Abstract
Background:
Breast cancer is the most common type of cancer amongst women throughout the world. Currently, there are various follow-up strategies implemented in Iran, which are usually dependent on clinic policies and agreement among the resident oncologists.
Purpose:
A cost-effectiveness analysis was performed to assess the cost-effectiveness of intensive follow-up versus standard models for early breast cancer patients in Iran.
Materials and methods:
This cross sectional study was performed with 382 patients each in the intensive and standard groups. Costs were identified and measured from a payer perspective, including direct medical outlay. To assess the effectiveness of the two follow-up models we used a decision tree along with indicators of detection of recurrence and metastasis, calculating expected costs and effectiveness for both cases; in addition, incremental cost-effectiveness ratios were determined.
Results:
The results of decision tree showed expected case detection rates of 0.137 and 0.018 and expected costs of US$24,494.62 and US$6,859.27, respectively, for the intensive and standard follow-up models. Tornado diagrams revealed the highest sensitivity to cost increases using the intensive follow-up model with an ICER=US$148,196.2.
Conclusion:
Overall, the results showed that the intensive follow-up method is not cost-effective when compared to the standard model.
Keywords: Breast cancer, cost-effectiveness-follow-up, intensive, standard
Introduction
Cancer is one of the most important issues of general health and economic concern throughout the world (Ferlayet al., 2007). Breast cancer is the most common type of cancer amongst women, whether in developed or developing countries. According to World Health Organization (WHO) approximately 508,000 individuals died of breast cancer in 2011. Although this disease is attributed to the developed countries, about 50% of theses cancer cases and 58% of cancer deaths had occurred in the developing countries (GLOBOCAN, 2012). Prevalence of this disease increases up to 1 to 2% per year in the developed countries, and up to 5% per year in the underdeveloped ones. In 2011, around 2.6 million people survived breast cancer in the United States and about 39,520 women lost their lives to this disease (Kolahdoozan et al., 2010). Prevalence of breast cancer has had a yearly increase in Iran and nowadays, it is recognized as the most common type of cancer among Iranian women. According to the latest statistics presented by the Ministry of Healthcare and Medical Education, 27 out of every 100,000 women have been diagnosed with breast cancer. This disease is considered a very serious one in Iran, since breast cancer affects Iranian women a decade sooner than women from western countries (Kolahdoozan et al., 2010). During the recent years, survival rates of the disease have increased as a result of proper screening programs and advances in adjuvants to surgery. Therefore, there has been tremendous growth in the number of cancer survivors needing follow-ups after treatment (NCCN, 2013). The main purpose of follow-up plan is early detection of recurrences in patients who had received standard treatments. Occurrence of metastasis can lead to a shorter life span and decreased quality of life in cancer patients. Most recurrence cases are witnessed within the first two to five years of follow ups (Ghohari et al., 2006; Moschetti et al., 2016). Therefore, in order to detect metastasis and recurrences at an early stage, outpatient follow-ups are done along with additional tests, depending on the clinic or hospital’s guidelines (Margenthaler et al., 2014).
There are standard follow-up guidelines specific to breast cancer patients. The National Comprehensive Cancer Network (NCCN) recommended follow-up plan is not classified based on cancer stage, however, they are performed for patients with stages 0 – III breast cancer (Margenthaler et al., 2012; Margenthaler et al., 2014).
Despite the huge number of cancer patients in Iran, including breast cancer patients, a good level of success has been achieved in treating the disease due to proper screening systems and increased awareness of society; this increases survival rates and thus the number of individuals needing long years of follow-ups (at least five years) would increase (Daroudi et al., 2015). Currently, there are various follow-up strategies implemented in Iran, which are usually dependent on clinic policies and agreements among the clinic’s oncologists. These strategies include the intensive and standard follow-up models recommended by NCCN’s guidelines. As our searches show, there hasn’t been a study in Iran with regards to cost-effectiveness of Intensive vs. Standard follow-up strategies, yet. In this study, in addition to determining cost-effectiveness of the mentioned models, the researchers aimed to evaluate the direct costs of prescribed tests and measures from the payer’s perspective using economic evaluation techniques; by determining a cost-effective model as the most convenient strategy. Through this, we might be able to take a step toward presenting a general guide for prescription of suitable tests. This consequently saves huge amounts of financial resources and may lead to imposition of less financial burden on the healthcare system.
Materials and Methods
The guidelines of the National Comprehensive Cancer Network (NCCN) recommend obtaining patient history, performing physical examinations every 3-6 months as well as annual mammography, chest radiography, blood tests, abdominal ultrasound, breast ultrasound, bone mineral density test, and pelvic ultrasound for those patients receiving letrazole and tamoxifen for two years and then after, if there is no recurrence, obtaining patient history and physical examination every 6-12 months and annual mammography, blood tests and bone scan is suggested. They have also recommend annual examinations specifically for women on Tamoxifen, and annual bone mineral density tests for patients who take aromatase inhibitors such as Letrazole; additional diagnostic tests should only be performed when an anomaly is witnessed in physical examinations, medical history or the mammograms (NCCN, 2013). On the other hand, the standard follow-up model merely consists of 3-6 months’ doctor visits and annual mammography for the first two years of follow up and if there is no recurrence, obtaining patient history and physical examination every 6-12 months and annual mammography (NCCN, 2013). Since the follow-up protocol of Imam Reza specialized clinic of Shiraz is similar to NCCN’s intensive follow-up model to a high degree, thus patients in this clinic were selected as the intensive group; and the patients at the Cancer Institute of Tehran’s Imam Khomeini hospital were selected as the standard group, because of the high resemblance between NCCN’s standard follow-up plan and this clinic’s follow-up protocols.
This cross-sectional study was an economic evaluation research among breast cancer patients who had received full treatment and at least five years of follow-ups from the mentioned clinics. Inclusion criteria included non-metastatic cancer in one or both breasts at the time of diagnosis, completion of surgical treatment, chemotherapy, radiation therapy and hormone therapy, and continuance of follow-ups until the year 2014. Exclusion criteria were incomplete treatment and lack of success in finding patients through phone calls.
When population variance and the variable’s probability of success are unknown and we are not able to use statistical formulas to estimate sample size, maximum sample size is determined by using Morgan’s table. Therefore, our study’s population was determined as about 75,000 individuals and sample size was estimated at 382 individuals in each group (764 patients in total). The reason why this sample size was selected was due to that fact that our population size was unknown and we only had access to file numbers and types of cancer in the clinics’ registry systems. Therefore, only after reading the file contents we could determine the non-occurrence of metastasis at the time of diagnosis, which was among the study’s inclusion criteria.
Using the data collection form, the demographic information of survivors and information regarding disease, treatment and number and types of diagnostic tests were extracted from the case records of treated patients under surveillance from 2008 to 2014. Because of the minimum 5-year follow-up criterion, the case records of patients who were diagnosed in the years 2007 to 2009 were assigned numbers and then 382 samples were randomly selected for each group. Based on the prognostic factors recorded in the patient’s records, such as Estrogen receptors (ERs), Progesterone receptors (PRs) and human epidermal growth factor receptor 2 (HER2), the survivors were divided into four groups of A, B, C and D, so that their follow-up process could be evaluated more accurately based on occurrence risk of metastasis, which would make them somewhat synchronized. Group A were ER and PR positive and HER2 negative; group B were ER and PR positive, as well as HER2 positive; group C were ER and PR negative and HER2 positive; and group D were both ER and PR negative and HER2 negative.
The time variable was determined as from the time of treatment completion and the first follow-up visit until observation of the first incident, whether it be recurrence of cancer in the breasts or metastasis in other parts of the body.
To compare cost-effectiveness of the standard and intensive follow-up models, we used a decision tree model along with the indicator of case detection or detection of recurrences and metastasis. First, we calculated expected costs and expected case detections based on this model, and then reached the incremental cost-effectiveness ratio. The incremental cost-effectiveness ratio (ICER) is defined as the difference between expected costs and expected case detections; it is used to increase research accuracy and reduce unreliability of sensitivity analysis. The decision tree model is presented in Figure 1.
Figure 1.
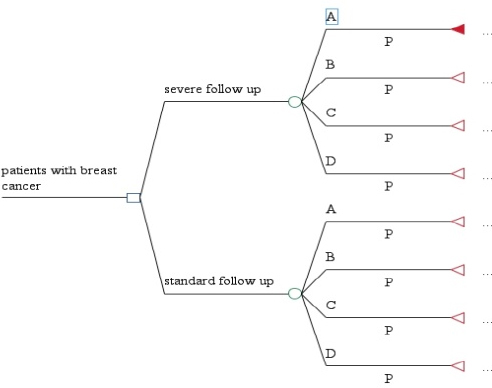
The Decision tree for Along with the Indicator of Case Detection or Detection of Recurrences and Metastasis. Group A: Estrogen Receptor (ER) and Progesterone Receptor (PR) positive and epidermal growth factor receptor 2 (HER2) negative, Group B: ER and PR positive and HER2 positive, Group C were ER and PR negative and HER2 positive, Group D: both ER and PR negative and HER2 negative
The decision tree shows the survivors’ final state across 2 conditions including relapse (metastasis) and no relapse (no metastasis). In the relapse condition, the indicator of case detection is presented based on the two states of by tests (detection of metastasis by diagnostic tests before appearance of clinical signs) and by physician (diagnosis of metastasis by the physician after appearance of clinical signs); in this study, the by test state is selected as the case detection indicator (effectiveness criterion). In this study, we aimed to see if the numerous diagnostic tests of the intensive follow-up model had been effective enough in early detection of metastasis and recurrences, or they just simply imposed additional costs on patients without really having the ability to detect recurrences and metastasis on time. To this end, we studied physician notes regarding each patient’s general condition and the results of each follow-up visit recorded in the records.
In this research, costs are calculated from the perspective of the payer (patients and insurance companies); in this regard, we calculated the direct costs of follow-ups for breast cancer patients, including costs of each diagnostic and lab test (mammography, blood test, chest radiography, abdominal ultrasound, pelvic ultrasound, breast ultrasound, and bone mineral density) over a year using the private fees published by the Ministry of Healthcare and Medical Education, and eventually reached at the total follow-up cost for each patient during the follow-up period 2008–2014. Since we only had access to patient records in this study, only the direct medical costs was entered into our calculations and indirect costs or direct non-medical costs weren’t used, because they weren’t included in the records and there were errors in the stated costs for previous years. Also, since both clinics were public and had similar number of visits at the same cost, we disregarded all costs relating to this variable.
Because the follow-ups were done during the period of 2008 to 2014, all costs were converted to values of the study year (2014), using the inflation rates reported by the Central Bank of Iran; therefore, since past data were converted to present values, there was no need to use discount rates in this study.
For analysis of demographic data, we entered the information from each section of the data collection form into the SPSS software package for Windows, Version 19 (SPSS Inc., Chicago, USA). Descriptive tests were used to estimate relative and absolute frequency of data based on both follow-up models, and the Mann-Whitney test determined the significant cost differences. This study’s event was determined as the period until occurrence of the first event, meaning that a recurrence or metastasis; values less than or equal to 5% were considered significant statistically. For economic analyses such as analysis of the cost-effectiveness diagram, calculation of the incremental cost-effectiveness ratio, drawing of the Tornado Diagram and sensitivity analysis of certainty, we used the Treeage Pro 2012 software (Treeage Software Inc., Williamstown, MA, USA).
Results
Of the 764 survivors in our study (382 individuals in each group), 91.4% were married, 76.7% were above 40 years old and 63.6% were residents of urban areas; regarding types of insurance, the highest frequency (39.1%) pertained to patients under social security insurance and 79.4% of the patients didn’t have any kind of extra insurance (supplementary). Most of the survivors (42.9%) were at the stage 3 of the disease, and 59.4% of them had been placed under the intensive follow-up plans. Comparing tumor size among patients revealed that 66.9% of them had 2-5 cm tumors, 73.0% of which were placed under the intensive follow-up plan. Furthermore, 34.3% of the patients had 1-3 involved lymph nodes, of which 39% were placed in the standard follow-up group. According to study findings, 68.2% of the patients were in the estrogen and progesterone receptor positive groups and 58.0% were positive for HER2. Therefore, 40.8% of the survivors belonged to group B (Table 1). Most of the survivors (61.5%) had had a mastectomy; 99.21% had been placed under chemotherapy and 91.6% had received radiation therapy. Among the studied patients, 86.0% had never experienced treatment with herceptin. However, 34.3% of them had received hormone therapy with letrazole and 30.7% had hormone therapy with tamoxifen in their treatment plan.
Table 1.
Relative and Absolute Frequency of Breast Cancer Survivors Classified Based on ER, PR and HER2 in the Intensive and Standard Follow-Up Models; and also Follow-Up Costs Based on Private Fees (US Dollars) Divided by the Follow-Up Groups 2008-2014
| Intensive | Follow-up | Group | Standard | Follow-up | Group | |
|---|---|---|---|---|---|---|
| Costs ($US) | Costs ($US) | |||||
| Group | Number of patients (%) | Total | Per patient | Number of patients (%) | Total | Per patient |
| A | 91 (23.8) | 27,526 | 302.4 | 121 (31.7) | 14,605 | 120.7 |
| B | 185 (48.5) | 62,348 | 337 | 127 (33.2) | 13,072 | 103 |
| C | 65 (17) | 16,690 | 256.7 | 71 (18.6) | 8,128 | 114.4 |
| D | 41 (10.7) | 12,443 | 407 | 63 (16.5) | 7,345 | 118.5 |
| Total | 382 (100) | 119,007 | - | 382 (100) | 43,150 | - |
Group A, ER and PR positive and HER2 negative; Group B, ER and PR positive and HER2 positive; Group C, ER and PR negative and HER2 positive; Group D, ER and PR negative and HER2 negative
Of the 764 survivors in this study, 31.9% had occurrence of metastasis, out of which 35.1% were placed under the standard follow-up plan. Since the time of detection of recurrences or metastasis is of importance, we studied this indicator. Our findings revealed that metastasis has been diagnosed in the third year of follow-up for 45.3% of the patients, from which 47.3% received standard follow-up care. 75.9% of the patients with metastatic relapse had been diagnosed by physician based on clinical signs. Most of the patients with metastasis (37.9%) had metastatic bone involvement. Findings also revealed that up to year 2014, 65.9% of the patients under the standard follow-up plan and 70.9% of the patients under intensive follow-up had survived without any recurrences. Among the mentioned variables, only the variable of detection method for recurrence and metastasis was statistically significant for the two follow-up groups; there were more early detections (detection of metastasis by diagnostic tests before the appearance of clinical signs) in the intensive group (p<0.001). On the other hand, 65 (17.0%) and 87 (22.0%) of intensive and standard treatment groups were died by the end of the study, respectively.
According to table 1 with regards to cost analysis, it was revealed that in the intensive follow-up group, the highest total follow-up cost belonged to group B (ER and PR positive and HER2 positive) with total cost of $ 62,345.05 US dollars, and the highest total cost in the standard follow-up group pertained to group A (ER and PR positive and HER2 negative) with total cost of $14,605.00; overall, the intensive follow-up model had a 2.7 times higher total cost ($1,190.1) than the standard model ($43,150.0). Highest mean follow-up cost per patient belonged to group D (ER and PR negative and HER2 negative) in the intensive group ($40.6), and group A in the standard group ($120.7). The mean follow-up cost difference was statistically significant between the two groups of intensive follow-up ($311.53) and standard follow-up ($112.9); the intensive group had a higher mean cost (p<0.001). Although, the mean costs of all four group were quite close in the standard group.
Discussion
The decision tree results presented in Table 2 shows expected case detection rate as 0.137 and 0.018 and expected costs as $24,494.6 and $6,859.3 for intensive and standard follow-up models, respectively. The cost-effectiveness analysis diagram (Figures 2, 3) indicates that comparing to the standard follow-up model, the intensive model increases costs and effectiveness by $17,635.3 and 0.119, respectively. Thus, for the purposes of decision-making, we calculated the incremental cost-effectiveness ratio (ICER) and compared it with its threshold. The calculated ICER indicates that for each unit of increase in effectiveness, the intensive follow-up model would increase costs by $148,196.2, comparing to the standard model.
Table 2.
Results from the Decision Tree Model Regarding Intensive and Standard Follow-up Models Among the Breast Cancer Survivors of Imam Reza Clinic of Shiraz and Cancer Institute of Tehran during the Years 2008-2014
| Follow-up Cost model | Expected Cost ($US) | Expected Effectiveness (case detection) | Cost Difference DC | Effectiveness DifferenceDE | Result |
|---|---|---|---|---|---|
| Intensive | 24,494.62 | 0.137 | 17,635.35 | 0.119 | There is a need for comparison of ICER with the threshold value |
| Standard | 6,859.27 | 0.018 |
Figure 2.
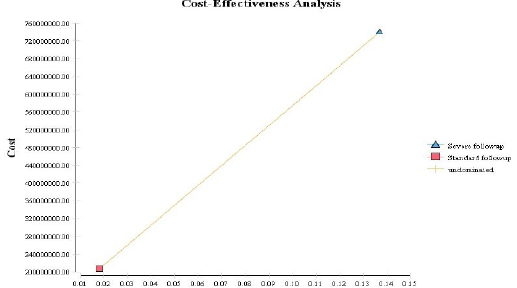
Cost-Effectiveness Analysis of the Standard and Intensive Follow-up Models for Breast Cancer Survivors
Figure 3.
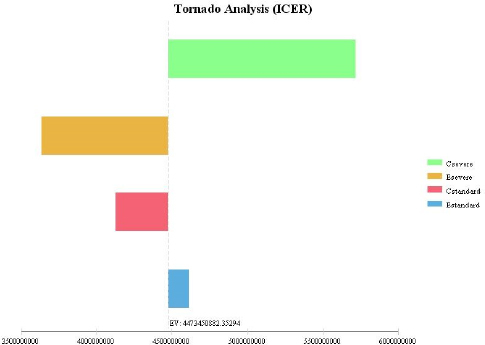
Tornado Analysis Relating Sensitivity of the Intensive and Standard Follow-up Models
We used World Health Organization’s (WHO) method to calculate the threshold value; therefore, if ICER was lower than three times Gross Domestic Product (GDP) per capita, the plan would be cost-effective (Koko et al., 2005). According to the World Bank’s report in 2014, our country’s GDP per capita equaled to $5,442.9 (Kolahdoozan et al., 2011). The threshold value is three times this amount, meaning $16,328.7 US dollars. Furthermore, since ICER was obtained as $148,196.2, an amount above the threshold ($16,328.7), therefore the intensive follow-up method is not cost-effective comparing to the standard model.
For the purposes of sensitivity analysis in this study, all variable values were increased by 20%; we drew the Tornado diagram based on this analysis. The Tornado diagram (Figure 3) reveals that study results had the highest sensitivity to cost increases in the intensive follow-up model (C intensive), and the lowest sensitivity to increases in the effectiveness of the standard follow-up model (E standard). Discussion
While there is talk regarding the limitations of healthcare budget in Iran, a developing country, it happens a lot that the scarce budget is wasted in follow-ups for no apparent reason, through unnecessary number of visits and unreasonable types of imaging such as MRIs and ultrasounds as well as various blood tests in each visit. These unnecessary tests result in imposing additional costs on patients as well as the totality of healthcare system (Baena et al., 2013). In recent years, the number of breast cancer survivors has increased as a result of proper screening plans and advances in adjuvants to surgery. When most breast cancer patients are diagnosed in early stages of the disease and have proper prognosis, there would be a need for many years of follow-ups, as well as huge amounts of healthcare resources. Therefore, it is important to discuss cost-effectiveness and the contents of these types of follow-ups (Kolahdoozan et al., 2010; Kimman et al., 2011; Van hezewijk et al., 2012). Economic evaluation compares the costs and outcomes of these interventions and plans for the purposes of proper resource allocation (Hatam et al., 2015).
This study aimed to evaluate cost-effectiveness of the intensive follow-up model in comparison with the standard one, amongst breast cancer survivors. Findings revealed that the mean costs of intensive and standard follow-up models to be $112.9 and $311.5 US dollars, respectively, which was higher in the intensive group. This shows a significant difference (p<0.001). Meanwhile, the highest mean follow-up costs per patient belonged to group D ($407.0) in the intensive group and group A ($120.7) in the standard group. Also, highest total follow-up costs pertained to group B ($62347.5) in the intensive group and group A ($14,605.00) in the standard group; overall, the total follow-up cost for the intensive group ($11,900.7) was approximately 2.7 times the total cost for the standard group ($143,150.0). Oltra et al., (2007) found mean follow-up costs per person to be €1,278 and €390 for the intensive and standard follow-up groups, respectively. Furthermore, they stated that the total follow-u costs as €24,567.0 in the standard group and €74,171.0 in the intensive group, which revealed that the follow-up costs was 3 times higher in the intensive model comparing to the standard one (Lauzier et al., 2013). Koko et al., (2005) confirmed the same results; in their study, the intensive model increased follow-up costs by 2.2 times (Rosselli Del Turco et al., 1994).
Lu et al., (2012), Margenthaler et al., (2014) and Kimman et al., (2007) all emphasized that a lower-intensity follow-up plan, aside from relieving the physicians of unnecessary burdens could lead to significant reduction of costs along with detection of metastases without any signs and recurrences in the same part of the body (Gohari et al., 2006; Van hezewijk et al., 2012; Lu et al., 2012). Although in the present study the less intensive follow-up plan reduced the costs, but it wasn’t effective in the detection of recurrences and metastasis without clinical signs.
According to our results, case detection rates were 0.137 and 0.018 for the intensive and standard groups, respectively. Considering the study findings, we can state that the intensive follow-up model had a higher number of early metastasis detections, which could be as a result of numerous diagnostic tests and visits to the specialists.
Since this study was the first one regarding cost-effectiveness of intensive vs. standard follow-up models for breast cancer survivors based on case detection, hence, we weren’t able to compare results with other studies. Nevertheless based on our results, the intensive follow-up model wasn’t cost-effective in comparison with the standard model; because the intensive model expected costs was $24,494.6 and case detection ratio of 0.138, while the standard model expected costs was $68,59.3 and case detection of 0.018. Therefore, comparison of the incremental cost-effectiveness ratio with its threshold revealed that with each unit of increase in effectiveness, the intensive model would cause a $148,196.2 increase in costs, compared to the standard model. Now since ICER is above the threshold, we can state that the intensive follow-up model isn’t cost-effective in comparison with the standard one.
In this study, in order to evaluate accuracy of the ICER results, we did sensitivity analysis, which enables us to determine the decisive parameters in the results of economic evaluation. Based on the results of the sensitivity analysis, which was performed with a 20% increase in all data, ICER had the highest sensitivity to the cost increases of the intensive model and the lowest sensitivity to increases in the effectiveness of the standard model. Subsequently, in this condition ICER would increase greatly in comparison to the initial value, therefore we would be able to comment on the cost-effectiveness of standard vs. intensive models with higher accuracy. On the other hand, ICER has low sensitivity to most parameters and this could increase generalizability of the results.
Toward generalization of this finding, we can extend this result to other follow-up clinics in our country, because they all use the same two follow-up strategies (Standard and Intensive) for breast cancer patients. However, we cannot positively extend this result to other countries because of the probable differences in costs and levels of coverage by their insurance organizations, patients’ ability to pay, incidence and prevalence of the disease and survival rates, clinical guidelines, and test fees as well as system and maximum amounts of payment.
All in all, our results showed that the intensive follow-up model isn’t cost-effective in comparison with the standard model, since its ICER is above WHO announced threshold value (three times GDP per capita).
Therefore, based on the result from evaluation of cost-effectiveness, we recommend that oncologists use the standard follow-up model for survivors, rather than the intensive one.
Acknowledgment
This research was performed by Ms. Mina Vazirzadeh in partial fulfillment of the requirements for obtaining an MSc in Health Administration at Shiraz University of Medical Sciences. This article is the result of a research project (93-7370) approved by the Deputy of Research of the aforementioned university. Also, we thank the Research Consultation Center (RCC) at Shiraz University of Medical Sciences for their invaluable assistance in editing this article.
Authors’ contribution to the manuscript
Nahid Hatam: Protocol/project management, Data management, Data analysis, Manuscript Writing and editing
Niloofar Ahmadloo: Patient management, Manuscript editing
Mina Vazirzadeh: Data management, Data collection, Data analysis, Manuscript Writing
Abdossaleh Jafari: Data management, Data analysis, Manuscript editing
Mehrdad Askarian: Protocol/project management, Data management, Data analysis, Manuscript Writing and editing
Compliance with Ethical Standards
Funding
This study was funded by Shiraz University of Medical Sciences (grant number 93-7370).
Conflict of Interest
None to declare
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
References
- Baena J M, Ramirez P, Cortes C, et al. Follow-up of long-term survivors of breast cancer in primary care versus specialist attention. Family practice. 2013;30:525–32. doi: 10.1093/fampra/cmt030. [DOI] [PubMed] [Google Scholar]
- Daroudi R, Akbari Sari A, Nahvijou A, et al. The economic burden of breast cancer in Iran. Iran J Public Health. 2015;44:1225–33. [PMC free article] [PubMed] [Google Scholar]
- Eichler HG KS, Gerth WC, Mavros P, Gerth WC, Mavros P. Use of cost-effectiveness analysis in health care resource allocation decision- making: how are cost- effectiveness thresholds expected to emerge? Value Health. 2004;7:518–28. doi: 10.1111/j.1524-4733.2004.75003.x. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Autier P, Boniol M, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–92. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- Gohari MR, Mahmoudi M, Kazem M, Pasha E, Khodabakhshi R. Recurrence in breast cancer analysis with frailty model. Saudi Med J. 2006;27:1187–93. [PubMed] [Google Scholar]
- Hatam N, Dehghani M, Habibian M, Jafari A. Cost-utility analysis of IEV drug regimen versus ESHAP drug regimen for the patients with relapsed and refractory hodgkin and non-hodgkin’s lymphoma in Iran. Iran J Cancer Prev. 2015;8:e4061. doi: 10.17795/ijcp-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer, World Health Organization. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. [Accessed 21 Sept 2015]. Available from: http://globocan.iarc.fr/Default.aspx .
- Kimman ML, Dirksen CD, Voogd AC, et al. Economic evaluation of four follow-up strategies after curative treatment for breast cancer: results of an RCT. EJC. 2011;47:1175–85. doi: 10.1016/j.ejca.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Kimman ML, Voogd AC, Dirksen CD, et al. Improving the quality and efficiency of follow-up after curative treatment for breast cancer: rational and study design of MaCare trial. BMC Cancer. 2007;7:1. doi: 10.1186/1471-2407-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koko R, Hakama M, Holli K. Follow-up cost of breast cancer patients with localized disease after primary treatment: a randomized trial. Breast Cancer Res Treat. 2005;93:255–60. doi: 10.1007/s10549-005-5199-2. [DOI] [PubMed] [Google Scholar]
- Kolahdoozan S, Sadjadi A, Radmard AR, Khademi H. five common cancers in Iran. Arch Iran Med. 2010;13:143–6. [PubMed] [Google Scholar]
- Lauzier S, Levesque P, Mondor M, et al. Out-of-pocket costs in the year after early breast cancer among Canadian women and spouses. JNCI. 2013;105:280–92. doi: 10.1093/jnci/djs512. [DOI] [PubMed] [Google Scholar]
- Lu W, Greuter MJ, Schaapveld M, et al. Safety and cost effectiveness of shorting hospital follow-up after breast cancer treatment. BJS. 2012;99:1227–33. doi: 10.1002/bjs.8850. [DOI] [PubMed] [Google Scholar]
- Margenthaler JA, Allam E, Chen L, et al. Surveillance of patients with the breast cancer after curative-intent primary treatment: current practice patterns. J Oncol Prac. 2012;8:79–83. doi: 10.1200/JOP.2011.000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margenthaler JA, Johnson FE, Cyr AE. Intensity of follow-up after breast cancer surgery: low versus high. Ann Surg Oncol J. 2014;21:733–7. doi: 10.1245/s10434-013-3251-8. [DOI] [PubMed] [Google Scholar]
- Moschetti I, Cinquini M, Lambertini M, Levaggi A, Liberati A. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst Rev. 2016;5:CD001768. doi: 10.1002/14651858.CD001768.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCCN clinical practice guidelines in oncology. [Accessed 4 Sept 2013]. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#breast .
- Oltra A, Santaballa A, Munarriz B, Pastor M, Montalar J. Cost-benefit analysis of a follow-up program in patients with breast cancer: a randomized prospective study. Breast J. 2007;13:571–4. doi: 10.1111/j.1524-4741.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- Rosselli Del Turco M, Palli D, Cariddi A, et al. Intensive diagnostic follow-up after treatment of primary breast cancer. A randomized trial. National research council project on breast cancer follow-up. JAMA. 1994;271:1593–7. doi: 10.1001/jama.271.20.1593. [DOI] [PubMed] [Google Scholar]
- The GIVIO Investigators. Impact of follow-up testing on survival and health-related quality of life in breast cancer patients. A multicenter randomized controlled trial. JAMA. 1994;271:1587–92. doi: 10.1001/jama.1994.03510440047031. [DOI] [PubMed] [Google Scholar]
- The world bank [online] 2016. [Accessed 13 july 2015]. Available from: http://databank.worldbank.org/data/reports.aspx?source=2&series=ny.gdp.pcap.cd&country=irn .
- Van hezewijk M, van den akker ME, van de velde CJH, Scholten AN, Hille E TM. Costs of different follow-up strategies in early breast cancer: a review of the literature. Breast J. 2012;21:693–700. doi: 10.1016/j.breast.2012.09.009. [DOI] [PubMed] [Google Scholar]


