Abstract
Objective:
Gimatecan is a new camptothecin (CPT) analogue that inhibits tumor growth by targeting DNA topoisomerase I (TOP I) and introducing strong and persistent DNA cleavage. Anti-tumor activity has been demonstrated with a wide range of solid tumors in previous preclinical and clinical studies. Here, we investigated for the first time the effects of gimatecan on the proliferation of hepatocellular carcinoma (HCC) cells both in vitro and in vivo.
Methods:
Anticancer efficacy of gimatecan were evaluated in a panel of HCC cell lines and corresponding mouse xenograft models. Inhibition of cell proliferation was measured by CellTiter-Glo cell viability assay. In vivo, gimatecan and control preparations were orally administered every four days, for a total of four times. Tumor volume and body weights of the mice were measured twice weekly.
Results:
In vitro cytotoxicity evaluation showed that gimatecan inhibited the proliferation of a large panel of HCC cell lines in a dose dependent manner, with IC50 values ranging between 12.1~1085.0 nM. In vivo evaluation in mouse xenograft models showed significant antitumor effects of gimatecan at 0.8mg/kg and 0.4mg/kg as compared to the control group.
Conclusion:
This study suggested that gimatecan may have the potential to be used as a chemotherapeutic agent for the treatment of HCC.
Keywords: Gimatecan, DNA topoisomerase I, hepatocellular carcinoma, antitumor, in vitro, in vivo
Introduction
Primary liver cancer is a cancer that originates in the liver, with over 90% of the cases pathologically classified as hepatocellular carcinoma (HCC) (Qin, 2012). As reported by GLOBOCAN 2012, liver cancer is the fifth most common cancer (782,451 cases per year representing 5.6% of all cancer cases) and the third leading cause of cancer-related deaths worldwide (with a mortality rate of 745,533 cases per year). In 2012, 83% of the newly diagnosed liver cancer occurred in less developed countries; China alone accounted for 50% of the new cases. The incidence and mortality rate in more developed countries are also rising. The annual percentage increase of liver cancer cases in the USA is the highest (2.4%) among the most common cancers (Mikhail et al., 2014).
Surgical interventions (e.g. surgical resection, liver transplantation, and locoregional therapies) have been playing a significant role in the treatment of HCC. However, more than 70% of patients diagnosed with HCC already have liver dysfunction and/or are diagnosed in advanced stages (III or IV), and cannot benefit from these interventions. Treatment of these advanced HCC patients mainly rely on non-surgical therapies although the long-term cure rate is low (less than 5%) (Mor et al., 1998; Mikhail et al., 2014). Sorafenib is the first and only medication approved by the FDA for the treatment of advanced HCC. However, a large percentage of patients (40.6%) still experience disease progression after sorafenib treatment (He and Goldenberg, 2013). In addition, the clinical efficacy of sorafenib in certain HCC patients appears to be less. For example, emerging evidence suggests that HCC patients with chronic HBV infection treated with sorafenib obtained less clinical benefits than those without HBV infection (Johnson et al., 2013; Cheng et al., 2013). Therefore, there is still a large unmet medical need to develop new therapeutic strategies to treat HCC.
Although the National Comprehensive Cancer Network (NCCN) has not recommended any chemotherapeutic agents as standard of care for the treatment of liver cancer, chemotherapeutic agents like platinum compounds, antimetabolites, and camptothecin derivatives have been studied intensively either as single agent or combination therapy (Brown, 2006). However, the clinical use of most of these chemotherapeutic agents is restricted by their modest efficacy and high toxicities. Therefore, new chemotherapeutic compounds and/or new treatment regimens with higher antitumor efficacy and lower toxicity are still very much in demand. To this end, a phase III clinical study of FOLFOX4 (infusional fluorouracil, leucovorin, and oxaliplatin) showed increasing trend of overall survival (OS) and significantly increased progression free survival (PFS) and response rate (RR) as compared to doxorubicin in China (Qin et al., 2013). Nevertheless, there is still a high unmet medical need to identify more efficacious and less toxic therapeutic interventions for these patients.
Gimatecan (7-[(E)-tert-butyloxyminomethyl]-camptothecin) is a novel camptothecin (CPT) and TOP I inhibitor with anti-tumoral activities. It has been shown that gimatecan exerts stronger and more durable deoxyribonucleic acid cleavages and possesses larger therapeutic index than other TOP I inhibitors (Pratesi et al., 2004). For example, a phase II study conducted in recurrent epithelial ovarian, fallopian tube or peritoneal cancer patients showed that gimetecan had at least comparable clinical efficacy and better safety results (with respect to hematological and gastrointestinal toxicities) as compared to two marketed CPT analogues, topotecan and irinotecan (Pecorelli et al., 2010). Although the anti-tumor activities of gimatecan have been demonstrated in a wide variety of xenografts, such as lung carcinoma, melanoma, osteosarcoma, and ovarian carcinoma etc (Pratesi et al., 2002), to our knowledge the effects of gimatecan on HCC have never been studied. Therefore, we investigated the in vitro and in vivo antitumor activities of gimatecan against hepatocellular carcinoma in the current study.
Materials and Methods
Cell lines
Human HCC cell lines (HCCLM3, Hep-G2, Hep3B, HuCCT1, Huh-1, Huh-7, JHH-7, MHCC97-H, PLC/PRF/5, SNU-761, SNU-878) were obtained from different sources (ATCC: American Type Culture Collection; ZHFU: Zhongshan Hospital Fudan University; JCRB: Japanese Collection of Research Bioresources; SIBS: Shanghai Institutes of Biological Sciences; and KCLB: Korean Cell Line Bank). All the cells were cultured in ATCC recommended growth media (HCCLM3, Hep G2, Huh-1, Huh-7, MHCC97-H, and PLC/PRF/5 in DMEM containing 10% fetal bovine serum (FBS); Hep3B in MEM containing 10% FBS and 0.01mM NEAA; HuCCT1 in RPMI1640 containing 10% FBS; JHH-7 in William’s E containing 10% FBS; SNU-761 and SNU-878 in RPMI1640 containing 10% FBS), in 5% CO2 at 37ºC. All culture media were obtained from GIBCO or Hyclone. HepG2 is HBV negative, but HCV positive; Huh-1, HCCLM3, and PLC/PRF/5 are HBV positive, and HCV negative.
Compounds
Gimatecan (7-[(E)-tert-butyloxyminomethyl]-camptothecin) was provided by Lee’s Pharmaceutical (Hong Kong) Limited. Stock solutions of gimatecan were dissolved in 100% DMSO (Amresco, USA) and stored in sterilized brown glass bottles at -20ºC. It was diluted with corresponding growth media (as described in cell lines section) to the desired concentration with a final DMSO concentration of 0.1% for in vitro studies. Stock solution of gimatecan was diluted with sterile injection water to the desired concentrations for in vivo studies.
Cell viability
Cells were harvested during the logarithmic growth period and seeded at a concentration of approximately 5×103 cells/80µl/well in 96-well plate. 20µl of compounds were dispensed in each well, with triplicate for each concentration. After 3 days (72h) drug treatment, 50μl CellTiter-Gl Reagent was added to each well. The contents were mixed for 2 minutes on an orbital shaker to induce cell lysis and incubated at room temperature for 10 minutes to stabilize luminescent signal. Cell number was then estimated by recording luminescence using Envision.
Treatments in mouse xenograft model
6-8 or 7-9 week-old female BALB/c nude and NOD/SCID mice were purchased from Shanghai Lingchang Biological Technology Co., Ltd, and were used for cell line derived xenograft (CDX) HCC models. Huh1 (5x106 cells), HCCLM3 (5×106 cells), Hep G2 (1×107 cells), and PLCPRF5 (1×107 cells) were inoculated subcutaneously on the right side of the mouse back, respectively. When tumor volume grew to approximately 150mm3, animals in each CDX study were randomly divided into 4 groups: control (10% DMSO) and gimatecan (0.1, 0.4, and 0.8mg/kg). Gimatecan and control were orally administered every four days, for a total of four times.
In vivo Analysis of Anticancer Activities
Tumor volumes were measured twice weekly in two dimensions using a caliper, and the volume was expressed in mm3 using the formula: V = 0.5 a × b2, where a and b are the long and short diameters of the tumor, respectively. Tumor weight was measured twice weekly and at study termination. Tumor volume inhibition (TVI) is an indication of antitumor effectiveness and expressed as: TVI (%) =100 × (1-T/C), where T and C were the mean tumor volume of the treated and control groups, respectively. Body weight loss (BWL) is described as mild (<10%), moderate (10-20%), and severe (>20%).
Statistical analysis
Summary statistics, including mean and the standard error of the mean (SEM), are provided for the tumor volume of each group at each time point. Statistical analysis of difference in tumor volume among the groups was conducted using Independent-Samples T Test. All data were analyzed in SPSS (Statistical Product and Service Solutions) version 18.0 (IBM, Armonk, NY, U.S.). P-values were rounded to three decimal places, with the exception that raw P-values less than 0.001 were stated as P<0.001. All tests were two-sided. P<0.05 was considered to be statistically significant.
Results
Inhibition of HCC cell proliferation in vitro
The anti-tumor activity of gimatecan was examined in a panel of HCC cell lines. After these cells were treated with various concentrations of gimatecan for 72 h, the number of viable cells was measured. As shown in Figure 1, gimatecan inhibited the growth of all the tested HCC cell lines in a dose dependent manner. The IC50 values of gimatecan ranged between 12.1~1085.0nM (Figure 2).
Figure 1.
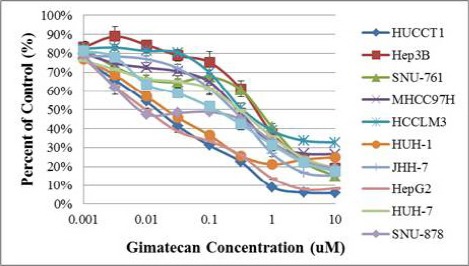
Gimatecan Inhibited HCC Cell Growth in Vitro.
Different HCC cell lines were cultured and incubated with various concentrations of gimatecan at 37 ºC for 72 hours. The percentages of viable cells were measured at the end the incubation period. 100% refers to the number of cells after 72 hours of incubation in the presence of the vehicle control (0.1% DMSO).
Figure 2.
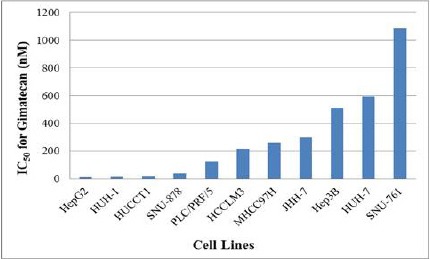
IC50 of Gimatecan in HCC Cell Lines.
Cancer cells were assessed by cell growth inhibition after treatment with gimatecan at a range of concentrations for 72 hr. IC50s refers to the 50% inhibition concentrations.
In vivo antitumor activities
In this study, the anti-tumor activity of gimatecan against Huh-1, HepG2, HCCLM3, and PLC/PRF/5 human liver cancer cells was evaluated in the respective mouse xenograft models. Huh-1, HCCLM3, and PLC/PRF/5 models are HBV positive, while HepG2 model is HCV positive. The results of tumor sizes in different treatment groups at different time points after tumor inoculation were recorded.
As shown in Figure 3, gimatecan demonstrated a dose-dependent inhibition of tumor growth for all four HCC cell lines inoculated in mice. The results analyzed by tumor weight were similar to that analyzed by tumor volume. As shown in Table 1, as compared with the vehicle group, the anti-tumor activities were significant at 0.8mg/kg and 0.4mg/kg for all 4 models. Treatment with gimatecan at 0.1 mg/kg produced minor antitumor activities.
Figure 3.
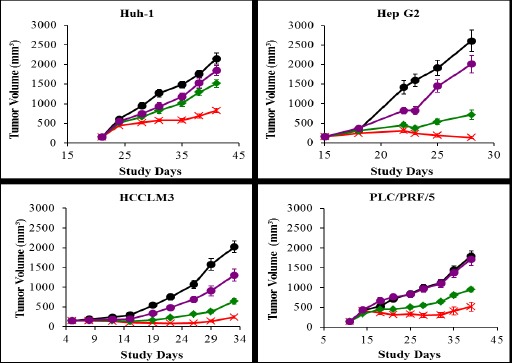
In vivo Anti-Tumor Activity of Gimatecan in HCC Xenograft Models.
Four types of HCC cell lines, Huh-1, Hep G2, HCCLM3, and PLCPRF5 were inoculated subcutaneously on the right side of the mouse back, respectively. In each tumor-bearing model mice were divided into four groups (n = 10 for each group) and orally administered with 0.8 mg/kg (×), 0.4 mg/kg (◆), and 0.1 mg/kg (●) gimatecan every four days for a total of four times, respectively. The control mice were administered with 10 µl 10%DMSO/g (●). Tumor volume was measured and expressed in mm3
Table 1.
Antitumor Activity of Gimatecan in the Treatment of Subcutaneous Liver Cancer Xenograft Model (by tumor volume). TVI (%) refers to the percentage tumor volume inhibition. aThe P value was determined against vehicle control.
| Model | Treatment | TVI(%) | P valuea |
|---|---|---|---|
| Huh-1 | Vehicle control | -- | -- |
| Gimatecan (0.8 mg/kg) | 62 | <0.001 | |
| Gimatecan (0.4 mg/kg) | 30 | 0.002 | |
| Gimatecan (0.1 mg/kg) | 14 | 0.140 | |
| HepG2 | Vehicle control | -- | -- |
| Gimatecan (0.8 mg/kg) | 95 | <0.001 | |
| Gimatecan (0.4 mg/kg) | 74 | <0.001 | |
| Gimatecan (0.1 mg/kg) | 24 | 0.101 | |
| HCCLM3 | Vehicle control | -- | -- |
| Gimatecan(0.8 mg/kg) | 88 | <0.001 | |
| Gimatecan(0.4 mg/kg) | 68 | <0.001 | |
| Gimatecan(0.1 mg/kg) | 34 | 0.014 | |
| PLC/PRF/5 | Vehicle control | -- | -- |
| Gimatecan(0.8 mg/kg) | 71 | <0.001 | |
| Gimatecan(0.4 mg/kg) | 47 | <0.001 | |
| Gimatecan(0.1 mg/kg) | 4 | 0.718 |
As shown in Figure 4, no severe body weight loss was observed in all gimatecan treated groups in the tested liver cancer model during dosing period. Moderate body weight loss was observed post cell inoculation, which was gradually recovered.
Figure 4.
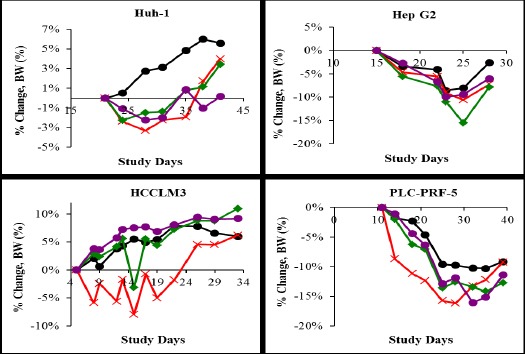
Gimatecan Administration Did Not Severely Affect Body Weights.
Four types of HCC cell lines, Huh-1, Hep G2, HCCLM3, and PLCPRF5 were inoculated subcutaneously on the right side of the mouse back, respectively. In each tumor-bearing model mice were divided into four groups (n = 10 for each group) and orally administered with 0.8 mg/kg (×), 0.4 mg/kg (◆), and 0.1 mg/kg (●) gimatecan every four days for a total of four times, respectively. The control mice were administered with 10 µl 10%DMSO/g (●). Mouse body weights were measured twice weekly and at study termination.
Discussion
In this study we demonstrated here for the first time the antitumor effects of gimatecan against HCC both in vitro and in vivo. The in vitro cytotoxicity data indicated dose-dependent inhibition of gimatecan against HCC cell lines. Difference in IC50s of gimatecan was detected among the different HCC cell lines tested. Such differences could be due to the differences in expression of multidrug resistance proteins in these different cell lines leading to different effective intracellular drug concentrations (Giglia et al., 2010), and subsequently different sensitivities to gimatecan.
In each of the four selected in vivo models, HepG2, Huh-1, HCCLM3, and PLC/PRF/5, gimatecan also showed antitumor activity in a dose-dependent fashion. According to our own preliminary study (unpublished data), gimatecan (2.5mg/kg, Q4dx4) was not well tolerated in both BALB/c nude and NOD/SCID mice while a lower dose of gimatecan (1mg/kg, Q4dx4) showed good tolerability. In order to avoid drug intolerance, doses of 0.8mg/kg, 0.4mg/kg, and 0.1mg/kg were selected to investigate the in vivo anti-HCC activity in our study. The TVI% of gimatecan (0.8mg/kg, Q4dx4) in the four HCC models varied between 62-95%. These effects of gimatecan were comparable to those reported earlier in other antitumor studies: when delivered at its maximum tolerated doses (2-3mg/kg, Q4dx4) to a large panel of human tumor xenografts, gimatecan resulted in TVI (%) between 75-100%. Based on the consistent anti-tumor effects of gimatecan both in vitro and in vivo and in different HCC cell lines, we believe that gimatecan warrants further investigation as a therapeutic agent against HCC.
References
- Brown KS. Chemotherapy and other systemic therapies for hepatocellular carcinoma and liver metastases. Semin Intervent Radiol. 2006;23:99–108. doi: 10.1055/s-2006-939845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: Results of a randomized phase III trial. J Clin Oncol. 2013;31:4067–75. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- Giglia JL, Antonia SJ, Berk LB, et al. Systemic therapy for advanced hepatocellular carcinoma: past, present, and future. Cancer Control. 2010;17:120–9. doi: 10.1177/107327481001700207. [DOI] [PubMed] [Google Scholar]
- He AR, Goldenberg AS. Treating hepatocellular carcinoma progression following first-line sorafenib: therapeutic options and clinical observations. Therap Adv Gastroenterol. 2013;6:447–58. doi: 10.1177/1756283X13498540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517–24. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- Mikhail S, Cosgrove D, Zeidan A. Hepatocellular carcinoma: systemic therapies and future perspectives. Expert Rev Anticancer Ther. 2014;10:1205–18. doi: 10.1586/14737140.2014.949246. [DOI] [PubMed] [Google Scholar]
- Mor E, Tur-Kaspa R, Sheiner P, Schwartz M. Treatment of hepatocellular carcinoma associated with cirrhosis in the era of liver transplantation. Ann Intern Med. 1998;129:643–53. doi: 10.7326/0003-4819-129-8-199810150-00013. [DOI] [PubMed] [Google Scholar]
- Pecorelli S, Ray-Coquard I, Tredan O, et al. Phase II of oral gimatecan in patients with recurrent epithelial ovarian, fallopian tube or peritoneal cancer, previously treated with platinum and taxanes. Ann Oncol. 2010;21:759–65. doi: 10.1093/annonc/mdp514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratesi G, De Cesare M, Carenini N, et al. Pattern of antitumor activity of a novel camptothecin, ST1481, in a large panel of human tumor xenografts. Clin Cancer Res. 2002;8:3904–9. [PubMed] [Google Scholar]
- Pratesi G, Beretta GL, Zunino F. Gimatecan, a novel camptothecin with a promising preclinical profile. Anticancer Drugs. 2004;15:545–52. doi: 10.1097/01.cad.0000131687.08175.14. [DOI] [PubMed] [Google Scholar]
- Qin SK. Guidelines on the diagnosis and treatment of primary liver cancer. Chin Clin Oncol. 2012;1:1–32. doi: 10.3978/j.issn.2304-3865.2012.07.01. [DOI] [PubMed] [Google Scholar]
- Qin SK, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501–8. doi: 10.1200/JCO.2012.44.5643. [DOI] [PubMed] [Google Scholar]


