Abstract
Aim:
To assess the diagnostic utility of serum and salivary interleukin 6 (IL-6) levels in the differential diagnosis of potentially malignant lesions and conditions (PMLs/PMCs) and oral squamous cell carcinoma (OSCC) in a high oral cancer prevalence region.
Methods:
After appropriate ethical clearance and informed consent, salivary and blood samples were collected from 100 participants in each group (OSCC, PMLs, and healthy controls). Serum and salivary IL-6 levels were measured by enzyme-linked immunosorbent assay and data were subjected to appropriate statistical analysis.
Results:
Significant differences in IL-6 concentration were noted between OSCC and PML/C patients in both serum and saliva, with salivary levels being 2 to 3 fold higher than serum values in all the groups. Receiver operating characteristic curve analysis demonstrated 96% specificity and 99% sensitivity for salivary IL-6 in differentiating PML from OSCC.
Conclusions:
The results of the present study suggest that the pro-inflammatory cytokine, IL-6, is elevated in the saliva of patients with OSSC compared to PMD and controls, and thus may prove to have diagnostic and/or prognostic significance.
Keywords: Cytokine, interleukin 6, oral squamous cell carcinoma, premalignant lesion, saliva
Introduction
Oral cancer remains a major global threat and is the sixth most common cancer worldwide. Although there is wide geographical variation, India is one of the countries where there is a high incidence rate (Warnakulasuriya S, 2009). Recent reports on oral cancer revealed that India accounts for a third of the world burden of oral cancer and ranks on the list of highest incidence oral cancer countries in the world and these countries collectively account for 80% of OSCC cases globally (Dikshit et al., 2012; Ferlay et al., 2013). This high prevalence is being attributed to the influence of carcinogens and region-specific epidemiological factors, especially tobacco and betel quid chewing (Tsantoulis et al., 2007). Even more worrying, is the fact that there is an alarming rise in the incidence rates of OSCC in younger people owing to the heavy abuse of tobacco and tobacco related products. An efficient screening program requires early and accurate diagnosis and treatment of patients with malignant OSCC. A major obstacle to early treatment of oral cancer is the lack of rapid accurate diagnostic methods that can be applied in low-income areas. Diagnosis of OSCC in resource-constrained settings is primarily based on clinical examination, histological findings of oral biopsy.
Histopathological studies require laboratories with skilled personell and are rather time consuming process. Patients monitored under screening programs will not be willing to undergo biopsies for histological diagnosis. In addition, histopathological procedure is expensive and needs experienced person for interpretation, thus, is not suitable for routine screening and monitoring. Moreover, the differential diagnosis of premalignant oral lesion (PML) and malignant OSCC has remained a difficult task mainly owing to the similarity between OSCC and PML in terms of their signs, symptoms, and presentation on imaging techniques. The prolonged asymptomatic nature and having an unspecific disease course are two most essential reasons for a very low curative resection rates (5%-10%) for OSCC. Indisputable confirmation of the benign nature of a tumor is an important factor that can markedly influence the disease management of the patient. Hence, an appropriate marker which will be simpler to use and cost effective in differential diagnosis of oral PML and OSCC is the immediate need for initiating an effective screening for oral cancer.
One may anticipate that clinical research conducted worldwide will result in the establishment of a relatively simple and reliable noninvasive diagnostic technique that is able to differentiate malignant and benign tumors quickly and cost effectively. A wide-variety of tumor markers derived from serum, oral tissue and saliva has been proposed for early diagnosis as well as to predict prognosis in OSCC patients (Rajkumar et al., 2014). Nevertheless, utility of those markers is often significantly limited by poor sensitivity, high false positive rates and lack of large scale validation. Among the various reported markers interleukin (IL-6) was found to be a prominent salivary marker for OSCC. In late 1990’s Ewa Jablonska et al., (1997) stated that IL-6 to be a most sensitive parameter in early stages and suggested its use as an additional marker in oral cancer (Ewa Jablonska et al., 1997). Many studies reported elevated IL-6 levels in saliva of OSCC patients. Studies reveal a higher IL-6 levels in serum and saliva of OSCC when compared with premalignant disease (PMD) also. In addition, strong association of IL-6 gene polymorphism has been reported (Singh et al., 2015).
Thus, the role of IL-6 in OSCC is well established as a pathogenic factor and biomarker. Furthermore, IL-6 has been suggested as one of the best biomarker for diagnosing OSCC (Lisa Cheng et al., 2014). Despite the vast number of research studies supporting the usage of IL-6 as a biomarker, there is no validation of this marker in differential diagnosis of OSCC and PMD. Therefore, the present study was planned to assess the diagnostic utility of serum and salivary IL-6 levels in differential diagnosis of OSCC and PMD in a high oral cancer prevalent area.
Materials and Methods
This study was approved by the Scientific Advisory Committee and Institutional Ethical Committee of SRM University, Chennai. According to ethical principles, including the World Medical Association Declaration of Helsinki (version, 2002), a written and informed consent was obtained from all the study participants before drawing blood and collecting saliva.
Study subjects recruitment
The recruitment of study subjects were done at outpatient department of SRM Dental College, referrals from SRM General Hospital and private dental clinics, Chennai during January 2009 and December 2012. Study subjects were recruited by professionally qualified, well trained and experienced Oral Pathologists. The demographic details and information on previous history were collected.
Exclusion and inclusion criteria for study subjects
Healthy control group
The study subjects were recruited from student and staff volunteers from SRM Dental College and Hospital. Subjects were recruited with the following eligibility criteria: ages 21–65, male or female, no current use of prescribed or non-prescribed medication, no chronic/acute illnesses, no oral lesions no acute or sub-acute inflammation or infection, no pathological dry mouth syndrome, or inability to collect sufficient saliva samples on a reliable basis. Pregnant and lactating subjects were also excluded.
PMD group
The study subjects were recruited from outpatient department of SRM Dental College, referrals from SRM General Hospital and private dental clinics, Chennai. Subjects were recruited with the following eligibility criteria: ages 21–90, male or female, Comprised of patients who had oral potentially malignant lesions/conditions which were clinically diagnosed and later histopathologically confirmed and who were not undergoing or having undergone any form of treatment for these lesions. Patients with underlying systemic illnesses and presence of oral inflammatory conditions such as gingivitis, periodontitis, and oral ulcers were excluded. A total of 159 subjects were screened for potentially malignant lesion, before confirming the lesion with histopathological analysis, required amount of saliva and blood was collected and stored. Only samples of confirmed cases with potentially malignant lesion were included in the study. Due to rarity of erythroplakia occurrence in South India region, only leukoplakia and oral sub mucous fibrosis (OSMF) was included for the study, 50 in each group (leukoplakia & OSMF) were included for the study. Diagnosis of OSMF was made by the clinical symptoms like difficulty in opening of mouth, palpable fibrotic bands and graded with the clinical grading system.
Oral squamous cell carcinoma (OSCC) group
The study subjects were recruited from outpatient department of SRM Dental College, referrals from SRM General Hospital and private dental clinics, Chennai. Subjects were recruited with the following eligibility criteria: ages 21–90, male or female, comprised of patients who had oral squamous cell carcinoma which were clinically diagnosed and who were not currently undergoing or having undergone any form of definitive therapy for OSCC in the form of radiation, chemotherapy or any other adjunctive treatments. Before confirming with tissue histopathological analysis, the required amount of saliva and blood was collected and stored. Sample was collected from total of 139 subjects, out of which 39 were excluded for various reason, either non-confirmed OSCC by tissue biopsy or had other systemic illness apart from OSCC. All oral lesions and conditions which report to the outpatient department are subjected to routine exfoliative cytology studies with Papanicoloau stain and potassium hydroxide stain to rule out presence of candida hyphae.
Sample collection
Saliva samples were collected between 9 and 11 A.M under non stimulatory condition. Participants were asked to refrain from eating, chewing and drinking at least one hour before collection. Salivary samples from patients with oral carcinoma and premalignant lesion were collected before any therapeutic procedure. Following collection, the saliva was immediately centrifuged to remove cell debris. Supernatants were then stored at -80ºC. 2 ml of peripheral blood were drawn from all study subjects through venipuncture. Blood was transferred to an empty vacutainer. Serum was then collected by centrifuging the coagulated blood and stored at -80ºC until further use.
IL-6 Estimation
Concentrations of salivary and serum IL-6 were quantified by commercially available ELISA kit (Diaclone, France). The assay was carried out according to the manufacturer’s instruction. Briefly, the kit was based on sandwich ELISA method and procedure is as follows; to the precoated IL-6 antibody microplate, standards and sample were added, incubated (for 2 hours) and washed with buffer. To the washed plate detection antibody bound with HRP conjugate was added. The unbound antibody was washed and a chromogen substrate was added to the wells resulting in the progressive development of a blue coloured complex with the conjugate. The colour development was then stopped by the addition of stop solution turning the resultant final product yellow. The intensity of colour developed is proportional to the IL-6 present which was measured in a microplate reader (Robonik ELISA plate reader) at a wavelength of 450nm. The optical density obtained was then used for calculation of IL-6 present in each sample. The detection range of the kit was from a minimum of 5pg to a maximum of 200 pg/ml. The results were expressed as pg/ml of saliva or serum.
Statistical analysis
All statistical analyses were carried out using GraphPad Prism for Windows ver. 5 (GraphPad Software, San Diego, CA, USA) and the statistical software program SPSS 18.0 (PASW statistics). Data were expressed as mean ± SD. A value of P<0.05 was considered statistically significant. Nonparametric Mann–Whitney U tests were performed to find the significance of the observed differences between groups. ROC curve analysis was performed, and the best cut off point was determined by the highest positive likelihood ratio (PLR) (sensitivity/ [1 − specificity]). The best cut-off value was defined as the test result with the highest sensitivity and specificity and that lied closest to the left upper corner of the curve. The area under the curve presented a direct measure of the diagnostic accuracy of the test.
Results
Clinical Features
Demographic and clinical characteristic of the patients are outlined in Table 1. Briefly age and sex matched 100 control subjects, 100 premalignant lesions which consisted 50 each of leukoplakia and OSMF and 100 OSCC subjects were included. All the premalignant and OSCC subjects either had tobacco/pan chewing/smoking or alcohol intake habits. The premalignant lesion were from buccal mucosa (n=52), vestibule (n=4) together in buccal mucosa and vestibule (n=35), alveolar mucosa (n=3), palate (n=2), tongue (n=4). Similarly, the OSCC lesion were from buccal mucosa (n=64), alveolar mucosa (n=11), palate (n=4), tongue (n=19) and lip (n=2). Based on the histopathological grading of OSCC, 36 patients had well differentiated, 31 had moderately differentiated and 33 had poorly differentiated lesion.
Table 1.
Demographics and subclassification of study groups
| Category | Control | Premalignant lesion | ||
|---|---|---|---|---|
| Leukoplakia | OSMF | OSCC | ||
| Subjects (n) | 100 | 50 | 50 | 100 |
| Age range (years) | 21-65 | 21-90 | 21-70 | 21-90 |
| Sex: Male | 65 | 29 | 42 | 68 |
| Female | 35 | 21 | 8 | 32 |
| Habits: | ||||
| Tobacco chewing | - | 28 | 42 | 34 |
| Smoking | - | 7 | - | 14 |
| Smoking and chewing | - | 14 | 8 | 38 |
| Smoking and alcohol | - | 1 | - | 9 |
| Alcohol only | - | - | - | 5 |
| Site of Lesion | ||||
| Buccal mucosa | - | 21 | 31 | 64 |
| Vestibule | - | - | 4 | - |
| Buccal mucosa and Vestibule | - | 20 | 15 | - |
| Alveolar mucosa | - | 3 | - | 11 |
| Palate | - | 2 | - | 4 |
| Tongue | - | 4 | - | 19 |
| Lip | - | - | - | 2 |
Grading and staging
Grading and clinical staging was done for both PMD (including both PML and PMC) and OSCC group. The overall distribution based on the histopathological grading of PML (leukoplakia) included 18 subjects with mild dysplasia, 20 subjects with moderate dysplasia and 12 subjects with severe dysplasia. For PMC (OSMF), clinical staging was carried, about 21 subjects were categorized under stage I, 25 patients were classified under stage II, and 4 patients were staged under stage III. The distribution based on histological grading of OSCC, 36 patients had well differentiated, 31 had moderately differentiated and 33 had poorly differentiated lesion. Also, the OSCC patients were categorized by clinical staging. There were 32 patients in stage I, in stage II there were 32 subjects, 25 subjects were categorized as in stage III and 11 patients were in stage IV.
Serum and salivary IL-6 levels in OSCC and PML/C group
The mean values of serum and salivary IL-6 concentrations for healthy controls, OSCC and PML/C are shown in Table 2. The median and interquartile range for serum IL-6 concentration in control subject is 0.9 (0.2 - 4.0), PML is 25.2 (5.6 - 59.4), PMC is 21.6 (10.3 - 48.6) and OSCC is 49.3 (28.0 - 101.0) ng/ml. Salivary IL-6 median and interquartile range for control subject is 10.8 (0.5 - 34.3), PML is 33.4 (13.9 - 82.4), PMC is 38.3 (17.4 - 86.8) and OSCC is 178.5 (62.3 - 242.5) pg/ml respectively. Significant differences in IL-6 concentration were noted between OSCC and PML/C patient in both serum and saliva (p<0.01). When compared to control group, the level of serum IL-6 in PML/C showed a significant difference of p<0.05. Salivary IL-6 also showed a significantly higher level in PML/C when compared to control group (p<0.05). Significantly higher concentrations of IL-6 were noted in OSCC patients than those of PML/C subjects (p<0.001). As expected, the salivary IL-6 levels were higher (2 to 3 fold) than serum (p<0.001) in all the groups (Table 2).
Table 2.
Comparison of IL-6 Levels in Serum and Saliva
| Study Subjects | Serum IL-6 (pg/ml) | Salivary IL-6 (pg/ml) |
|---|---|---|
| Healthy Control (n=100) | 1.0±0.7 | 10.8±6.7 |
| Premalignant Lesion subjects (n=50) | 25.3±9.9 | 35.3±14.3 |
| Premalignant Condition subjects (n=50) | 25.0±6.3 | 38.3±12.3 |
| Oral squamous cell carcinoma (n=100) | 52.0±19.2 | 178.0±28.3 |
The levels of serum and salivary IL-6 were assessed by ELISA in all the study subjects. The concentrations are expressed in picogram per milliliter, and the values represent mean ± SEM.
IL-6 levels based on histological grading
For 100 patients with OSCC, IL-6 concentrations were compared according to histological differentiation (Figure 1A and B). The median and interquartile range of serum IL-6 concentration were 33.3 (0.8 - 53.1), 49.7 (29.4 - 66.1) and 68.6 (41.8-101.3) pg/ml for well differentiated, moderately differentiated (MD) and poorly differentiated (PD) lesions, respectively (figure 1A). Surprisingly, in serum IL6 a significant down regulation was observed with PD compared to WD and MD (p<0.05). Contrarily, salivary IL-6 concentration did not show any significant difference between the histological grading in OSCC. Median and interquartile range of salivary IL-6 concentrations were 145.4 (5.6 – 178.9) for WD, 167.8 (10.4 - 204.3) for MD and 195.5 (6.4 – 242.5) pg/ml for PD lesion, respectively (Figure 1B). No significant difference was evident for serum or salivary IL-6 concentration in PML group based on histological grading. With PMC clinical staging (Figure 3A and B) decrease in IL-6 levels was observed in both serum and saliva from stage I to stage III. But, significant decrease was found only win serum stage III, IL-6 level when compared to stage I (p<0.05).
Figure 1.
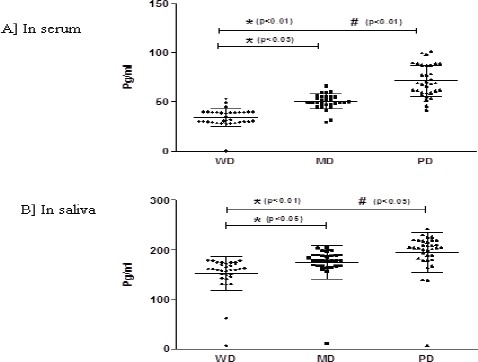
A and B. IL-6 Levels Based on Histological Grading in OSCC.
The levels of IL-6 compared in serum (A) and saliva (B) of OSCC subjects based on histological grading [well differentiated (WD), moderately differentiated and poorly differentiated (PD)]. Each dot represents IL-6 level of each individual subject and the horizontal line represents mean value. The statistical significance is shown as * when compared to WD and # compared to MD. p<0.05 was considered to be statistically significant.
Figure 3.
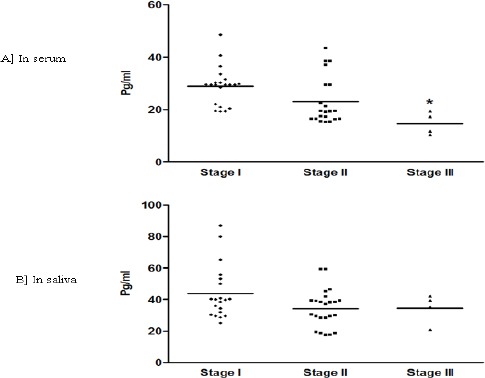
A and B: IL-6 Levels Based on Clinical Staging in PMC.
The levels of IL-6 compared in serum (E) and saliva (F) of PMC subjects based on clinical staging. Each dot represents IL-6 level of each individual subject and the horizontal line represents mean value. The statistical significance is shown as * when compared to stage I. p<0.05 was considered to be statistically significant
Figure 2.
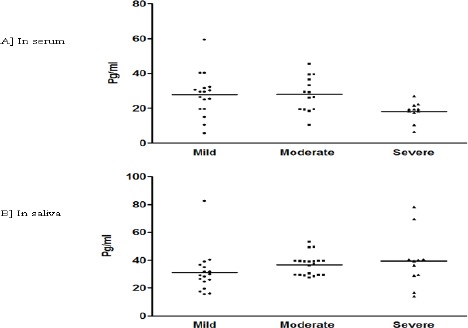
A and B: IL-6 Levels Based on Histological Grading in PML.
The levels of IL-6 compared in serum (C) and saliva (D) of PML subjects based on histological grading [Mild dysplasia, Moderate dysplasia and severe dysplasia]. Each dot represents IL-6 level of each individual subject and the horizontal line represents mean value.
IL-6 levels based on clinical staging in OSCC
Based on the clinical staging of OSCC patients, level of IL-6 in serum and saliva were also compared (Figure 4A and B). There was no statistical difference in serum and salivary IL-6 based on clinical staging of OSCC.
Figure 4.
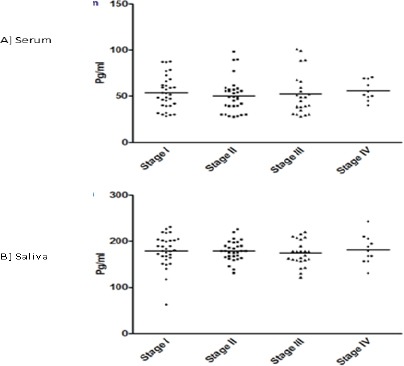
A and B: IL-6 Levels Based on Clinical Staging in OSCC.
The levels of IL-6 compared in serum (A) and saliva (B) of OSCC subjects based on clinical staging. Each dot represents IL-6 level of each individual subject and the horizontal line represent mean value
Diagnostic utility of salivary IL-6 versus serum IL-6
To determine the sensitivity and specificity of serum and salivary IL-6 in diagnosing OSCC, ROC curves were constructed. ROC curves compared the ability of the serum / salivary IL-6 to differentiate between patients with OSCC and PML/C group. At a cut off value of >75 pg/ml, salivary IL-6 shows 96% specificity and 99% sensitivity in differentiating PML from OSCC. On the other hand, a cut off value of 35 pg/ml in serum IL-6 level showed 86% specificity while the sensitivity was shown to be 82%. Also, PPV and NPV were above 80% for serum and above 90% for salivary IL-6 (Table 3). Curve comparing the diagnostic ability of salivary and serum IL-6 in detecting OSCC using ROC curve is illustrated (figure 5). The AUC for salivary IL-6 is 0.943(95% CI: 0.911 – 0.975) and serum IL-6 is 0.960 (95% CI:0.922 – 0.998).
Table 3.
Diagnostic Utility of Salivary and Serum IL-6
| Diagnostic parameters | Saliva | Serum |
|---|---|---|
| Cut off value | 75 pg/ml | 35pg/ml |
| Sensitivity | 99.0% | 82.0% |
| Specificity | 96.0% | 86.0% |
| Area under Curve | 0.9 | 1.0 |
| PPV | 96.2% | 85.4% |
| NPV | 98.9% | 82.7% |
The diagnostic utility is assessed in terms of specificity, sensitivity, positive predictive value (PPV)
Figure 5.
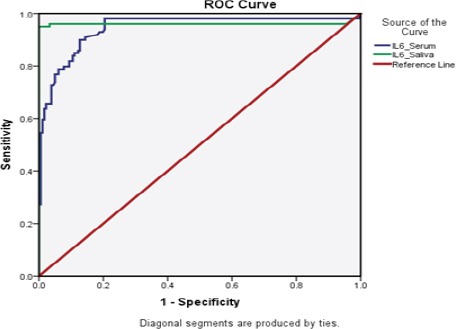
Diagnostic Utility of IL-6 Assessed by ROC Curve Analysis.
ROC curve analysis of IL-6 marker in saliva and serum for predicting OSCC when compared to PML
Clinicopathologic correlation with serum and salivary biomarkers in OSCC
The level of IL-6 in serum and saliva was correlated with clinicopathologic factors using the univariate analysis employing Spearman rank test and is illustrated in table 4. Clinicopathologic factors included for correlation were age, sex, habits, site of lesion, histopathologic grading and clinical staging. No significant correlation between age, sex, habit and site of lesion and serum/salivary IL-6 levels. A positive significant correlation was found with grading and serum IL-6 (p<0.01) levels. Salivary IL-6 was found to show significant correlation with histopathological grading (p<0.05). No significant correlation was found in IL-6 levels of serum and saliva with clinical staging.
Table 4.
Correlation between Biomarker Levels (Serum and Saliva) and Clinicopathological Factors in OSCC Patients
| Factors | Sample size (total n=100) | IL-6 (cut off- 35pg/ml) | IL-6 (cut off: 35pg/ml) |
|---|---|---|---|
| Serum | Saliva | ||
| Age | |||
| £40 years | 42 | 32 (10 > cut off) | 42 (0 > cut off) |
| >40 years | 58 | 50 (8 > cut off) | 57 (1> cut off) |
| p < 0.091 | p < 0.086 | ||
| Sex | |||
| Male | 68 | 54 (14 > cut off) | 67 (1 > cut off) |
| Female | 32 | 28 (4 > cut off) | 32 (0 > cut off) |
| p < 0.083 | p < 0.079 | ||
| Habit | |||
| Tobacco chewing | 34 | 29 (5 > cut off) | 34 (0> cut off) |
| Smoking only | 14 | 12 (2 > cut off) | 13 (1> cut off) |
| Smoking & chewing | 38 | 30 (8 > cut off) | 38(0 > cut off) |
| Alcohol only | 9 | 07 (2 > cut off) | 09 (0 > cut off) |
| Smoking & chewing | 5 | 04 (1 > cut off) | 05(0 > cut off) |
| p < 0.068 | p < 0.075 | ||
| Site of lesion | |||
| Buccal mucosa | 64 | 52 (12 > cut off) | 64 (0 > cut off) |
| Alveolar mucosa | 11 | 10 (2 > cut off) | 11 (0 > cut off) |
| Palate | 4 | 04 (0 > cut off) | 04 (0 > cut off) |
| Tongue/Lip | 21 | 17 (4> cut off) | 20 (1> cut off) |
| p < 0.083 | p < 0.636 | ||
| Histopathological grading | |||
| Well differentiated | 36 | 24 (12 > cut off) | 35 (1 > cut off) |
| Moderately differentiated | 31 | 27(4 > cut off) | 31(4 > cut off) |
| Poorly differentiated | 33 | 31 (2 > cut off) | 33 (2 > cut off) |
| p < 0.01* | p < 0.05* | ||
| Clinical staging | |||
| Stage I | 32 | 26 (6> cut off) | 32 (0> cut off) |
| Stage II | 32 | 26 (6 > cut off) | 32(0 > cut off) |
| Stage III | 25 | 21(4 > cut off) | 24(1 > cut off) |
| Stage IV | 11 | 09 (2> cut off) | 11(0> cut off) |
| p < 0.094 | p < 0.085 |
Discussion
From the scientific literature until now, this is the largest prospective study that demonstrates the equal potential of serum IL-6 and saliva as a diagnostic medium for detecting OSCC. The temporal association between histopathologic grading and IL-6 levels in both serum and saliva confirms that IL-6 may be a valuable biomarker for predicting the severity of OSCC in this population. Patients with high levels of IL-6 levels may benefit from closer follow-up to have a better chance of identifying recurrences at an early stage. Though, the mean value of salivary IL-6 level was almost 3 fold higher than serum mean IL-6 levels, the ROC curve analysis and specificity and sensitivity calculation reveals serum IL-6 to be best in predicting oral cancer compared to salivary IL-6. Those with higher IL-6 levels could be candidates for more aggressive intervention to prolong survival. However, in premalignant disorder there was no significant difference between serum and salivary IL-6 levels.
Increasing evidence implicating IL-6 as a diagnostic marker for OSCC and other cancer types is emerging in this and other studies. Studies in solid tumors like gastric, renal cell, colorectal, prostate, non-small cell lung, melanoma, head and neck SCC as well as hematologic malignancies like myeloma and non-Hodgkin’s lymphoma indicate the potential diagnostic significance of increased IL-6 levels. Several studies have demonstrated associations between serum IL-6 genotypes and cancer risk. (Sugiyama et al., 1996; Kim et al., 2003; Rose-John et al., 2006; Lippitz et al., 2013; Maccio et al., 2013; Ohishi et al., 2014; Tripsianis et al., 2014).
The mechanism by which serum IL-6 contributes to or reflects cancer progression and biology is likely due to its dual effects on tumor initiation by paracrine or autocrine mechanisms and to its additional inhibitory effects on the immune response directed against the tumor (Lederle et al., 2011). IL-6 inhibits dendritic cell differentiation, thus inducing immune tolerance of tumors and facilitating metastatic spread (Menetrier-Caux et al., 1998; Bharadwaj et al., 2007). The source of IL-6 in cancer patient’s sera has been shown predominately to emanate from the tumor itself, but monocytes in head and neck SCC patients have been shown to secrete higher levels than monocytes from healthy individuals (Kross et al., 2005; Kross et al., 2007). It is well documented that monocyte functional abnormalities and impaired cellular immunity are frequent and early characteristics of patients with OSCC (Eskinazi et al., 1987; Lam-ubol et al., 2010). An interesting hypothesis is raised, which proposes that IL-6 secretion from both tumor and monocytes in the tumor microenvironment results in an immune-tolerant situation that allows the tumor to thrive.
Interleukin-6 first demonstrated as a marker for oral cancer (St John et al., 2004) and subsequently in 2005 it was shown that salivary IL-6 had significant higher levels in OSCC compared to serum IL-6 (Vucicevic Boras., 2005). Present study also showed significantly high salivary IL-6 level than serum IL-6 in OSCC. In a study by Brailo et al, higher salivary IL-6 level was shown in oral leukoplakia compared to healthy subjeccts (Brailo et al., 2006), similar results was also observed in the present study. In addition, compared to IL-6 level in OSCC, the IL-6 level in premalignent disorder was significantly lower. Similar observation was reported by Rhodus et al (Rhodus et al., 2005). However, when analysed with ROC curve, area under curve value showed serum IL-6 to be better in predicting OSCC. This is the first study which has compared the serum and salivary IL-6 diagnostic utility by ROC curves. When taken together our present findings shows serum IL-6 level to be best in differentiating premalignant disorder from OSCC.
Correlation studies between IL-6 levels and histopathologic grading showed a positive association. IL-6 level was found to be increasing from well differentiating to moderately differentiating to poorly differentiating lesion in serum and saliva as well. This suggests that IL-6 level as a marker which can be associated with disease aggressiveness and severity as in other studies. However, there was no increase in IL-6 level with PML grading and staging of PMC. Although, various studies has shown association of IL-6 levels with clinical staging (Duffy et al., 2008; Van Tubergen et al., 2011; Chen et al., 2012; Chang et al., 2013; Panneer Selvam et al., 2015), the present study did not show any association or increase in IL-6 level with clinical staging in OSCC. This could be due to the fact that vast majority of patients in OSCC belongs to stage I and II rather than later stages.
The main limitation of this study is that it didn’t include other premalignant disorder like erythroplakia and oral lichen planus in premalignant group. The reason for their exclusion is their rare occurrence and moreover leukoplakia and OSMF are the PML’s which are stated to transit to malignant form at a high rate compared to others. So, this should not undervalue the outcome of the study. It is also important to note that the known increasing effect that tobacco smoking/chewing has on OSCC. But it could not account for the difference in IL-6 levels between the cancer patients and the PMD subjects, as the numbers of smokers among these two groups were similar and moreover it is reported that smoking has no effect on IL-6 concentration (Hockertz et al., 1994; Chiu et al., 2011).
Taken together, the results of the present study suggest that this pro-inflammatory cytokines are elevated in the saliva of patients with OSSC compared to PMD and compared to controls, and thus proves to be a validated diagnostic and/or prognostic significance which needs to be further confirmed by large population size at multicentre level.
Acknowledgements
The authors thank the Patients and healthy volunteers in the study for their willingness to contribute towards this study.
References
- Bharadwaj U, Li M, Zhang R, Chen C, Yao Q. Elevated interleukin-6 and G-CSF in human pancreatic cancer cell conditioned medium suppress dendritic cell differentiation and activation. Cancer Res. 2007;67:5479–88. doi: 10.1158/0008-5472.CAN-06-3963. [DOI] [PubMed] [Google Scholar]
- Brailo V, Vucićević-Boras V, Cekić-Arambasin A, et al. The significance of salivary interleukin 6 and tumor necrosis factor alpha in patients with oral leukoplakia. Oral Oncol. 2006;42:370–3. doi: 10.1016/j.oraloncology.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Chang KP, Kao HK, Wu CC, et al. Pretreatment interleukin-6 serum levels are associated with patient survival for oral cavity squamous cell carcinoma. Otolaryngol Head Neck Surg. 2013;148:786–91. doi: 10.1177/0194599813478573. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Sung WW, Lin YM, et al. Gender difference in the prognostic role of interleukin 6 in oral squamous cell carcinoma. PLoS One. 2012;7:50104. doi: 10.1371/journal.pone.0050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Spiegelman D, Dockery DW, et al. Secondhand smoke exposure and inflammatory markers in nonsmokers in the trucking industry. Environ Health Perspect. 2011;119:1294–300. doi: 10.1289/ehp.1003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikshit R, Gupta PC, Ramasundarahettige C, et al. Cancer mortality in India: a nationally representative survey. Lancet. 2012;379:1807–16. doi: 10.1016/S0140-6736(12)60358-4. [DOI] [PubMed] [Google Scholar]
- Duffy SA, Taylor JM, Terrell JE, et al. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113:750–7. doi: 10.1002/cncr.23615. [DOI] [PubMed] [Google Scholar]
- Eskinazi DP, Perna JJ, Mihail R. Mononuclear cell subsets in patients with oral cancer. Cancer. 1987;60:376–81. doi: 10.1002/1097-0142(19870801)60:3<376::aid-cncr2820600315>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Ewa J, Leszek P, Zyta G. Serum levels of IL-1β, IL-6, TNF-α, sTNFR1 and CRP in patients with oral cavity cancer. Pathol Oncol Res. 1997;3:126–9. doi: 10.1007/BF02907807. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer 2013; 2012. [accessed on 05/08/2015]. Available from: http://globocan.iarc.fr . [Google Scholar]
- Hockertz S, Emmendörffer A, Scherer G, et al. Acute effects of smoking and high experimental exposure to environmental tobacco smoke (ETS) on the immune system. Cell Biol Toxicol. 1994;10:177–90. doi: 10.1007/BF00757561. [DOI] [PubMed] [Google Scholar]
- Kim HK, Song KS, Park YS, et al. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. Eur J Cancer. 2003;39:184–91. doi: 10.1016/s0959-8049(02)00596-8. [DOI] [PubMed] [Google Scholar]
- Kross KW, Heimdal JH, Olsnes C, Olofsson J, Aarstad HJ. Head and neck squamous cell carcinoma spheroid- and monocyte spheroid-stimulated IL-6 and monocyte chemotactic protein-1 secretion are related to TNM stage, inflammatory state and tumor macrophage density. Acta Otolaryngol. 2005;125:1097–104. doi: 10.1080/00016480510038031. [DOI] [PubMed] [Google Scholar]
- Kross KW, Heimdal JH, Olsnes C, Olofsson J, Aarstad HJ. Tumour-associated macrophages secrete IL-6 and MCP-1 in head and neck squamous cell carcinoma tissue. Acta Otolaryngol. 2007;127:532–9. doi: 10.1080/00016480600951384. [DOI] [PubMed] [Google Scholar]
- Lam-ubol A, Hopkin D, Letuchy EM, et al. Squamous carcinoma cells influence monocyte phenotype and suppress lipopolysaccharide-induced TNF-alpha in monocytes. Inflammation. 2010;33:207–23. doi: 10.1007/s10753-009-9175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederle W, Depner S, Schnur S, et al. IL-6 promotes malignant growth of skin SCCs by regulating a network of autocrine and paracrine cytokines. Int J Cancer. 2011;128:2803–14. doi: 10.1002/ijc.25621. [DOI] [PubMed] [Google Scholar]
- Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14:218–28. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- Lisa Cheng YS, Jordan L, Gorugantula LM, et al. Salivary interleukin-6 and -8 in patients with oral cancer and patients with chronic oral inflammatory diseases. J Periodontol. 2014;85:956–65. doi: 10.1902/jop.2013.130320. [DOI] [PubMed] [Google Scholar]
- Maccio A, Madeddu C. The role of interleukin-6 in the evolution of ovarian cancer: clinical and prognostic implications - a review. J Mol Med (Berl) 2013;91:1355–68. doi: 10.1007/s00109-013-1080-7. [DOI] [PubMed] [Google Scholar]
- Menetrier-Caux C, Montmain G, Dieu MC, et al. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–91. [PubMed] [Google Scholar]
- Ohishi W, Cologne JB, Fujiwara S, et al. Serum interleukin-6 associated with hepatocellular carcinoma risk: a nested case-control study. Int J Cancer. 2014;134:154–63. doi: 10.1002/ijc.28337. [DOI] [PubMed] [Google Scholar]
- Panneer Selvam N, Sadaksharam J. Salivary interleukin-6 in the detection of oral cancer and precancer. Asia Pac J Clin Oncol. 2015;11:236–41. doi: 10.1111/ajco.12330. [DOI] [PubMed] [Google Scholar]
- Rajkumar K, Dineshkumar T, Rajashree P, et al. Association of serum and salivary tumor necrosis factor-αwith histological grading in oral cancer and its role in differentiating premalignant and malignant oral disease. Asian Pac J Cancer Prev. 2014;15:7141–48. doi: 10.7314/apjcp.2014.15.17.7141. [DOI] [PubMed] [Google Scholar]
- Rhodus NL, Ho V, Miller CS, Myers S, Ondrey F. NF-kappaB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect Prev. 2005;29:42–5. doi: 10.1016/j.cdp.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227–36. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- Singh PK, Chandra G, Bogra J, et al. Association of interleukin-6 genetic polymorphisms with risk of OSCC in Indian population. Meta Gene. 2015;15:142–51. doi: 10.1016/j.mgene.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John MA, Li Y, Zhou X, Denny P, Ho CM, Montemagno C, et al. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:929–35. doi: 10.1001/archotol.130.8.929. [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Inoue K, Ogawa H, et al. The expression of IL-6 and its related genes in acute leukemia. Leuk Lymphoma. 1996;21:49–52. doi: 10.3109/10428199609067579. [DOI] [PubMed] [Google Scholar]
- Tripsianis G, Papadopoulou E, Anagnostopoulos K, et al. Coexpression of IL-6 and TNF-alpha: prognostic significance on breast cancer outcome. Neoplasma. 1996;61:205–12. doi: 10.4149/neo_2014_026. [DOI] [PubMed] [Google Scholar]
- Tsantoulis PK, Kastrinakis NG, Tourvas AD, Laskaris G, Gorgoulis VG. Advances in the biology of oral cancer. Oral Oncol. 2007;43:523–34. doi: 10.1016/j.oraloncology.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Van Tubergen E, Vander Broek R, Lee J, et al. Tristetraprolin regulates interleukin-6, which is correlated with tumor progression in patients with head and neck squamous cell carcinoma. Cancer. 2011;117:2677–89. doi: 10.1002/cncr.25859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VucicevićBoras V, Cikes N, Lukać J, Virag M, Cekić-Arambasin A. Salivary and serum interleukin 6 and basic fibroblast growth factor levels in patients with oral squamous cell carcinoma. Minerva Stomatol. 2005;54:569–73. [PubMed] [Google Scholar]
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–16. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]


