Abstract
Objectives:
To describe the survival experience of cervix cancer patients in a screened rural population in India.
Methods:
Included 558 cervical cancer patients diagnosed in 2000-2013 in a cohort of 100,258 women invited for screening during 2000-2003. The primary end point was death from cervical cancer. We used the Kaplan-Meier method to estimate cumulative observed survival and Cox proportional hazards regression to assess the effect of patient characteristics on survival after diagnosis.
Results:
Of the 558 cases included, 143 (26%) and 114 (20%) were diagnosed in stages IA and IB respectively; 252 (45.2%) were dead, and 306 (54.8%) were alive at the last follow-up. The overall 5-year observed survival was 60.5%. The 5-year survival of stage IA patients was 95.1% and 5.3% for stage IV patients. All surgically treated stage IA patients, 94.1% of stage IB patients receiving intracavitary radiotherapy, 62% of stage IIB, 49% of stage III and 25% of stage IV patients receiving radiotherapy survived for 5 years.
Conclusion:
Higher 5-year survival in our study than elsewhere in India is due to the high proportion of early stage cancers detected by screening combined with adequate treatment, resulting into a favourable prognosis.
Keywords: Survival, cervical cancer, developing countries
Introduction
Cervical cancer is an important public health problem in India, where 123,000 new cases and 68,000 deaths are estimated to occur annually, accounting for one fifth of cancers in women in India (Ferlay et al., 2013). Despite the fact that India contributes one-fifth of global burden of disease, there are no cervical screening programs on-going in the country except in the state of Tamil Nadu and public health policies aiming at effective integration of screening and vaccination for human papillomavirus (HPV) are yet to evolve in any region of India. More than four-fifths of cervical cancer patients in India present in advanced clinical stages with less than half of the cases surviving 5-years (Sankaranarayanan et al., 2010; Sankaranarayanan and Swaminathan 2011). Integrated HPV vaccination of girls aged 9-14 years and effective screening programs through the early detection of high-grade cervical intraepithelial neoplasia (CIN 2-3) and their effective treatment leading to prevention of invasive cancer is the cornerstone of cervical cancer control.
Five-year survival from cervical cancer varies between 35% and 60% in different regions of India (Sankaranarayanan et al., 2010; Gajalakshmi et al., 2000; Jayant et al., 1996; Yeole et al., 2004; Shanta et al., 1998; Nandakumar et al., 1998; Jayant et al., 1998; Swaminathan et al., 2009). Three main factors influence the magnitude of survival rates in cohorts of cervical cancer patients: 1) the relative proportion of patients with early vs. advanced disease, 2) the age distribution of patients, and 3) access to diagnostic and treatment facilities and availing the main treatment modalities, namely surgery, radiotherapy and chemotherapy and their combinations integrated in specific protocols.
Clinical stage at presentation is a very important predictor for long-term survival. Five-year survival exceeds 90% for early stages such as stage IA and IB whereas less than 20% of those diagnosed with advanced disease such as stages III and IV survive for 5 years (Sankaranarayanan et al., 2010; Yeole et al., 2004; Shanta et al., 1998; Nandakumar et al., 1998; Jayant et al., 1998; Swaminathan et al., 2009). Lack of early detection initiatives, poor awareness among physicians and the public, advanced clinical disease at diagnosis and inadequate access to treatment are the main reasons for the poor survival in the different regions (Sankaranarayanan et al., 2010; Yeole et al., 2004; Shanta et al., 1998; Nandakumar et al., 1998; Jayant et al., 1998; Swaminathan et al., 2009). The prognostic value of stage at presentation may vary in different populations due to other determinants such as socio-economic factors, age at presentation (general health, competing morbidity), availability, accessibility and affordability of effective treatment and varying adherence to prescribed treatment and follow-up care. We describe in this manuscript the survival experience of a cohort of cervical cancer patients diagnosed in women invited for screening in a randomized cervical cancer screening trial carried out in Osmanabad district, Maharashtra state in Western India (Sankaranarayanan et al., 2009).
Materials and methods
Study design
The survival study was conducted in the context of the cluster randomized controlled trial carried out in Osmanabad district, Maharashtra State, India (hereafter referred to as the trial) to evaluate the effectiveness of a single round of screening with visual inspection with acetic acid (VIA), or conventional cytology (Pap smear), or human papilomavirus (HPV) testing in reducing cervical cancer mortality as compared to a control group receiving usual care (Sankaranarayanan et al., 2009). The study design, methods and results of the trial have already been published (Sankaranarayanan et al., 2009). In this trial, 131,746 eligible women aged 30-59 years living in 52.0 clusters were enrolled and were randomly allocated to four groups: the control group receiving usual existing care (13 clusters, 31,488 women), HPV testing group screened with a single round of HPV screening (13 clusters, 34,126 women), VIA group screened with single round of visual screening (13 clusters, 34,074 women) and the cytology group receiving a single Pap smear (13 clusters, 32,058 women). Women in the control group as well as all women invited for screening were educated on signs and symptoms of cervical cancer, early detection and prevention and were advised on how and where to avail of screening, early diagnosis and treatment services in the district. The women in the groups allocated to screening were invited for testing during 2000-2003 and women tested positive were offered diagnostic investigations consisting of colposcopy triage, directed biopsies of colposcopically abnormal lesions and those diagnosed with CIN and invasive cervical cancer were offered treatment. The study cohorts were followed-up for cervical cancer incidence and death.
The eligible cases for this survival study include all cervical cancer patients diagnosed with cervical cancer (International Classification of Diseases for Oncology 10th revision, C53) between the years 2000 and 2013 among the three cohorts of women who were invited to be screened by HPV testing, VIA or cytology in the trial (in total, 100,258 women aged 30-59 years at study entry) 79,506 (79.3%) of the invited women received screening.
Women diagnosed with cervical cancer following screening tests or symptoms and clinical diagnosis were staged and treated in the Nargis Dutt Memorial Cancer Hospital (NDMCH), Barshi, which coordinated the screening trial, or in a cancer hospital or other tertiary hospitals in the region.
Clinical stage at presentation of patients was assessed by pelvic examination, bimanual palpation, cystoscopy, ultrasonography of the pelvis, x-rays of the pelvis and chest. The clinical stages were categorized according to the stage categories of the International Federation of Gynaecology and Obstetrics (FIGO) staging system as stage IA, IB, IIA, IIB, IIIA, IIIB, IVA, IVB (Benedet et al., 2000). If no staging details were available, the stage was recorded as unknown by us. Histological type of tumour was categorized as squamous cell carcinoma or adenocarcinoma. Age at diagnosis was grouped into five-year age groups. Formal education of the participants was categorized as none, primary school and above primary. Patients in good general health with stage IA tumours were predominantly treated with radical or simple hysterectomy. Patients with stages IB and IIA disease in good general health and without competing morbidity received radical hysterectomy or intracavitory radiotherapy, whereas those with bulky disease and poor surgical risks were treated with a combination of external radiotherapy and intracavitary radiation; concurrent chemotherapy with weekly cisplatinum. To a maximum of 3-5 courses was administered if the patients were in good general health. Patients with stages IIB, III and IV disease were predominantly treated with a combination of external radiotherapy and intracavitary radiotherapy with or without concurrent chemotherapy with cisplatinum. The type of treatment offered was categorized into surgery, radiotherapy, a combination of treatments and treatment details not known.
Information on incident cervical cancer cases in the study cohorts and their vital status was regularly updated by the staff of the screening trial, as part of the continuing evaluation of the impact of the single round of screening with the different screening tests, by matching the incident cervical cancer cases registered by the Osmanabad district population-based cancer registry and by matching with death records kept by the district mortality registration system, scrutiny of hospital records and by conducting house visits in the villages included in the study to collect information on proven/likely cancer cases which might have been missed by the cancer and mortality registries. The Osmanabad district cancer registry uses active case finding methods recommended by the International Agency for Research on Cancer (IARC) and the International Association of Cancer Registries (IACR) (Jensen et al., 1991) and covers the resident population of the entire district. The registry staff members regularly visit hospitals and diagnostic centres within and outside the registry area, all primary health centres within the registry area, and the cancer treatment centres in Mumbai, Pune and Aurangabad to actively collect information on cancer cases diagnosed in the resident population of Osmanabad district. Cause of death for each death was assessed case-by-case by the cancer registry staff by taking into account the information from hospital records, death certificates, house visits and interviewing relatives/friends. Information on the incident cervical cancer cases in the screening cohort of women (100,258 women aged 30-59 years at entry) and their vital status were abstracted from the screening study database.
The primary endpoint was death from cervical cancer. The vital status of all included patients was established as dead or alive or lost to follow-up on 31st December 2013. Survival time was defined from the date of diagnosis to date of death, for the patients who had died, or date of last follow-up for those who were still alive or lost to follow-up. Cumulative observed survival was estimated using the Kaplan-Meier method and Cox proportional hazards regression was used to assess the effect of patient characteristics on patient survival after diagnosis. Only those variables significant in the univariate analysis were included in the multivariate regression model.
Results
There were 558 women diagnosed with invasive cervical cancer during 2000-2013 from among 100,258 women randomized to receive a single round of one of the screening tests in the trial. The patient characteristics, vital status and hazard ratios (relative risk) for death in terms of age, household income, education, histology, clinical stages and treatment received are given in Table 1. Three-fourths of the patients had no formal education, half were diagnosed in stage I, almost all cases had squamous cell carcinoma and one-third of patients were treated by surgery only. The proportion of cases in stage I was higher in HPV and cytology arms compared to the VIA arm. Of the 163 cases diagnosed in the HPV screening arm, 87 (53%) were diagnosed in stage I; among the 180 cases in the cytology arm, 91 (51%) cases and among the 215 cases in the VIA arm, 79 cases (37%) were diagnosed in stage I. By 31st December 2013, the cut off date for follow-up of vital status in the study, 252 (45.2%) patients were dead, 306 (54.8%) were alive and none were lost to follow-up. The median follow-up time of the cancer patients was 5.3 years (interquartile range: 1.3-11.2 years).
Table 1.
Patient Characteristics and Relative Risk of Death from Cervical Cancer
| Characteristics | No. of patients | No. of deaths (%) | Crude ratio | hazard (95%CI) | Adjusted ratio | hazard (95%CI)* | |||
|---|---|---|---|---|---|---|---|---|---|
| Patients assessed | 558 | 252 | -45.2 | ||||||
| Age at diagnosis (years) | |||||||||
| 30-34 | 36 | 5 | -13.9 | 1.0 | 1.0 | ||||
| 35-39 | 80 | 42 | -52.5 | 5.3 | (2.1 | 13.3) | 4.2 | (1.6 | 10.9) |
| 40-44 | 106 | 44 | -41.5 | 4.4 | (1.7 | 11.2) | 3.2 | (1.2 | 8.3) |
| 45-49 | 114 | 63 | -55.3 | 6.4 | (2.6 | 15.9) | 4.6 | (1.8 | 11.7) |
| 50-54 | 103 | 43 | -41.8 | 4.7 | (1.9 | 11.9) | 2.9 | (1.1 | 7.5) |
| 55+ | 119 | 55 | -46.2 | 5.3 | (2.1 | 13.1) | 3.1 | (1.2 | 8.1) |
| Education | |||||||||
| None | 389 | 165 | -42.4 | 1.0 | |||||
| Primary | 57 | 21 | -36.8 | 0.8 | (0.5 | 1.3) | |||
| Above primary | 54 | 21 | -38.9 | 0.9 | (0.5 | 1.4) | |||
| Type of house | |||||||||
| Thatched | 161 | 66 | -41.0 | 1.0 | |||||
| Tiled | 311 | 134 | -43.1 | 1.1 | (0.8 | 1.5) | |||
| Concrete | 52 | 24 | -46.2 | 1.3 | (0.8 | 2.1) | |||
| Income (Indian rupees)** | |||||||||
| <2000 | 333 | 147 | -44.1 | 1.0 | |||||
| 2000+ | 191 | 77 | -40.3 | 0.9 | (0.7 | 1.2) | |||
| Marital status | |||||||||
| Currently married | 428 | 167 | -39.0 | 1.0 | |||||
| Not currently married | 62 | 32 | -51.6 | 1.6 | (1.1 | 2.3) | |||
| Total number of pregnancies | |||||||||
| None | 44 | 30 | -68.2 | 1.0 | 1.0 | ||||
| 01-Mar | 152 | 52 | -34.2 | 0.3 | (0.2 | 0.5) | 0.6 | (0.4 | 1.0) |
| 4+ | 329 | 143 | -43.5 | 0.4 | (0.3 | 0.6) | 0.7 | (0.5 | 1.1) |
| Histology | |||||||||
| Squamous cell carcinoma | 536 | 238 | -44.4 | 1.0 | 1.0 | ||||
| Adenocarcinoma | 22 | 14 | -63.6 | 2.5 | (1.4 | 4.2) | 1.8 | (0.9 | 3.4) |
| FIGO stage | |||||||||
| IA | 143 | 29 | -20.3 | 1.0 | 1.0 | ||||
| IB | 114 | 34 | -29.8 | 1.8 | (1.1 | 3.0) | 1.7 | (0.9 | 2.9) |
| IIA | 33 | 16 | -48.5 | 5.6 | (3.0 | 10.4) | 5.6 | (2.8 | 11.0) |
| IIB | 56 | 32 | -57.1 | 4.5 | (2.7 | 7.4) | 4.6 | (2.47 | 8.5) |
| III | 123 | 88 | -71.5 | 7.8 | (5.1 | 11.9) | 6.3 | (3.6 | 10.9) |
| IV | 19 | 18 | -94.7 | 30.4 | (16.6 | 55.4) | 28.6 | (13.7 | 59.8) |
| Stage details not known | 70 | 35 | -50.0 | 6.1 | (3.7 | 10.0) | 2.9 | (1.6 | 5.5) |
| Treatment | |||||||||
| Surgery | 161 | 28 | -17.4 | 1.0 | 1.0 | ||||
| Radiotherapy | 144 | 75 | -52.1 | 4.1 | (2.6 | 6.3) | 0.9 | (0.5 | 1.7) |
| Combination | 69 | 27 | -39.1 | 3.0 | (1.8 | 5.1) | 1.4 | (0.8 | 2.6) |
| Treatment details not known | 184 | 122 | -66.3 | 7.9 | (5.2 | 11.9) | 3.2 | (1.9 | 5.5) |
CI, confidence interval;
Adjusted on characteristics significant on the univariate analysis;
1 US dollar ≈ 45 rupees in 2,000
The risk of death was significantly increased in women with adenocarcinoma compared to those with squamous cell carcinoma in the multivariate model (hazard ratio (HR) = 1.1; 95% CI = 0.9-3.4) (Table 1). As expected, stage at diagnosis was the strongest predictor of the survival outcome with increased risk of death observed for advanced stage at diagnosis; the HR of death for stage III and IV disease were 6.3 (95% CI:3.6-10.9) and 28.6 (95% CI:13.66-59.8), respectively as compared to stage IA disease (Table 1). The stage-specific Kaplan Meier survival curves for the cervical cancer patients are shown in Figure 1.
Figure 1.
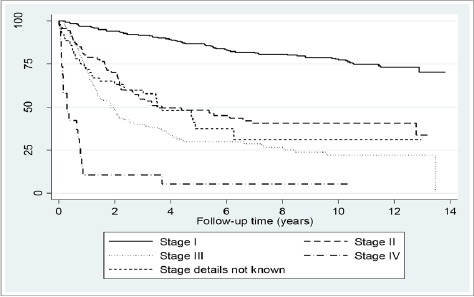
Survival from Cervical Cancer
The overall 5-year observed survival was 60.5%. The observed 1-, 3- and 5-year survival estimates for different histology types and stages by the different patient characteristics that were significant independent predictors of survival in the multivariate analysis are given in Table 2. Marked declines in survival were observed for women presenting with advanced stage at diagnosis and those with adenocarcinoma. The 5-year survival of patients with stage IA disease was 95.1% and patients for stage IV was 5.3%. The 5-year survival of adenocarcinoma cases was 16.6% (based on 22 cases only) as opposed to 61.9% for squamous cell carcinoma. Of the 22 cases of adenocarcinoma, 8 were diagnosed in the HPV arm, 9 in the cytology and 5 in the VIA arm.
Table 2.
1-, 3- and 5-Year Survival Rates of Cervical Cancer Patients
| Characteristics | Number of cases | Observed survival (%) | ||
|---|---|---|---|---|
| 1-year | 3-year | 5-year | ||
| Patients assessed | 558 | 82.4 | 68.0 | 60.5 |
| Cancer type | ||||
| Squamous cell carcinoma | 536 | 82.6 | 68.8 | 61.9 |
| Adenocarcinoma | 22 | 75.7 | 46.6 | 16.6 |
| Stage | ||||
| IA | 143 | 99.3 | 97.9 | 95.1 |
| IB | 114 | 93.7 | 83.5 | 75.8 |
| IIA | 33 | 72.0 | 46.9 | 46.9 |
| IIB | 56 | 82.1 | 58.3 | 48.8 |
| III | 123 | 71.2 | 39.9 | 29.8 |
| IV | 19 | 10.5 | 10.5 | 5.3 |
| Stage details not known | 70 | 71.6 | 59.8 | 37.5 |
| Treatment | ||||
| Surgery | 161 | 98.1 | 96.8 | 92.9 |
| Radiotherapy | 144 | 85.2 | 63.0 | 55.5 |
| Combination | 69 | 88.1 | 72.3 | 63.8 |
| Treatment details not known | 184 | 64.1 | 44.7 | 33.0 |
Five-year observed survival rates by different treatment modalities for each stage category are given in Table 3. The 5-year survival rates of stage IA patients, who received radical surgery, were 100% and of stage IB patients, receiving intracavitary radiotherapy were 94.1%; 62% of stage IIB, 49% of stage III and 25% of stage IV patients receiving radiotherapy survived for 5 years.
Table 3.
Number of Cervical Cancer Patients and 5-Year Observed Survival by Stage of Disease and Type of Treatment Offered
| FIGO stage | Treatment | Number | 5-year survival (%) |
|---|---|---|---|
| IA | Radical hysterectomy only | 68 | 100 |
| Simple hysterectomy only | 33 | 93.9 | |
| Combination of surgery/radiotherapy/chemotherapy | 20 | 95 | |
| Treatment details not known | 22 | 81.6 | |
| All treatment modalities including combination and treatment details not known | 143 | 95.1 | |
| IB | Radical hysterectomy only | 55 | 82.1 |
| Intracavitary radiotherapy only | 17 | 94.1 | |
| External beam radiotherapy plus intracavitary radiotherapy only | 9 | 40 | |
| Combination of surgery/radiotherapy/chemotherapy | 15 | 66.7 | |
| Treatment details not known | 18 | 64.9 | |
| All treatment modalities including combination and treatment details not known | 114 | 75.8 | |
| IIA | Radical hysterectomy only | 5 | 100 |
| External beam radiotherapy plus intracavitary radiotherapy only | 10 | 63.5 | |
| Combination of surgery/radiotherapy/chemotherapy | 7 | 85.7 | |
| Treatment details not known | 11 | - | |
| All treatment modalities including combination and treatment details not known | 33 | 46.9 | |
| IIB | External beam radiotherapy plus intracavitary radiotherapy only | 37 | 61.8 |
| Combination of surgery/radiotherapy/chemotherapy | 6 | - | |
| Treatment details not known | 13 | 28.9 | |
| All treatment modalities including combination and treatment details not known | 56 | 48.8 | |
| III | External beam radiotherapy plus intracavitary radiotherapy only | 58 | 48.6 |
| Combination of surgery/radiotherapy/chemotherapy | 15 | 23.5 | |
| Treatment details not known | 50 | 7.5 | |
| All treatment modalities including combination and treatment details not known | 123 | 29.8 | |
| IV | External beam radiotherapy plus intracavitary radiotherapy only | 4 | 25 |
| Combination of surgery/radiotherapy/chemotherapy | 4 | - | |
| Treatment details not known | 11 | - | |
| All treatment modalities including combination and treatment details not known | 19 | 5.3 | |
| Stage details not known | Combination of surgery/radiotherapy/chemotherapy | 11 | 53 |
| Treatment details not known | 59 | 35 | |
| All treatment modalities including combination and treatment details not known | 70 | 37.5 | |
| All cases | Radical hysterectomy only | 128 | 92.6 |
| Simple hysterectomy only | 33 | 93.9 | |
| Intracavitary radiotherapy only | 17 | 94.1 | |
| External beam radiotherapy plus intracavitary radiotherapy only | 118 | 52.6 | |
| Combination of surgery/radiotherapy/chemotherapy | 78 | 58.4 | |
| Treatment details not known | 184 | 33 | |
| All treatment modalities including combination and treatment details not known | 558 | 60.5 |
Discussion
The cohort of cervical cancer patients in our study is unique given that they are from a screened population and that more than half have been diagnosed in early stages and the treatment details were known for 67% of the patients. More than four-fifths of the women population from which these cases occurred was exposed to a single round of screening with HPV testing, VIA or cytology, leading to a higher proportion of cases being diagnosed in early stages when treatment is more effective as compared to populations receiving routine care. A large proportion of cancer cases were diagnosed in stage IA before the symptoms occurred, providing considerable lead time leading to improved survival and cure. Participation in the screening trial provided equitable access to women belonging to the different socio-economic categories (indicated by household income, education and type of house) for diagnosis, treatment and follow-up care.
We report a higher 5-year survival for cervical cancer in our cohort of women due to the above factors, as compared to the survival rates reported from other population-based cohorts of cervical cancer patients in India (Table 4). Five-year observed survival ranged between 33.1% and 54% for cervical cancer patients of all ages diagnosed during 1990-1999 in other urban and semi-urban populations in India such as Dindigul district (Swaminathan et al., 2009), Karunagappally (Jayalekshmi et al., 2011), Chennai (Swaminathan et al., 2011), Mumbai (Yeole et al., 2011) and Bhopal (Dikshit et al., 2011). Furthermore, the observed survival in our current patient cohort was two times higher than the corresponding experience in a neighbouring area around Barshi (Jayant et al., 2011). The Barshi Rural Cancer Registry reported a five-year survival of 32.2% for cervical cancer based on 406 patients of all ages diagnosed during 1993-2000 (Jayant et al., 2011) (Table 4). In the control arm of the current trial, in which women received health education and better than existing healthcare, we observed a five-year survival of 43.2% (192 cases). The survival experience with the control group of our trial was similar to that observed in the populations in Mumbai (Yeole et al., 2011) and Karunagappaly (Jayalekshmi et al., 2011) in India. Compared to the survival experience reported from low-income countries in Sub-Saharan Africa such as The Gambia (Bah et al., 2011), Uganda (Wabinga et al., 2011) and Zimbabwe (Chokunonga et al., 2011) and low middle-income countries such as Thailand (Sumitsawan et al., 2011) and the Philippines (Laudico and Mapua 2011), the survival observed by us in the screened population was much higher. The survival experience of patients with unknown staging details indicates that most of them are likely to have advanced stage III disease in view of their similar survival (30% vs. 38% 5-year survival) experience (Table 2).
Table 4.
Proportion of Localized Disease Stage and 5-Year Observed Survival Rates in This Study and Different Populations in India and Asia
| Population | Years | No. of total cases | Proportion of Cases in Localized stage | 5-year absolute survival |
|---|---|---|---|---|
| This study | ||||
| Patients assessed in screening group | 2000-2013 | 558.0 | 46.1 | 60.5 |
| Women in the control group | 2000-2013 | 192.0 | 20.3 | 43.2 |
| India* | ||||
| Barshi, Paranda and Bhum | 1993-2000 | 406 | 12.6 | 32.2 |
| Bhopal | 1991-1995 | 332 | 28.3 | 33.1 |
| Chennai | 1990-1999 | 4,438 | 6.4 | 54.0 |
| Karunagappally | 1991-1997 | 170 | 15.3 | 46.7 |
| Mumbai | 1992-1999 | 4,436 | 27.9 | 42.2 |
| Dindigul District | 2003 | 223 | - | 35.0 |
| Philippines* | ||||
| Manila | 1994-1995 | 377 | 21.5 | 34.0 |
| Singapore* | 1993-1997 | 984 | 45.5 | 59.9 |
| Thailand* | ||||
| Chiang Mai | 1993-1997 | 885 | 26.1 | 58.8 |
| Khon Kaen | 1993-1997 | 554 | 17.3 | 53.3 |
| Lampang | 1990-2000 | 1,079 | 31.2 | 62.6 |
| Songkhla | 1990-1999 | 780 | 22.3 | 60.4 |
All before the screening was undertaken (Registry data)
Improvements in cervical cancer survival observed in most developed countries can be ascribed to the success of screening programs, greater awareness among physicians and the public of the signs and symptoms of the disease and to improvements in multimodality therapy for different stages of disease (Ries et al., 2008; Berrino et al., 2007; Ponten et al., 1995). The higher survival observed in our screened cohort of women in rural India is mainly due to earlier detection and improved access to treatment. The survival outcomes in stage IIA and stage IIB disease in our study was similar indicating possible misclassification between these two stages.
Our results confirm that FIGO stages are strongly correlated with survival outcome and early stages of the disease are associated with an exceptionally favourable prognosis provided they are adequately treated whereas survival for stage III and IV cancers were dismally low. It also proves that among all determinants to assess the overall prognostic outlook of a cervical cancer patient and to plan the treatment, clinical stage is by far the most important variable, although the clinical stage is largely a function of time elapsed since onset of the disease, rather than an indicator of the biological aggressiveness of cervical cancer. The relative risks of death from cervical cancer increased and survival rates declined steadily for advanced stage categories.
Treatment modalities in our study varied with the clinical extent of cancer but, most importantly, were also dependent on clinico-pathological profiles such as barrel shaped cervix, a patient’s age, general health and presence of other medical conditions. In our study, 61% of the early stage (stage I) cervical cancer patients received surgery alone and an additional 24% received either radiotherapy alone or a combination of treatments resulting in an observed 5-year survival exceeding 90%, which confirms the clinical importance of diagnosing cervical cancers at an early stage and treating them adequately.
Treatment details were not available for 184 patients; however, the high survival for stage I patients with unknown treatment details indicates that they may have been treated elsewhere and we failed to get those treatment details. On the other hand, the very low or no survival at 5.0 years for those with advanced cancers not having treatment details indicates that those patients may have dropped out of treatment. The significant number of patients with unknown treatment details even in a controlled setting like ours with committed inputs indicates that the proportion of patients with inadequate treatment should be high in routine healthcare settings in India.
Our study, like in several previous studies (Ries et al., 2008; Berrino et al., 2007; Movva et al., 2008; Kosary 1994; Chen et al., 1999), has demonstrated poor survival for women diagnosed with adenocarcinoma compared with those diagnosed with squamous cell carcinoma. The 3-year observed survival was 47% for adenocarcinomas and 69% for squamous cell carcinomas. A similar trend was observed in a previous study showing the 5-year relative survival figures as 55% and 67%, respectively (Kosary 1994). It is well established that adenocarcinomas are inadequately prevented by screening. We could not ascertain the influence of the grades of differentiation for squamous cell carcinomas as this information was not available for most cases.
Our results further demonstrate that poor survival is explained largely by advanced disease stage at diagnosis and histological category of tumour. It is important for public health officials and policy makers to improve access to early detection services in low- and medium-resource rural areas that would result in equitable survival for all women diagnosed with cervical cancer.
Conflict of interest
All authors have no conflict of interest.
References
- Bah E, Sam O, Whittle H, et al. Cancer survival in the Gambia 1993-1997. In: Sankaranarayanan R, Swaminathan R, editors. ‘Cancer survival in Africa, Asia, the Caribbean and Central America. IARC Scientific Publications volume 162’. Lyon: International Agency for Research on Cancer; 2011. pp. 97–106. [Google Scholar]
- Benedet JL, Bender H, Jones H, et al. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000;70:209–62. [PubMed] [Google Scholar]
- Berrino F, De Angelis R, Sant M, et al. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995-99: results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773–83. doi: 10.1016/S1470-2045(07)70245-0. [DOI] [PubMed] [Google Scholar]
- Chen RJ, Lin YH, Chen CA, et al. Influence of histologic type and age on survival rates for invasive cervical carcinoma in Taiwan. Gynecol Oncol. 1999;73:184–90. doi: 10.1006/gyno.1999.5364. [DOI] [PubMed] [Google Scholar]
- Chokunonga E, Borok MZ, Chirenje ZM, et al. Cancer survival in Harare, Zimbabwe 1993-1997. In: Sankaranarayanan R, Swaminathan R, editors. ‘Cancer survival in Africa, Asia, the Caribbean and Central America. IARC Scientific Publications volume 162’. Lyon: International Agency for Research on Cancer; 2011. pp. 249–55. [PubMed] [Google Scholar]
- Dikshit R, Kanhere S, Surange S. Cancer survival in Bhopal, India 1991-1995. In: Sankaranarayanan R, Swaminathan R, editors. ‘Cancer survival in Africa, Asia, the Caribbean and Central America. IARC Scientific Publications volume 162’. Lyon: International Agency for Research on Cancer; 2011. pp. 107–13. [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, et al. ‘GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet]’. Lyon: International Agency for Research on Cancer; 2013. [accessed on 10/04/2016]. Available from: http://globocan.iarc.fr . [Google Scholar]
- Gajalakshmi V, Rajaraman S, Shanta V. A survival study of cervical cancer in Chennai, India. Indian J Cancer. 2000;37:158–64. [PubMed] [Google Scholar]
- Jayalekshmi P, Gangadharan P, Sebastian P. Cancer survival in Karunagappally, India 1991-1997. In: Sankaranarayanan R, Swaminathan R, editors. ‘Cancer survival in Africa, Asia, the Caribbean and Central America. IARC Scientific Publications volume 162’. Lyon: International Agency for Research on Cancer; 2011. pp. 125–32. [Google Scholar]
- Jayant K, Nene BM, Dinshaw KA, et al. Cancer survival in Barshi, India 1993-2000. In: Sankaranarayanan R, Swaminathan R, editors. ‘Cancer survival in Africa, Asia, the Caribbean and Central America. IARC Scientific Publications volume 162’. Lyon: International Agency for Research on Cancer; 2011. pp. 101–6. [Google Scholar]
- Jayant K, Nene BM, Dinshaw KA, et al. Survival from cervical cancer in Barshi registry, rural India. In: Sankaranarayanan R, Black RJ, Parkin DM, editors. ‘Cancer survival in developing countries’. Lyon: International Agency for Research on Cancer; 1998. pp. 69–77. [PubMed] [Google Scholar]
- Jayant K, Rao RS, Nene BM, et al. Improved survival in cervical cancer cases in a rural Indian population. Br J Cancer. 1996;74:285–87. doi: 10.1038/bjc.1996.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen OM, Parkin DM, Maclennan R, et al. ‘Cancer Registration Principles and Methods. No.95. Lyon: IARC Scientific Publications, International Agency for Research on Cancer; 1991. [Google Scholar]
- Kosary CL. FIGO stage, histology, histologic grade, age and race as prognostic factors in determining survival for cancers of the female gynecological system: an analysis of 1973-87 SEER cases of cancers of the endometrium, cervix, ovary, vulva, and vagina. Semin Surg Oncol. 1994;10:31–46. doi: 10.1002/ssu.2980100107. [DOI] [PubMed] [Google Scholar]
- Laudico A, Mapua C. Cancer survival in Manila, Philippines 1994-1995. In: Sankaranarayanan R, Swaminathan R, editors. ‘Cancer survival in Africa, Asia, the Caribbean and Central America. IARC Scientific Publications volume 162’. Lyon: International Agency for Research on Cancer; 2011. pp. 147–54. [Google Scholar]
- Movva S, Noone AM, Banerjee M, et al. Racial differences in cervical cancer survival in the Detroit metropolitan area. Cancer. 2008;112:1264–71. doi: 10.1002/cncr.23310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandakumar A, Anantha N, Venugopal TC. Population-based survival from breast and cervical cancer and lymphoreticular malignancies in Bangalore, India. In: Sankaranarayanan R, Black RJ, Parkin DM, editors. ‘Cancer survival in developing countries’. Lyon: International Agency for Research on Cancer; 1998. pp. 61–8. [PubMed] [Google Scholar]
- Ponten J, Adami HO, Bergstrom R, et al. Strategies for global control of cervical cancer. Int J Cancer. 1995;60:1–26. doi: 10.1002/ijc.2910600102. [DOI] [PubMed] [Google Scholar]
- Ries LAG, Melbert D, Krapcho M, et al. Cancer Statistics Review 1975-2005. Bethesda, MD: National Cancer Institute; 2008. National Cancer Institute: Bethesda, MD. Based on November 2007 SEER data submission, posted to the SEER web site. http://seer.cancer.gov/csr/1975_2005 . [Google Scholar]
- Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385–94. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R, Swaminathan R. ‘Cancer survival in Africa, Asia, the Caribbean and Central America. Vol. 162. Lyon: IARC Scientific Publications, International Agency for Research on Cancer; 2011. [PubMed] [Google Scholar]
- Sankaranarayanan R, Swaminathan R, Brenner H, et al. Cancer survival in Africa, Asia, and Central America: a population-based study. Lancet Oncol. 2010;11:165–73. doi: 10.1016/S1470-2045(09)70335-3. [DOI] [PubMed] [Google Scholar]
- Shanta V, Gajalakshmi CK, Swaminathan R. Cancer survival in Chennai (Madras), India. In: Sankaranarayanan R, Black RJ, Parkin DM, editors. ‘Cancer survival in developing countries’. Lyon: International Agency for Research on Cancer; 1998. pp. 89–100. [Google Scholar]
- Sumitsawan Y, Srisukho S, Sastraruji A, et al. Cancer survival in Chiang Mai, Thailand 1993-1997. In: Sankaranarayanan R, Swaminathan R, editors. ‘Cancer survival in Africa, Asia, the Caribbean and Central America. IARC Scientific Publications volume 162’. Lyon: International Agency for Research on Cancer; 2011. pp. 199–209. [PubMed] [Google Scholar]
- Swaminathan R, Rama R, Nalini S, Shanta V. Cancer survival in Chennai (Madras), India 1990-1999. In: Sankaranarayanan R, Swaminathan R, editors. ‘Cancer survival in Africa, Asia, the Caribbean and Central America. IARC Scientific Publications volume 162’. Lyon: International Agency for Research on Cancer; 2011. pp. 115–24. [Google Scholar]
- Swaminathan R, Selvakumaran R, Esmy PO, et al. Cancer pattern and survival in a rural district in South India. Cancer Epidemiol. 2009;33:325–31. doi: 10.1016/j.canep.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Wabinga H, Parkin DM, Nambooze S, Amero J. Cancer survival in Kampala, Uganda 1993-1997. In: Sankaranarayanan R, Swaminathan R, editors. ‘Cancer survival in Africa, Asia, the Caribbean and Central America. IARC Scientific Publications volume 162’. Lyon: International Agency for Research on Cancer; 2011. pp. 243–7. [Google Scholar]
- Yeole BB, Kumar AV, Kurkure A, et al. Population-based survival from cancers of breast, cervix and ovary in women in Mumbai, India. Asian Pac J Cancer Prev. 2004;5:308–15. [PubMed] [Google Scholar]
- Yeole BB, Kurkure AP, Sunny L. Cancer survival in Mumbai, India 1992-1999. In: Sankaranarayanan R, Swaminathan R, editors. ‘Cancer survival in Africa, Asia, the Caribbean and Central America. IARC Scientific Publications volume 162’. Lyon: International Agency for Research on Cancer; 2011. pp. 133–42. [Google Scholar]


