Abstract
Cancer, a worldwide epidemic disease with diverse origins, involves abnormal cell growth with the potential to invade other parts of the body. Globally, it is the main cause of mortality and morbidity. To overcome the drawbacks of the commercially available chemotherapies, natural products-loaded nano-composites are recommended to improve cancer targetability and decrease the harmful impact on normal cells. This study aimed at exploring the anti-cancer impacts of Moringa oleifera seed oil in its free- (MO) and nano-formulations (MOn) through studying whether it mechanistically promotes mitochondrial apoptosis-mediating cell death. Mitochondrial-based cytotoxicity and flow cytometric-based apoptosis analyses were performed on cancer HepG2, MCF7, HCT 116, and Caco-2 cell lines against normal kidney BHK-21 cell line. The present study resulted that MOn triggered colorectal cancer Caco-2 and HCT 116 cytotoxicity via mitochondrial dysfunction more powerful than its free counterpart (MO). On the other side, MOn and MO remarkably induces HCT 116 mitochondrial apoptosis, while sparing normal BHK-21 cells with minimal cytotoxic effect. The present results concluded that nano-micelle of Moringa oleifera seed oil (MOn) can provide a novel therapeutic approach for colorectal and breast cancers via mitochondrial-mediated apoptosis, while sparing normal and even liver cancer cells a bit healthy or with minimal harmful effect. Intriguingly, MOn induced breast cancer not hepatocellular carcinoma cell death.
Keywords: Moringa oleifera, seed oil, Nano-micelle, mitochondrial apoptosis, cancer
Introduction
Moringa oleifera, which is originally located in Asia then spread in many parts of Africa, is belongs to family Moringaceae (Anwar et al., 2007). This family contains around 13 species conveyed from tropical to subtropical districts and going in size from little herbs to huge trees (Janick and Paull, 2008). The most commonly known species globally is the Moringa oleifera Lam., otherwise called horseradish or drumstick tree (Awodele et al., 2012). This species is the most broadly developed of the Moringaceae and was used by the old Egyptians, Romans, and Greeks in botanical medicine. Customarily, all parts and products of Moringa oleifera are palatable, and have for quite some time been expended as vegetable and used to tackle numerous ailments, for example, cancers and liver disorders (Abd-Rabou, 2016; Rao and Mishra, 1998).
Moringa oleifera seeds contain 33-41% w/w vegetable oil (Rashid et al., 2008). This oil contains every single unsaturated fat contained in olive oil, aside from linolieic and was medicinally utilized due to its worthy substitute (Lalas and Tsaknis, 2002). Moreover, it is broadly utilized as a part of cooking, lighting, hairdressing; furthermore, cleanser and scent commercial enterprises (Ghazali and Mohammed, 2011). Intriguingly, it has been found to show antimicrobial and anticancer properties (Siddhuraju and Becker, 2003; Chuang et al., 2007).
Mechanistically wise, Moringa seeds contain a dimeric cationic proteins (13 kDa) naturally have coagulant and antimicrobial properties, as well as generally is used for decontamination and treatment of high turbid water (Anwar et al., 2007). The seeds of Moringa oleifera contain 19 to 47% substantial oils (Ahmad et al., 1989), which present a marvelous sort of nutritious supplements. The fat from the seeds is not only utilized as vegetable oil, for cooking which have imperviousness to oxidative debasement, but also it has a defensive activity against poisonous impacts (Sanchez-Machado et al., 2010).
Malignancy is a standout amongst the most unsafe ailments among nations. In 2012, 14.1 million growth cases have been accounted around the world, from which 52.5% men and 45.4% ladies (Sultana et al., 2014). These percentages are expected to be steadily increased by around 70% in 2035 (Ferlay et al., 2014). Colon growth, the third most regular tumor around the world, represents the fourth happening malignancy among all age bunches (Al-Kuraya et al., 2006). Liver malignancy is considered as the third reason for disease mortality around the world (Farooq et al., 2013). Among women, the most common site diagnosed for cancer was breast (De Martel et al., 2012; WHO, 2015).
However, chemotherapy regimens are currently available for cancer treatments (Huang et al., 2014), there are many physiological and psychological burdens. Thus, the new trend of utilizing phytochemical mixes has been an expanding pattern to use of conventional home grown medications as potential anticancer candidates (Abd-Rabou et al., 2012; Ahmed et al., 2015; Awodele et al., 2012). To increase the targetability of such phytochemical products, nanotechnology with potential applications in health and drug delivery for cancer therapy played a crucial role to solve this obstacle (Abd-Rabou, 2016; Park et al., 2008).
However, common cancer prevention agents; vitamin C, tocopherols, flavonoids, and other phenolic mixes are not introduced in certain Moringa species (Laandrault et al., 2001), Moringa oleifera is one such plant that has been recognized to contain normal cell reinforcements (Iqbal and Bhanger, 2006; Siddhuraju and Becker, 2003).
Eventually, Moringa oleifera has anticancer properties and antihepatotoxic (Asare et al., 2012). Besides, its cancer prevention agent action in vivo and in vitro, basically because of the vicinity of high measures of polyphenols (Fahey, 2005; Tatiana et al., 2013). Therefore, this study is aimed at exploring the mitochondrial apoptosis-mediating cytotoxic impact of nano-micelle of Moringa oleifera seed oil on different cancer cell lines in vitro.
Materials and Methods
Materials
The seed oil of Moringa oleifera was cordially obtained from the Egyptian Scientific Society of Moringa (ESSM), National Research Center, Dokki, Cairo, Egypt. Tween80, ethanol, dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from (Sigma-Aldrich, USA). Dulbecco’s modified Eagle’s medium (DMEM), Roswell Park Memorial Institute (RPMI-1640) medium, and Hanks’ Modified Eagle (Hanks’ MEM) medium were supported by (Lonza, USA). Fetal bovine serum (FBS), penicillin/streptomycin (P/S), phosphate buffered saline (PBS), L-glutamine, trypsin/EDTA, Annexin V were obtained from (Life Technologies, USA).
Preparation of Moringa oil-loaded nano-micelle (MOn)
Moringa oleifera seed oil in water (MO/W) liquid microemulsion was prepared by dissolving tween80 (surfactant) in ethanol (co-surfactant) at 2 : 1 weight ratio, respectively, followed by equilibration and titration into water. Therefore, the surfactant and co-surfactant were dissolved in water to make 5 wt% surfactant solution then 1 wt% MO was titrated into the water/surfactant solution followed by equilibration at room temperature till it gave a transparent clear nano-particulate until the system was transparent under vortexing. The monophasic nano-formulation was formed spontaneously using microemulsion method at room temperature according to (Kumar et al., 2011).
Malvern Zeta Sizer analysis
Particle size and zeta potential of the MOn was determined by photon correlation spectroscopy (PCS) using a Zeta Sizer (Nano ZS, Malvern Instruments, UK). For size measurements, 1 ml of the nano-micelle was measured for a minimum of 120 s. Similarly for zeta potential measurements, the samples were placed in an electrophoretic cell. Nano-micelle was maintained at a constant temperature of 25 °C.
Cell culture
Human breast cancer MCF7, colorectal cancer HCT 116 and Caco-2, hepatoma HepG2, and normal kidney BHK-21 cell lines were purchased from American Type Culture Collection (ATCC, USA) and cultured using Dulbecco’s modified Eagle’s medium (DMEM), Roswell Park Memorial Institute (RPMI-1640) medium, and Hanks’ Modified Eagle (Hanks’ MEM) medium, supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin (P/S), and 1% L-glutamine obtained from Life Technologies, Gibco (Grand Island, NY). Cells were cultured in 5% CO2 at 37°C and then treated with 0.25% (w/v) trypsin/EDTA to affect cell release from the culture flask. After washing the cells with phosphate buffered saline (PBS), cells were suspended in the media.
MTT-based mitochondrial function
The anti-cancer activities and the mitochondrial-based cytotoxicity (Van Meerloo et al., 2011) of Moringa seed oil (MO) and its nano-micelle (MOn) were evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay using HepG2, MCF7, HCT 116, Caco-2, and BHK-21 cells.
The principle of the MTT test is based on the measurement of the viability of cells via metabolic activity. Yellow water-soluble MTT is metabolically reduced in viable cells to a blue-violet insoluble formazan. The number of viable cells correlates to the color intensity determined by photometric measurements after dissolving the formazan in dimethyl sulfoxide (DMSO). Briefly, the cells were cultured in 96-well plates at a density of 1×104 cells/well. MO and MOn with different concentrations (0, 20, 40, 60, 80, 100 µg/mL) were added in the media over those cells. After a day incubation, MTT (Sigma) dissolved in PBS was added to each well at a final concentration of 5 mg/ml, and the samples were incubated at 37°C for 4 h. Water-insoluble dark blue formazan crystals that formed during MTT cleavage in actively metabolizing cells were then dissolved in DMSO. Absorbance was measured at 455 nm, using a microplate reader (Model 500; BIORed Instrument Inc., USA). The cell viability (%) was calculated and compared with the controls according to this equation.

Flow cytometric analysis
We selected HCT 116 cell line to study the underlying apoptotic mechanism, because we found that MOn had the highest cytotoxic effect upon this cancer type, scoring the lowest half maximal inhibitory concentration of cell growth (IC50= 49.1µg/mL). Accordingly, we used Annexin V to stain flipped phospholipids on the HCT 116 cell membranes. This approach was used in determination of apoptosis after treatment with the MO and MOn at 24 h of drug incubation. Annexin V kit was purchased from (ThermoFisher Scientific). We used the IC50s of the MO and MOn to treat HCT 116 cells, then apoptosis was measured and analyzed using flow cytometer (Beckman Coulter instrument, USA).
Statistical analysis
The results were presented as the mean of three independent experiments and the standard error (SE). One way analysis of variance (ANOVA) was used for the analysis of the test results at the significance level of p-value <0.05 (OriginPro 8 software). The IC50 was obtained using polynomial concentration–response curve fitting models (OriginPro 8 software).
Results
Nano-micelle characterization
It was observed that Moringa seed oil nano-micelle (MOn) has nano-size (85 nm; Figure 1a), polydispersity index (PDI= 0.1), nano-stability with accurate cumulants fit, raw correlation data, and phase plot as illustrated in (Figures 1b-d).
Figure 1.
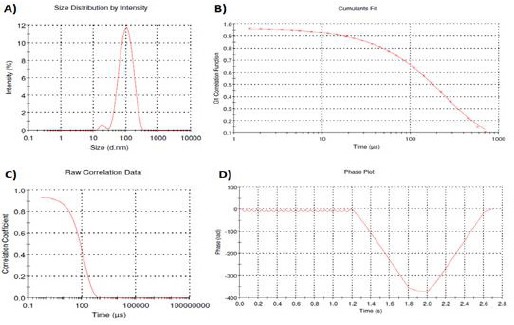
Characterization of Moringa Seed Oil Nano-Micelle (MOn). A) Size distribution of MOn in intensity was measured. Cumulants fit (B) is a simple method of analysing the autocorrelation function generated by a dynamic light scattering (DLS) experiment. The calculation is defined in ISO 13321 and ISO 22412 as it is a moment expansion that can produce a number of fitting values. Raw correlation curve (C) and phase plot (D) were also illustrated. The accuracy of the measured data in a DLS experiment is depdeing on the correlation curve which should be a smooth, single exponential decay function for a mono-size particle dispersion. Size= 85 nm and Polydispersity Index (PDI)= 0.1
MOn induces Caco-2 mitochondrial cell death
MOn and its free counterpart (MO) have been applied on Caco-2 cell line at different concentrations (0, 20, 40, 60, 80, 100 µg/mL) using MTT-based mitochondrial assay. The cytotoxicities of MOn were able to kill about 50% of the colon cancerous Caco-2 cells at 60 up to 100 µg/mL through mitochondrial dysfunction compared to its free counterpart which kill just around 40% of the cancer cells at the highest dosage used (100 µg/mL) as illustrated in (Figure 2).
Figure 2.
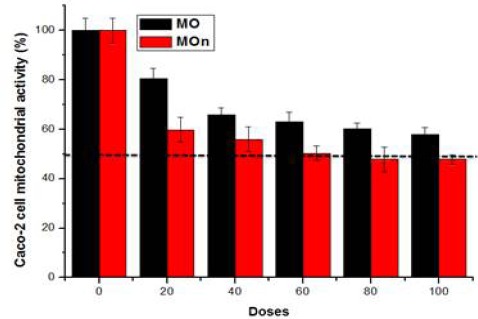
The Cytotoxic Effects of Moringa Seed Oil Nano-Micelle (MOn) and Its Free Counterpart on Caco-2 Colon Cancer Cell line. MOn significantly induces colon cancer Caco-2 cell death compared to Moringa seed oil itself. The results were presented as the mean of three independent experiments and the standard error (SE). There were statistically significant decreases when comparing MOn and MO concentrations with the control (P<0.05)
MOn remarkably induces HCT 116 mitochondrial apoptosis, sparing normal cells with minimal effect
Figure 3 shows that MOn and its free counterpart (MO) triggers mitochondrial dysfunction in colorectal HCT 116 cancer cells using MTT assay. MOn and its free counterpart (MO) have been applied on HCT 116 cell line at different concentrations (0, 20, 40, 60, 80, 100 µg/mL) using MTT-based mitochondrial assay. The cytotoxicities of MOn were able to reach the half maximal inhibitory concentration of cell growth (IC50) at 49.1 µg/mL, while there is undetectable IC50 in case of its free counterpart which kills less than 40% of the colon cancer cells at the highest dosage used (100 µg/mL) as illustrated in (Figure 3a). To study the underlying machinery of colon cancer HCT 116 cell death, MOn was tested up on HCT 116 cells using flow cytometry system. Annexin V-based apoptosis in HCT 116 cell line was tested up on treatment with 100 µg/mL MOn and MO against untreated control cells after 24 h incubation as illustrated in (Figure 3b). The underlying mechanism of MOn and MO are mainly dependent on triggering mitochondrial apoptosis (608.1% and 567.5%, respectively) compared to control (100%). Therefore, the flow cytometric analyses for apoptosis measurement using Annexin V showed a significant induction of apoptosis-mediated cell death by MO, especially the MOn compared to the untreated cells. On the other side, Figure 4 shows that MOn significantly lowers the cytotoxic effect by enhancing the mitochondrial function of MO itself when applied up on normal kidney BHK-21 cells, where there was an IC50= 85.9 µg/mL up on MO treatment and an undetectable IC50 up on MOn therapy, suggesting an improvement in the MO targetability after formulating it in a nano-micelle.
Figure 3.
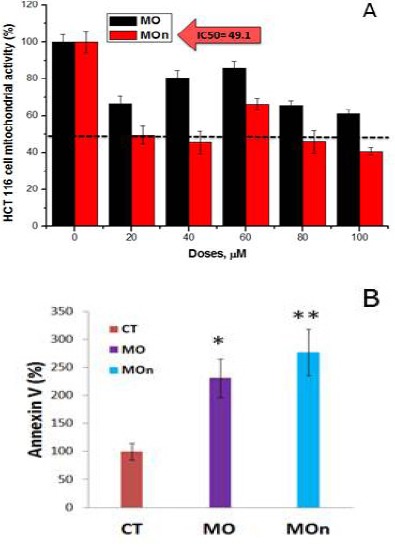
Moringa Seed Oil Nano-Micelle (MOn) Induces Mitochondrial-Mediated Apoptosis in HCT 116 Colon Cancer Cell Line. A) MOn and its free counterpart (MO) triggers mitochondrial dysfunction in HCT 116 cancer cells using MTT assay. B) Flow cytometric analyses for apoptosis measurement using Annexin V. To study the underlying mechanism of colon cancer HCT 116 cell death, MOn was tested upon those cells, showing a significant induction of apoptosis-mediated cell death compared to MO. The results were presented as the mean of three independent experiments and the standard error (SE). There were high statistically significant decreases when comparing MOn concentrations with the control (P<0.01) and statistically significant decreases when comparing MOn concentrations with the control (P<0.05)
Figure 4.
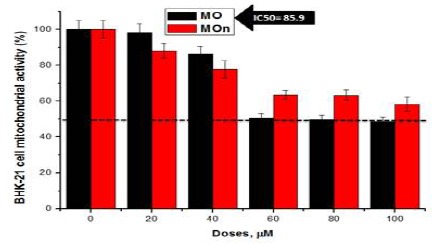
Nano-Formulation of MO (MOn) Significantly Lowers the Cytotoxic Effect by Enhancing the Mitochondrial Function of MO Itself when Applied up on Normal Kidney BHK-21 Cells. The results were presented as the mean of three independent experiments and the standard error (SE). There were statistically significant decreases when comparing MOn concentrations with the control (P<0.05)
MOn induce MCF7 not HepG2 cell death
Figs. 5 and 6 show the cytotoxic effects of MOn and MO on breast MCF7 and liver HepG2 cancer cell lines, respectively. It was reported that MOn had an IC50= 86.5 µg/mL compared to MO with an undetectable IC50 with over 70% cell viability at 100 µg/mL in case of MCF7 cells, while there were no IC50 detected in case of HepG2 cells up on both MOn and Mo treatments, reaching approximately 60% cell viabilities at 100 µg/mL.
Figure 5.
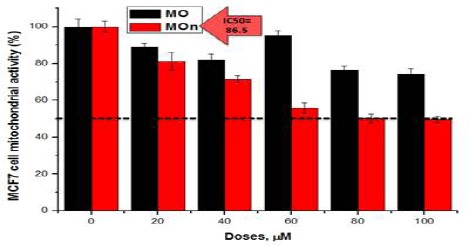
Moringa Seed Oil Nano-Micelle (MOn) Improves the Cytotoxic Effect of Its Free Counterpart on MCF7 Breast Cancer Cell Line. The results were presented as the mean of three independent experiments and the standard error (SE). There were statistically significant decreases when comparing MOn concentrations with the control (P<0.05)
Figure 6.

Moringa Seed Oil Nano-Micelle (MOn) and MO Itself Have the Same Slight Cytotoxic Effects Upon Liver Cancer HepG2 Cell Line. The results were presented as the mean of three independent experiments and the standard error (SE). There were statistically significant decreases when comparing MOn and MO concentrations with the control (P<0.05)
Figure 7.
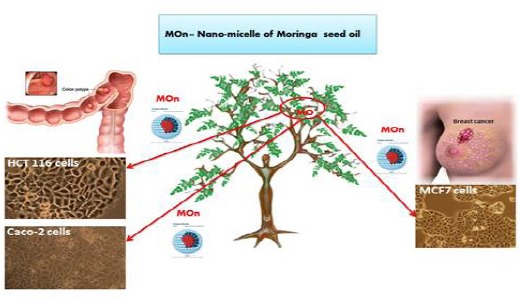
Schematic Diagram of the Impact of Moringa Seed Oil Nano-Micelle (MOn) on Different Cancer Cells. This diagram shows that MOn effectively kills MCF7, HCT 116, and Caco-2 not HepG2 cell lines compared to its free MO counterpart. While in the case of normal BHK-21 cells, the nano-form does not induce the MO cytotoxic effect and activates the mitochondrial activity
Discussion
In the present study, we found that nano-micelle of Moringa oleifera seed oil (MOn) can provide a novel therapeutic strategy for colorectal cancers (Caco-2 and HCT 116 cell lines) and breast cancer (MCF7 cell line) through mitochondrial-mediated apoptosis, while sparing normal BHK-21 cells and even liver cancer (HepG2 cell line) with minimal cytotoxic effect.
Normal restorative plants and their bioactive constituents have the pharmaceutical power to display their cytotoxic effects against malignant cells (Cragg and Newman, 2005). Moringa oleifera has been exceedingly used in medicine due to its nutritional advantages (Janick and Paull, 2008). In spite of the fact that Moringa oleifera has been generally explored for its anticancer feature, rare data can be found with respect to its cytotoxic action of its seed oil (MO) and no data regarding to its nano-composite (MOn).
In the current results on cancer HepG2, MCF7, HCT 116, and Caco-2 cell lines against normal kidney BHK-21 cell line, we not only applied the Moringa seed oil itself (MO) but also its nano-micelle (MOn). We observed that MOn induced colorectal cancer Caco-2 and HCT 116 cytotoxicity and cell death through mitochondrial dysfunction more powerful than its free counterpart (MO). On the other hand, MOn and MO remarkably induce HCT 116 mitochondrial apoptosis, while sparing normal BHK-21 cells with minimal effect. Some examinations came to support our results of Moringa oleifera seed oil (MO) by assessing the antiproliferative impact of MO on various cell lines including HeLa, HepG2, MCF7, Caco-2, and L929. They demonstrated that different doses of MO extraordinarily diminished the suitability of various cell lines relying up on the concentration of MO after 24 h of incubation. In addition, our set of data was supported by two other publications acquired some measurements of various convergences of MO on various cell lines, demonstrating that HeLa cells have been noticed to have the most noteworthy decline in their viability followed by MCF7, L929, and Caco-2 (Al-Oqail et al., 2013; Sharaf-Eldin et al., 2015). Intriguingly, our data showed that MOn was specific for breast cancer MCF7 cell death but not for hepatic cancer HepG2 cell death. On the contrary, HepG2 cells death was remarkably induced in previous studies (Al-Oqail et al., 2013; Sharaf-Eldin et al., 2015).
Mechanistically wise, the GC-MS investigation of the Moringa oleifera seed oil indicated that the most constituent was Naphthalene with approximately 35.65 %, which gives MO the capability to scavenge free radicals. Knowing that, 1.4 mg/ml MO is the best dose which shows the most scavengingforce of free radicals. This demonstrates that MO has a chelation property to provide a potential security against oxidative harm (Hussein et al., 2014).
Notwithstanding, Moringa seed yielded 42.23% oil content (Manzoor et al., 2007) which is impressively very higher than that reported for some normal seed oil crops; cotton (15.0–24.0%), soybean (17.0–21.0%), and somewhat practically identical with safflower (25.0-40.0%). Knowing that, the oils yielded from various Moringa genotypes in Egypt and other nations depend on developing conditions, topographical states of the areas, aging stage, collecting time of the seeds, and extraction techniques utilized (Pritchard, 1991). Consequently, this percentage of each yielded-oil reflects the anti-oxidant potential for scavenging free radicals.
On the other hand, the importance of unsaturated fat analogs present in Moringa oleifera against cancer has been sounded. Authors indicated that the oil acquired from Moringa oleifera seeds has dose dependent cytotoxic impacts, where it contains 49.1-50.9% unsaturated fats (Abdulkarim et al., 2005; Heo et al., 2014). Unsaturated fats have been accounted to show anticancer effect against numerous cancerous cells in vitro and in vivo cancer-induced models (Scheim, 2009). Mustafa et al., (2004) explored the anticancer potential of various unsaturated fat analogs, which showed cytotoxic impacts against some distinctive cancerous cell lines. Moreover, Ravichandran and Johnson (1999) proposed that the antitumor impact of the unsaturated fats in the Moringa seed oil is identified with the expanded lipid peroxidation, due to expanded creation of free radicals. The impact of various doses of seed oil on the morphology of cancer cell lines demonstrated a focus subordinate impact, where the cells began to lose their ordinary morphological structure with prompt cell separation and in turn programmed cell death. This evidence is in agreement with our observations suggested that MOn and its free counterpart (MO) triggers mitochondrial dysfunction in colorectal HCT 116 cancer cells and that apoptosis in those cells was elicited up on treatment with 100 µg/mL MOn and MO compared to untreated control cells after 24 h incubation. Thus, the underlying mechanism of MOn and MO are mainly dependent on triggering mitochondrial programed cell death; apoptosis, while sparing normal kidney BHK-21 cells with minimal effect. This discussion suggested an improvement in the MO targetability after formulating it in the nano-micelle (MOn).
Overall, nano-micelle of Moringa oleifera seed oil (MOn) can deliver a new therapeutic strategy for colorectal and breast cancers through mitochondrial-mediated apoptosis, while sparing normal BHK-21 cells with minimal cytotoxic effect. This will help us get closer to the day when cancers are no longer global health care obstacles.
Acknowledgement
We are thankful to the project “Recent approaches in the utilization of Moringa oleifera and Moringa peregrina as a good nutritional, medicinal and industrial plant in Egypt” (ID. 5979), Science and Technology Development Fund (STDF) Academy of Scientific Research of the Ministry of Scientific Research, for partial financial support.
References
- Abd-Rabou AA, Zoheir K, Ahmed HH. Potential impact of curcumin and taurine on human hepatoma cells using huh-7 cell line. Clin Biochem. 2012;45:1519–21. doi: 10.1016/j.clinbiochem.2012.06.032. [DOI] [PubMed] [Google Scholar]
- Abd-Rabou AA. Calcium, a cell cycle commander, drives colon cancer cell diffpoptosis. Ind J Clin Biochem. 2016:1–10. doi: 10.1007/s12291-016-0562-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulkarim SM, Lai OM, Muhammad SKS, Long K, Ghazali HM. Some physic-chemical properties of Moringa oleifera seed oil extracted using solvent and aqueous enzymatic methods. Food Chem. 2005;93:253–63. [Google Scholar]
- Ahmad MB, Rauf A, Osman MJ. Oil technologies’ Association of India. 1989;3:46–7. [Google Scholar]
- Ahmed HH, Abd-Rabou AA, Hassan AZ, Kotob SE. Phytochemical analysis and anti-cancer investigation of boswellia serrata bioactive constituents in vitro. Asian Pac J Cancer Prev. 2015;16:7179–88. doi: 10.7314/apjcp.2015.16.16.7179. [DOI] [PubMed] [Google Scholar]
- Al-Kuraya KS, Bavi PP, Ezzat AA. Colorectal carcinoma from Saudi Arabia: Analysis of MLH-1, MSH- 2 and p53 genes by immunohistochemistry and tissue microarray analysis. Saudi Med J. 2006;27:323–8. [PubMed] [Google Scholar]
- Al-Oqail MM, Farshori NN, Al-Sheddi ES. In vitro cytotoxic activity of seed oil of fenugreek against various cancer cell cines. Asian Pac J Cancer Prev. 2013;14:1829–32. doi: 10.7314/apjcp.2013.14.3.1829. [DOI] [PubMed] [Google Scholar]
- Anwar F, Latif S, Ashrat M. M. oleifera: A food plant with multiple medicinal uses. Phytother Res. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- Asare GA, Gyan B, Bugyei K, Adjei S. Toxicity potentials of the nutraceutical Moringa oleifera at supra-supplementation levels. J Ethnopharmacol. 2012;139:265–72. doi: 10.1016/j.jep.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Awodele O, Oreagbaa IA, Odomaa S, Teixeira da Silva JA, Osunkaluc VO. Toxicological evaluation of the aqueous leaf extract of Moringa oleifera Lam. (Moringaceae) J Ethnopharmacol. 2012;139:330–6. doi: 10.1016/j.jep.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Chuang PH, Lee CW, Chou JC. Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresour Technol. 2007;98:232–6. doi: 10.1016/j.biortech.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Cragg GM, Newman DJ. Plant as source of anticancer agents. J Ethnopharmacol. 2005;100:72–9. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- De Martel C, Ferlay J, Franceschi S. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lanc Oncol. 2012;13:607–15. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- Fahey JW. Moringa oleifera: A Review of the Medical Evidence for Its Nutritional, Therapeutic, and Prophylactic Properties. Part 1. Trees for Life J. 2005;1:1–33. [Google Scholar]
- Farooq M, Hozzein WN, Elsayed EA. Identification of histone deacetylase I protein complexes in liver cancer cells. Asian Pac J Cancer Prev. 2013;14:915–21. doi: 10.7314/apjcp.2013.14.2.915. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN. Inter J Cancer. 2014;136:359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Ghazali HM, Mohammed AS. Moringa (Moringa oleifera) seed oil: composition, nutritional aspects and health attributes. In: Preedy V, Watson R, Patel V, editors. ‘nuts & seeds in health and disease prevention’. USA: Elsevier Academic Press; 2011. pp. 787–93. [Google Scholar]
- Heo BG, Park YJ, Park YS. Anticancer and antioxidant effects of extracts from different parts of indigo plant. Indust Crops Products. 2014;56:9–16. [Google Scholar]
- Huang YJ, Zhang YY, Liu G. Preliminary evaluation of the in vitro efficacy of 1, 2-di(quinazolin-4- yl) diselane against SiHa cervical cancer cells. Asian Pac J Cancer Prev. 2014;15:6301–6. doi: 10.7314/apjcp.2014.15.15.6301. [DOI] [PubMed] [Google Scholar]
- Hussein MA, Gobba NA, El Bishbishy MH. Composition, in vitro antioxidant and antitumor properties of essential oil from the seeds of Moringa oleifera. Inter J Pharm Scien. 2014;4:532–40. [Google Scholar]
- Iqbal S, Bhanger MI. Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. J Food Comp Anal. 2006;19:544–51. [Google Scholar]
- Janick J, Paull RE. The encyclopedia of fruit and nuts. Cambridge, UK: CABI; 2008. pp. 509–10. [Google Scholar]
- Kumar B, Jain SK, Prajapati SK. Effect of penetration enhancer DMSO on in-vitro skin permeation of acyclovir transdermal microemulsion formulation. Inter J Drug Deliv. 2011:83–94. [Google Scholar]
- Laandrault N, Pouchert P, Ravel P. Antioxidant activities and phenolic level of French wines from different varieties and vintages. J Agric Food Chem. 2001;49:3341–3. doi: 10.1021/jf010128f. [DOI] [PubMed] [Google Scholar]
- Lalas S, Tsaknis J. Extraction and identification of natural Antioxidant from the seeds of the Moringa oleifera tree variety of Malaw. J Am Oil Chem Soc. 2002;79:677–83. [Google Scholar]
- Manzoor M, Anwar F, Iqball T, Bhanger MI. Physico-chemical characterization of Moringa concanensis seeds and seed oil. J Am Oil Chem Soc. 2007;84:413–9. [Google Scholar]
- Mustafa J, Khan SI, Ma G, Walker LS, Khana IA. Synthesis and anticancer activities of fatty acid analogs of podophyllotoxin. Lipids. 2004;39:167–72. doi: 10.1007/s11745-004-1215-5. [DOI] [PubMed] [Google Scholar]
- Park JH, Lee S, Kim JH. Polymeric nanomedicine for cancer therapy. Prog Polym Sci. 2008;33:113–37. [Google Scholar]
- Pritchard JLR. Analysis and properties of oilseeds. In: Rossell J.B, Pritchard J.L.R, editors. Analysis of Oilseeds Fats and Fatty Foods. London, New York: Elsevier Applied science; 1991. pp. 80–98. 127. [Google Scholar]
- Rao KS, Mishra SH. Antiinflammatory and hepatoprotective activities of fruits of Moriga pterygosperma gaertn. Ind J Nat Prod. 1998;14:23–6. [Google Scholar]
- Rashid U, Anwar F, Moser BR, Knothe G. Moringa oleifera: A possible source for biodiesel. Bioresour Technol. 2008;99:8175–9. doi: 10.1016/j.biortech.2008.03.066. [DOI] [PubMed] [Google Scholar]
- Ravichandran D, Johnson CD. Anticancer effects of essential fatty acids. In: Johnson CD, editor. ‘pancreatic disease’. London: Springer-Verlag; 1999. pp. 325–37. [Google Scholar]
- Sanchez-Machado DI, Nuñez JA, Reyes MC. Nutritional quality of edible parts of Moringa oleifera. Food Anal Methods. 2010;3:175–80. [Google Scholar]
- Scheim DE. Cytotoxicity of unsaturated fatty acids in fresh human tumor explants: concentration thresholds and implications for clinical efficacy. Lipids Health Dis. 2009;8:54–9. doi: 10.1186/1476-511X-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharaf-Eldin E, Asian M. In vitro Evaluation of Cytotoxic Activities of Essential Oil from Moringa oleifera Seeds on HeLa, HepG2, MCF-7, CACO-2 and L929 Cell Lines. Asian Pac J Cancer Prev. 2015;16:4671–5. doi: 10.7314/apjcp.2015.16.11.4671. [DOI] [PubMed] [Google Scholar]
- Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem. 2003;51:2144–55. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Sultana S, Asif HM, Nazar HMI. Medicinal plants combating against cancer-a green anticancer approach. Asian Pac J Cancer Prev. 2014;15:4385–94. doi: 10.7314/apjcp.2014.15.11.4385. [DOI] [PubMed] [Google Scholar]
- Tatiana M, Filomena N, Emilia M, Florinda F. Chemical omposition and Biological Activity of the Essential Oil from Leaves of Moringa oleifera Lam. Cultivated in Mozambique. Molecules. 2013;18:10989–1000. doi: 10.3390/molecules180910989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol Biol. 2011;731:237–45. doi: 10.1007/978-1-61779-080-5_20. [DOI] [PubMed] [Google Scholar]
- WHO Cancer Updated February. Fact sheet N°297. 2015. http://www.who.int/mediacentre/factsheets/fs297/en/


