j.brachy.2015.12.001. [DOI] [PubMed] [Google Scholar]
- AJCC Ophthalmic oncology task force. International validation of the American joint committee on Cancer’s 7th edition classification of Uveal Melanoma. JAMA Ophthalmol. 2015;133:376–83. doi: 10.1001/jamaophthalmol.2014.5395. [DOI] [PubMed] [Google Scholar]
- American brachytherapy society - ophthalmic oncology task force. The American brachytherapy society consensus guidelines for plaque brachytherapy of uvealmelanoma and retinoblastoma. Brachytherapy. 2014;13:1–14. doi: 10.1016/j.brachy.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Caujolle JP, Mammar H, Chamorey E, et al. Proton beam radiotherapy for uveal melanomas at nice teaching hospital:16 years’ experience. Int J Radiat Oncol Biol Phys. 2010;78:98–103. doi: 10.1016/j.ijrobp.2009.07.1688. [DOI] [PubMed] [Google Scholar]
- Collaborative ocular Melanoma study group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol. 2006;124:1684–93. doi: 10.1001/archopht.124.12.1684. [DOI] [PubMed] [Google Scholar]
- Damato EM, Damato BE. Detection and time to treatment of uveal melanoma in the United Kingdom: an evaluation of 2,384 patients. Ophthalmology. 2012;119:1582–9. doi: 10.1016/j.ophtha.2012.01.048. [DOI] [PubMed] [Google Scholar]
- Diener-West M, Reynolds SM, Agugliaro DJ, et al. Collaborative ocular Melanoma study group Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative ocular Melanoma study group report No. 26. Arch Ophthalmol. 2005;123:1639–43. doi: 10.1001/archopht.123.12.1639. [DOI] [PubMed] [Google Scholar]
- Dithmar S, Diaz CE, Grossniklaus HE. Intraocular melanoma spread to regional lymph nodes: report of two cases. Retina. 2000;20:76–9. doi: 10.1097/00006982-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Finger PT. Radiation therapy for choroidal melanoma. Survey ophthalmol. 1997;42:215–32. doi: 10.1016/s0039-6257(97)00088-x. [DOI] [PubMed] [Google Scholar]
- Ge YR, Tian N, Lu Y, et al. Occupational cooking and risk of uveal melanoma: a meta-analysis. Asian Pac J Cancer Prev. 2012;13:4927–30. doi: 10.7314/apjcp.2012.13.10.4927. [DOI] [PubMed] [Google Scholar]
- Helgadottir H, Höiom V. The genetics of uveal melanoma: current insights. Appl Clin Genet. 2016;9:147–55. doi: 10.2147/TACG.S69210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic P, Mihajlovic M, Djordjevic-Jocic J, et al. Ocular melanoma: an overview of the current status. Int J Clin Exp Pathol. 2013;6:1230–44. [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch PK, Lommatzsch R. Treatment of juxtapapillary melanomas. Br J Ophtalmol. 1991;75:715–7. doi: 10.1136/bjo.75.12.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallone S, De Vries E, Guzzo M, et al. Descriptive epidemiology of malignant mucosal and uveal melanomas and adnexal skin carcinomas in Europe. Eur J Cancer. 2012;48:1167–75. doi: 10.1016/j.ejca.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Shields CL, Naseripour M, Shields JA, Freire J, Cater J. Custom-designed plaque radiotherapy for nonresectable iris melanoma in 38 patients: tumor control and ocular complications. Am J Ophthalmol. 2003;135:648–56. doi: 10.1016/s0002-9394(02)02241-9. [DOI] [PubMed] [Google Scholar]
- Shields CL, Furuta M, Thangappan A, et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009;127:989–98. doi: 10.1001/archophthalmol.2009.208. [DOI] [PubMed] [Google Scholar]
- Shields CL, Kaliki S, Furuta M, Mashayekhi A, Shields JA. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8,033 cases. Retina. 2012;32:1363–72. doi: 10.1097/IAE.0b013e31824d09a8. [DOI] [PubMed] [Google Scholar]
- Shields CL, Kaliki S, Furuta M, Shields JA. Diffuse versus nondiffuse small (≤3 mm thickness) choroidal melanoma: Comparative Analysis in 1,751 Cases. The 2012 F. Phinizy calhoun lecture. Retina. 2013;33:1763–76. doi: 10.1097/IAE.0b013e318285cd52. [DOI] [PubMed] [Google Scholar]
- Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881–5. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- Tarmann L, Wackernagel W, Avian A, et al. Ruthenium-106 plaque brachytherapy for uveal melanoma. Br J Ophthalmol. 2015;99:1644–9. doi: 10.1136/bjophthalmol-2015-306666. [DOI] [PubMed] [Google Scholar]
- Wackernagel W, Holl E, Tarmann L, et al. Local tumour control and eye preservation after gamma-knife radiosurgery of choroidalmelanomas. Br J Ophthalmol. 2014;98:218–23. doi: 10.1136/bjophthalmol-2013-304031. [DOI] [PubMed] [Google Scholar]
- Wilson MW, Hungerford JL. Comparison of episcleral plaque and proton beam radiation therapy for the treatment of choroidal melanoma. Ophthalmology. 1999;106:1579–87. doi: 10.1016/S0161-6420(99)90456-6. [DOI] [PubMed] [Google Scholar]


