Abstract
Objective:
To investigate the impact of a Croton tiglium extract on cellular proliferation and apoptosis in a non-small cell lung cancer cell line (A549) in vitro.
Methods:
A Croton tiglium seed methanol extract was prepare and assessed for effects on A549 cells regarding cellular proliferation, apoptotic rates, and expression of apoptosis related genes and proteins using real-time PCR and immunofluorescence.
Results:
The tested Croton tiglium extract inhibited A549 cell proliferation in a dose- and time-dependent manner, with significant elevation of apoptotic indexes at various concentrations after 24 h. In addition, rates in both early and late stages were higher in treated than untreated groups, the 100 μg/ml dose causing the highest levels of apoptosis. RT-PCR showed that A549 cells treated with 100 μg/ml Croton tiglium extract for 24 h has markedly higher Bax mRNA expression levels and obviously lower Bcl-2 expression levels than controls, equivalent results being observed for proteins by immunofluorescence. However, the mRNA expression levels of Fas and caspase-8 were not significantly altered.
Conclusion:
A Croton tiglium extract can inhibit proliferation of A549 cells and promote apoptosis though Bax/Bcl-2 pathways.
Keywords: Lung cancer, A549 cells, croton tiglium, apoptosis, Bax, Bcl-2
Introduction
Non-small cell lung cancer (NSCLC) accounts for about 85% of lung cancer which is the leading cause for tumor-associated deaths all over the world (McKeage et al., 2010; Wang et al., 2015). At present, the 5-year survival rate after surgery for NSCLC is still unsatisfactory in clinic, because most patients are in advanced stages with poor therapeutic efficacy when diagnosed. Though there has been great development in clinical treatment of NSCLC, such as -radio- and chemotherapy, which would lead to severe toxic side-effects and complications simultaneously (Ding et al., 2013; Then et al., 2012; Yilmaz et al., 2014; Xu et al., 2015). Therefore, seeking more potential anticancer drugs is of great significance for NSCLC patients. The challenge is still to develop a new and highly effective antineoplastic agents.
Medicinal plants represent a rich source of drug leads and they have been utilized as novel compounds for the treatment of many diseases, including cancer, because of their ability to inhibit cancer cell growth and induce apoptosis (Kuo et al., 2010; Li et al., 2015). Croton, a large genus of Euphorbiaceae, is widely distributed in tropical regions of South-East Asia and China. Many researchers previously reported the pharmacological and physiological actions of Croton species on anti-inflammatory, anticonvulsant and wound healing properties (Mudium and Kolasani, 2014; Lima et al., 2015; Mota et al., 2015; Jang et al., 2016). It has been claimed that the growth inhibitory or antiproliferative effects of Croton tiglium extract are associated with its cytotoxic and anti-tumor activity (Sandoval et al., 2002). Mature Croton tiglium contains large amounts of natural medical components, in which croton alkaloid, flavonoids and diterpenes are anticancer agents (Mudium and Kolasani, 2014; Pal et al., 2014; Madrigales-Ahuatzi and Perez-Gutierrez, 2015).
However, the mechanisms of these beneficial roles have not been elucidated adequately for ethnomedical fields. In addition, the study on application of Croton in lung cancer is lacking. In this study, we investigated the regulating effects of Croton tiglium extract on A549 cell line’s proliferation and apoptosis, and further explored the molecular mechanism.
Materials and Methods
Preparation of Croton tiglium seed extract
Croton tiglium seeds (dried in Electric thermostatic drying oven 180ºC for 90 min) were washed with ethylether to eliminate seed oils and extracted with MeOH at 50ºC for 5 hrs and filtered. The combined extracts were evaporated under reduced pressure. The aqueous suspension was centrifuged and the supernatant was reconcentrated to yield the crude extract as an yellow solution. Three major compounds were isolated from the medicinal plant Croton tiglium in Yunnan of China with modified technique and identified as described previously (Kim et al., 1994; El-Mekkawy et al., 2000; Thuong et al., 2014). Compound 1-3 were determined as follows: (1) Isoguanosine, (2) 12-O-Acetylphorbol-13-tigliate, (3)13-O-Acetylphorbol-20-linoleate. The purities of the compounds used for biological experiments were determined by HPLC and were of >90.0%. Diterpenoids were dissolved in dimethyl sulfoxide (DMSO, Sigma) at the concentration of 1 mg/ml and diluted in culture medium before use in experiments.
Cell cultures
The A549 human lung cancer cell line was obtained from Shanghai Institute of Biochemistry and Cellular Biology Chinese Academy of Sciences (Shanghai, China), and was maintained in RPMI 1640 culture medium (PAA, USA) with 10% fetal bovine serum, 100 U/ml penicillin and 100 U/ml streptomycin in a humidified atmosphere with 5% CO2 saturated humidity at 37ºC.
Cell viability assay
In logarithmic phase, A549 cells were made into mono-cellular suspension and seeded in a 96-well plate with RPMI 1640 medium (supplemented with 10% FBS) at a density of 1×104 cells/well for totally 200 μl for a 24 h incubation. The cells were treated with Croton tiglium extract with a series of concentrations (0, 10, 50, 100 and 200 μg/ml) and cultured in 2% FBS medium for 12, 24, and 48 h. The morphology of cells were observed and photographed under the inverted microscope (×100). 20 μl 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenylte-trazolium bromide (MTT, 5 mg/ml, Sigma, USA) were added to each well and incubated for an additional 4 h. Then, the medium was removed and 150 μl DMSO were added to each well. The absorbance (A) was measured at A490 nm by a Multiskan MS microplate reader. All experiments were conducted in parallel with vehicle controls (0.1% DMSO). Four parallel holes were established in each group and the study was repeated for 3 times to obtain the mean values. Cell survival rate (%) = (treatment group absorbance/control group absorbance) × 100%. The IC 50 value was defined as a concentration of compound that reduces absorbance by 50% in comparison with vehicle-treated well and five concentrations were applied to determine an IC 50 value of Croton tiglium extract on A549.
Flow cytometric analysis of apoptosis
Following treatment with 0, 10, 50, 100 and 200μg/ml Croton tiglium extract for 24 h, A549 cells were harvested from 6-well culture plate at a concentration of 2×105 cells/ml, washed by PBS twice and stained by 5 μl annexin-V-fluorescein isothiocyanate (Annexin V/FITC) and propidiumiodide (PI) for 15 min at room temperature in the dark. The apoptotic rates were analyzed by flow cytometry (BD Biosciences, San Jose, CA, USA). Each assay was repeated three times.
Real-time for detection of Bax, Fas, Caspase-8, Bcl-2 mRNA expression level
The A549 cells were planted on the 6-well plate at a density of 5 × 104 per hole. After cultured with three doses of Croton tiglium extract (0, 10 and 100 μg/ml) in non-serum medium for 24 h, the cells were collected and 1 mg total RNA from each sample was reverse transcribed into cDNA using a PrimeScript RT Reagent Kit (Takara, Shiga, Japan), according to the manufacturer’s instructions. Oligonucleotide primers and probes were designed according to Gene Bank by Shanghai Biological Engineering Co. The primer sequences and reaction conditions are listed in Table 1. Real-time PCR cycles consisted of: 2 minutes at 50°C, 4 minutes at 95°C for polymerase activation, 45 cycles of 10 seconds at 95°C (denaturation) 5 seconds at 54°C, 5 seconds at 72°C and 15 seconds at 83°C (annealing and extension). Finally, melting was curried out at 72-95°C (0.5°C increments) for 5 seconds for each step (Porichi et al., 2009). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) of each sample served as intrinsic positive control. The threshold cycle (CT) of each sample was normalized to the intrinsic control GAPDH. Each sample was run in duplicate. The relative level of the gene of interest was obtained using the Equation 1/2 (Ct for gene - Ct for GAPDH), and the final relative expression ratio of each gene in each group was obtained by the geometric mean of three samples in each group.
Table 1.
Real-Time PCR Primers
| Gene | Primers 5’-3’ | Fragment length |
|---|---|---|
| Bax | F-GAGGAACTGGACAGTAACATGGAGCT | 83bp |
| R-CGGCCCCAGTTGAAGTTGC | ||
| Bcl-2 | F-GCCGGTTCAGGTACTCAGTCATC | 149bp |
| R-GTCACCTTCACCGTTCCA | ||
| Fas | F-AGGTGGACCAGCTAACCAAC | 103bp |
| R-AGCATCTCCTCCTGCAATTT | ||
| Caspas-8 | F-TCTGGAGCATCTGCTGTCTG | 294bp |
| R-CCTGCCTGGTGTCTGAAGTT | ||
| GAPDH | F-GCACCGTCAAGGCTGAGAAC | 105bp |
| R-ATGGTGGTGAAGACGCCAGT |
F, forward; R, reverse
Immunofluorescence for detection of Bax, Bcl-2, Fas, Caspase-8 protein expression level
The logarithmic phase A549 cells on the 24-well plate were treated with three doses of Croton tiglium extract (0, 10 and 100 μg/ml) for 24 h. The slides were washed in PBS three times for 5 min and subsequently exposed to a fixative containing 4% formaldehyde. After treatment with 0.2% Triton X-100 for 10 min at room temperature, the slides were immersed in 5% bovine serum albumin for 30 min at 37°C. Following the instructions from the manufacturer, the cells were then incubated with anti-Bax antibody (BA0315, Wuhan Boster Biological Technology Ltd. Hubei, China), anti-Bcl-2 antibody (BM0200, Wuhan Boster Biological Technology Ltd. Hubei, China), anti-Fas antibody (BM004, Wuhan Boster Biological Technology Ltd. Hubei, China) and anti-Caspase-8 antibody (BA3721, Wuhan Boster Biological Technology Ltd. Hubei, China) overnight at 4°C. They were then incubated with a donkey anti-mouse IgG-FITC or bovine anti-rabbit IgG-PE secondary antibody for 2 h at 37°C in the dark. Cells were washed in PBS and then stained with 4’-6’-diamidino-2-phenylindole (DAPI) solution for 10 min at 37 ºC. After a further rinse in PBS, cells were observed by Confocal Laser Scanning Microscope (Olympus LX71 Microscope). Each slice were randomly selected with five high magnification views(× 200), using Image-Pro Plus 6.0 software to analyze the average optical density value as the expression level of cellular proteins.
Statistical analysis
SPSS 13.0 software was used for statistical analysis, and the data were evaluated using ANOVA for multi-group comparisons and the SNK-q test for paired-comparisons. Data are represented as means ± SD. P<0.05 was considered to be statistically significant.
Results
Effect of Croton tiglium extract on the proliferation of A549 cells
Compound 1-3 were tested for their cytotoxic activity against human lung cancer cell lines A549. The results indicated that compound 1, Isoguanosine, significantly suppressed the survival of lung cancer cells, showing an IC 50 value of 97.6 μg/ml at 95% confidence interval, while compound 2 and 3 did not exhibit cytotoxicity against A549 cells in this study (data not shown). Therefore, compounds 1 as representatives of active apoptosis inducer was used for further experiments.
A549 cells were treated with 0, 10, 50, 100 and 200 μg/ml Croton tiglium extract for 12, 24 and 48 h, respectively, and the MTT assay showed that various concentrations Croton tiglium extract could exert a significant inhibitory effect on the proliferation of A549 cells compared with the control (0 μmol/l) and the same concentration points of the 12 and 48 h are also significant (P<0.05) as compared with the control (0 h). Consequently, the inhibition of cell viability by Croton tiglium extract was dose- and time-dependent (Figure 1A). Brightfield light microscopy images show that most cells exhibit gradually distressed, shrunken and nucleus pycnotic morphology exposed to increasing concentration of Croton tiglium extract after 24 h (Figure 1B).
Figure 1.
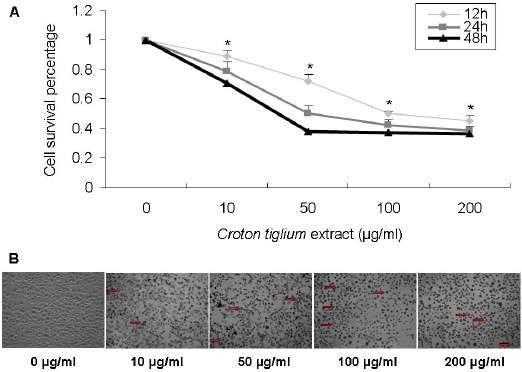
Effect of Croton Tiglium Extract on the Proliferation of A549 Cells.
(A) Cell proliferation and viability were determined by an MTT assay. Cell viability was observed with Croton tiglium extract at various concentrations (10, 50, 100 and 200 μg/ml) for 12, 24 and 48 h. Data are presented as means ± standard deviation (n=3, triplicate). *: P<0.05 compared with control (0 μg/ml and 0 h).
(B) The morphological changes of A549 cells with Croton tiglium extract after 24 h. Cell viability was observed with Croton tiglium extract treatment at various concentrations (10, 50, 100 and 200 μg/ml) for 24 h under the inverted microscope (×100)
Detection of apoptosis in A549 Cells
To examine whether Croton tiglium extract was able to induce apoptosis in lung cancer cells, the Annexin-FITC/PI double-staining method was used to assess the apoptosis rates. When A549 cells were treated with Croton tiglium extract at doses of 0, 10, 50, 100 and 200 μg/ml for 24 h, the apoptotic rates (%) in early and late stages of each dosage were all evidently increased than untreated group (Figure 2 A, B). It is notable that the proportion of late apoptotic cells were significantly increased with the increasing concentrations of Croton tiglium extract from 10 to 100 μg/ml in a dose-dependent manner (P<0.05). Moreover, the highest proportion of early apoptotic cells was 45.9% with 100 μg/ml Croton tiglium extract treatment which was significant as compared with control (P<0.05) (Figure 2B). Apoptosis was not detected at 12 h and 48 h intervals.
Figure 2.
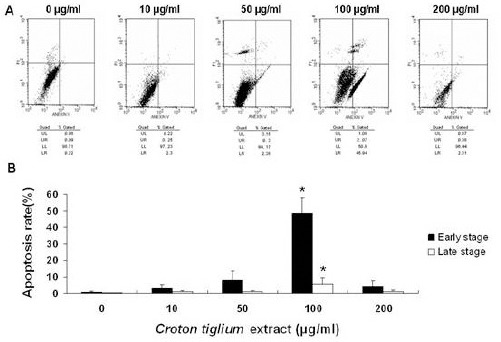
Croton Tiglium Extract Induces Apoptosis in A549 Cells.
(A) A549 cells treated with various concentrations of Croton tiglium extract were double-stained with annexin V and propidium iodide and analyzed by flow cytometry. The gate setting distinguished between living [lower left (LL)], necrotic [upper left (UL)], early apoptotic [lower right (LR)] and late apoptotic [upper right (UR)] cells. The representative data was shown from three separate assays.
(B) Quantitative data indicated that increasing the Croton tiglium extract concentration led to dose-dependent apoptosis in A549 cells. Data are presented as means ± standard deviation (n=3, triplicate). *: P<0.05 compared with control (0 μg/ml)
Effect of Croton tiglium extract on the expression of apoptosis-related genes and proteins
After confirming that Croton tiglium extract induces A549 cell apoptosis especially at the concentration of 100 μg/ml, we attempted to unveil the underlying mechanisms. It was well recognized that Bax family and Caspase-8 activation play an important role in cell apoptosis. A549 cells were exposed to increasing concentrations of Croton tiglium extract (0, 10 and 100 μg/ml) for 24 h, the mRNA expression level of Bax, Bcl-2, Fas and Caspase-8 were measured by real-time PCR. Compared with the negative control, Croton tiglium extract increased the mRNA expression of the pro-apoptotic gene Bax and decreased the expression of anti-apopotic gene Bcl-2 in A549 cells particularly at the 100 μg/ml treatment dose (Figure 3). However, the levels of Fas and Caspase-8 mRNA were not statistically significant as compared with the control.
Figure 3.
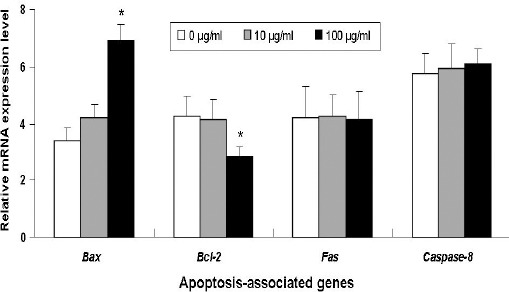
The Effect of Croton Tiglium Extract on the Expression of Apoptosis-Related Genes in A549 Cells.
The relative level of each gene obtained by normalization with GAPDH in the control group was set arbitrarily at 1, and the level in other groups was calculated accordingly. *: P<0.05 compared with control (0 μg/ml).
Using immunofluorescence to determine the effect of Croton tiglium extract with 100μg/ml on apoptosis-related proteins, it displayed that Croton tiglium extract could significantly (P<0.05) increase the Bax protein and decrease the Bcl-2 protein expressions (Figure 4).
Figure 4.
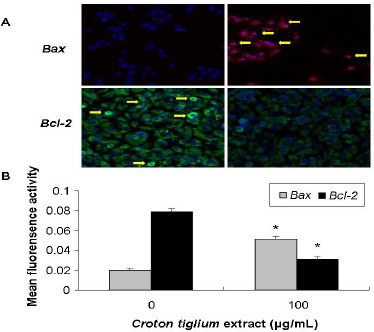
Immunostaining for Apoptosis-Related Protein in A549 Cells Treated with Croton Tiglium Extract.
(A) Representative immunofluorescence staining photomicrographs of Bax and Bcl-2 in A549 cells with 0 or 100 μg/ml Croton tiglium extract for 24 h, visualized by confocal microscope (×200).
(B) Mean fluorescence activity of Bax and Bcl-2 expressions in A549 cells analyzed by image-pro plus 6.0 software. *: P<0.05 compared with control (0 μg/ml).
Discussion
Natural plants and herbs are widely used over the years in the treatment of many diseases. A number of plants of the family Euphorbiaceae have been used to treat cancers and tumors from at least the time of Hippocrates, and scientific references have been published in several countries (Kupchan et al., 1976; Itokawa et al., 1991; Iancu et al., 2000). Croton, a member of the family Euphorbiaceae, is one of the most widely used medicinal plants of the South American tropics. The Croton genus includes several species, such as Croton lechleri, Croton palanostigma and Croton draconoides, all of which contain very rich natural medical components (Sandoval et al., 2002). The studies on Croton tiglium extract in the treatment of cancer research is controversial. The key ingredients and main functions of Croton tiglium extract are various, even diametrically opposite because of the different separating and extracting methods. For example, the Croton oil is a complex mixture of several volatiles, fatty acids and toxic proteins (Kim et al., 2015). Despite their pharmacological effects of Croton species reported, it was also known to be associated with promotion of tumorigenesis (Arroyo and Holcomb, 1965). On the other hand, Croton alkaloid and diterpenoids have been found to inhibit cell proliferation or induce programmed cell death for controlling the growth of malignant cells (Xu et al., 1995; Kuo et al., 2007; Thuong et al., 2014). Usually the toxicity of Croton tiglium seeds is due to the presence of phorbol esters and crotonic acid. In our study, these constituents are oil soluble that may have been removed during the process for reference (Pal et al., 2014; Tang et al., 2015). Reduction of these constituents content after the purification could reduce the toxicity of Croton tiglium seeds and contribute to improve the therapeutic activity.
Apoptotic genes regulate apoptosis by the action of their pro- and anti-apoptotic products. Among the most important proteins are Fas, Caspase and Bcl-x family proteins. In vitro, many drug components for anti-tumor proliferation and activity are achieved through the Bax/Bcl-2 pathways (Cai et al., 2014; Lee et al., 2014; Tang et al., 2015). In vivo, there is a high variability in the expression of apoptotic genes associated with clinical and pathologic individualities of tumor progression and metastasis. Interestingly, protein expression of anti-apoptotic Bcl-2 and pro-apoptotic Bax has both been associated with longer survival in NSCLC patients. However, the association between Bcl-2 and Bax protein expression and clinical prognostic factors is variable (Stark et al., 2007; Zhu et al., 2011; Toffart et al., 2014).
Our study found that Croton tiglium extract could significantly inhibit A549 cellular proliferation dosage-dependently. Moreover, this study also verified the effect of Croton tiglium extract on A549 cellular apoptosis, including apoptotic indexes, apoptotic rates and apoptosis-associated gene expressions by many methods. Determining the appropriate time to measure apoptosis is very important to researchers attempting to explore mechanisms of cell death in response to compounds, notably in drug discovery, because many biochemical events do not occur at all stages of apoptotic cells. It is noteworthy to mention that Croton tiglium extract could evidently induce apoptosis in A549 cells at a concentration of 100 μg/ml for 24h. but this was not markly observed in other dosages and time intervals. Additionally, this study suggests that Croton tiglium extract could elevate Bax genes and reduce Bcl-2 genes to induce cellular apoptosis. However, no obvious changes were observed in Fas and Caspase-8 gene expression. We speculate that the anticancer effects of Croton tiglium extract should be achieved in proper dosage, too low or too high concentration would cause ineffective or side effects in vitro. The mechanism may be achieved by up regulation of Bax and down regulation of Bcl-2 genes at molecular levels simultaneously. Overall, it can be anticipated that there is no relevant difference in the Fas and Caspase-8 mRNA and protein expression in A549 cells treated with Croton tiglium extract. At this stage, more insights into cell signal transduction pathway, e.g. the Bax/Bcl-2 activation and interaction, are needed before reasonable conclusions can be drawn from the existing data. Thus it appears that the suitable concentrations of Croton tiglium extract are effective on killing cancer cells in vitro and further study should be conducted in vivo.
In conclusion, our results demonstrate that the Croton tiglium extract could inhibit the proliferation of A549 cells by regulating apoptosis related genes expression in vitro. It has potential to provide biologically active compounds for treating NSCLC and deserves additional evaluation criteria as a new plant-derived anticancer agent.
Funding Statement
This work was supported by Annual Chinese Medical Research.
Program of Qingdao Municipality (No.2015-ZYY022).
References
- Arroyo ER, Holcomb J. Isolation and structure elucidation of a highly active principle from croton oil. Chem Ind. 1965;8:350–1. [PubMed] [Google Scholar]
- Cai Y, Sheng ZY, Chen Y, Bai C. Effect of Withaferin A on A549 cellular proliferation and apoptosis in non-small cell lung cancer. Asian Pac J Cancer Prev. 2014;15:1711–4. doi: 10.7314/apjcp.2014.15.4.1711. [DOI] [PubMed] [Google Scholar]
- Ding NH, Li JJ, Sun LQ. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr Drug Targets. 2013;14:347–56. doi: 10.2174/13894501113149990198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mekkawy S, Meselhy MR, Nakamura N, et al. Anti-HIV-1 phorbol esters from the seeds of Croton tiglium. Phytochemistry. 2000;53:457–64. doi: 10.1016/s0031-9422(99)00556-7. [DOI] [PubMed] [Google Scholar]
- Iancu C, Mistry SJ, Arkin S, Atweh GF. Taxol and anti-stathmin therapy: a synergistic combination that targets the mitotic spindle. Cancer Res. 2000;60:3537–41. [PubMed] [Google Scholar]
- Itokawa H, Ichihara Y, Mochizuki M, et al. A cytotoxic substance from Sangre de Grado. Chem Pharm Bull. 1991;39:1041–2. doi: 10.1248/cpb.39.1041. [DOI] [PubMed] [Google Scholar]
- Jang WS, Jyoti MA, Kim S, et al. In vitro activity of diterpenoids from the Vietnamese medicinal plant Croton tonkinensis. J Nat Med. 2016;70:127–32. doi: 10.1007/s11418-015-0937-1. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee SJ, Han YB, et al. Isolation of isoguanosine from Croton tiglium and its antitumor activity. Arch Pharm Res. 1994;17:115–8. doi: 10.1007/BF02974234. [DOI] [PubMed] [Google Scholar]
- Kim JY, Yun JW, Kim YS, et al. Mutagenicity and tumor-promoting effects of Tiglium seed extract via PKC and MAPK signaling pathways. Biosci Biotechnol Biochem. 2015;79:374–83. doi: 10.1080/09168451.2014.980217. [DOI] [PubMed] [Google Scholar]
- Kuo PC, Shen YC, Yang ML, et al. Crotonkinins A and B and related diterpenoids from Croton tonkinensis as anti-inflammatory and antitumor agents. J Nat Prod. 2007;70:1906–9. doi: 10.1021/np070383f. [DOI] [PubMed] [Google Scholar]
- Kuo YF, Su YZ, Tseng YH, et al. Flavokawain B, a novel chalcone from Alpinia pricei Hayata with potent apoptotic activity: Involvement of ROS and GADD153 upstream of mitochondria-dependent apoptosis in HCT116 cells. Free Radic Biol Med. 2010;49:214–26. doi: 10.1016/j.freeradbiomed.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Kupchan SM, Uchida I, Branfman AR, Dailey RG, Jr, Fei BY. Antileukemic principles isolated from euphorbiaceae plants. Science. 1976;191:571–2. doi: 10.1126/science.1251193. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Yue CH, Lin YJ, et al. Antitumor activity of acriflavine in lung adenocarcinoma cell line A549. Anticancer Res. 2014;34:6467–72. [PubMed] [Google Scholar]
- Li Q, Ren FQ, Yang CL, et al. Anti-proliferation effects of isorhamnetin on lung cancer cells in vitro and in vivo. Asian Pac J Cancer Prev. 2015;16:3035–42. doi: 10.7314/apjcp.2015.16.7.3035. [DOI] [PubMed] [Google Scholar]
- Lima GS, Castro-Pinto DB, Machado GC, Maciel MA, Echevarria A. Antileishmanial activity and trypanothione reductase effects of terpenes from the Amazonian species Croton cajucara Benth (Euphorbiaceae) Phytomedicine. 2015;22:1133–7. doi: 10.1016/j.phymed.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Madrigales-Ahuatzi D, Perez-Gutierrez RM. Evaluation of Anti-inflammatory Activity of Seeds of Phalaris canariensis. Drug Res. 2016;66:23–7. doi: 10.1055/s-0035-1548764. [DOI] [PubMed] [Google Scholar]
- McKeage MJ, Jameson MB Investigators ASSG. Comparative outcomes of squamous and non-squamous non-small cell lung cancer (NSCLC) patients in phase II studies of ASA404 (DMXAA) - retrospective analysis of pooled data. J Thorac Dis. 2010;2:199–204. doi: 10.3978/j.issn.2072-1439.2010.02.04.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota EF, Rosario DM, Silva Veiga AS, et al. Biological activities of Croton palanostigma Klotzsch. Pharmacogn Mag. 2015;11:601–6. doi: 10.4103/0973-1296.160449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudium R, Kolasani B. Anticonvulsant effect of hydroalcoholic seed extract of croton tiglium in rats and mice. J Clin Diagn Res. 2014;8:24–6. doi: 10.7860/JCDR/2014/7022.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal PK, Nandi MK, Singh NK. Detoxification of croton tiglium L. seeds by Ayurvedic process of Sodhana. Anc Sci Life. 2014;33:157–61. doi: 10.4103/0257-7941.144619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porichi O, Nikolaidou ME, Apostolaki A, et al. BCL-2, BAX and P53 expression profiles in endometrial carcinoma as studied by real-time PCR and immunohistochemistry. Anticancer Res. 2009;29:3977–82. [PubMed] [Google Scholar]
- Sandoval M, Okuhama NN, Clark M, et al. Sangre de grado Croton palanostigma induces apoptosis in human gastrointestinal cancer cells. J Ethnopharmacol. 2002;80:121–9. doi: 10.1016/s0378-8741(02)00013-2. [DOI] [PubMed] [Google Scholar]
- Stark AM, Hugo HH, Tscheslog H, Mehdorn HM. p53, BCL-2 and BAX in non-small cell lung cancer brain metastases: a comparison of real-time RT-PCR, ELISA and immunohistochemical techniques. Neurol Res. 2007;29:435–40. doi: 10.1179/016164107X165282. [DOI] [PubMed] [Google Scholar]
- Tang Y, Zhang X, Qi F, et al. Afatinib inhibits proliferation and invasion and promotes apoptosis of the T24 bladder cancer cell line. Exp Ther Med. 2015;9:1851–6. doi: 10.3892/etm.2015.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Then C, von Einem JC, Muller D, et al. Toxic epidermal necrolysis after pemetrexed and cisplatin for non-small cell lung cancer in a patient with sharp syndrome. Onkologie. 2012;35:783–6. doi: 10.1159/000345109. [DOI] [PubMed] [Google Scholar]
- Thuong PT, Khoi NM, Ohta S, et al. Ent-kaurane diterpenoids from Croton tonkinensis induce apoptosis in colorectal cancer cells through the phosphorylation of JNK mediated by reactive oxygen species and dual-specificity JNK kinase MKK4. Anticancer Agents Med Chem. 2014;14:1051–61. doi: 10.2174/1871520614666140127111407. [DOI] [PubMed] [Google Scholar]
- Toffart AC, Timsit JF, Couraud S, et al. Immunohistochemistry evaluation of biomarker expression in non-small cell lung cancer (Pharmacogenoscan study) Lung cancer. 2014;83:182–8. doi: 10.1016/j.lungcan.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Wang S, Su X, Bai H, et al. Identification of plasma microRNA profiles for primary resistance to EGFR-TKIs in advanced non-small cell lung cancer (NSCLC) patients with EGFR activating mutation. J Hematol Oncol. 2015;8:127. doi: 10.1186/s13045-015-0210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Qu C, Ma Z. The effects of antitumor agents of croton alkaloids and cisplatin on human red blood cell membranes. Zhonghua zhong liu za zhi. 1995;17:115–7. [PubMed] [Google Scholar]
- Xu YH, Mei JS, Zhou J. Randomized study of gefitinib versus pemetrexed as maintenance treatment in patients with advanced glandular non-small cell lung cancer. Int J Clin Exp Med. 2015;8:6242–6. [PMC free article] [PubMed] [Google Scholar]
- Yilmaz S, Adas YG, Hicsonmez A, et al. Evaluation of the radiation pneumonia development risk in lung cancer cases. Asian Pac J Cancer Prev. 2014;15:7371–5. doi: 10.7314/apjcp.2014.15.17.7371. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Lv H, Xie Y, et al. Enhanced tumor suppression by an ING4/IL-24 bicistronic adenovirus-mediated gene cotransfer in human non-small cell lung cancer cells. Cancer Gene Ther. 2011;18:627–36. doi: 10.1038/cgt.2011.31. [DOI] [PubMed] [Google Scholar]


