Abstract
Background:
An upward trend has been noted for the incidence of prostate cancer (PCa) in Vietnam, but information is limited on modifiable factors associated with this form of cancer. This case-control study was conducted to ascertain any relationship between habitual tea consumption and PCa risk.
Materials and Methods:
Two hundred and fifty-three incident patients with histologically confirmed PCa and 419 (340 community-based and 79 hospital-based) controls, matched by age, were recruited in Ho Chi Minh City during 2013-2015. Information on frequency, quantity and duration of tea consumption, together with demographics, habitual diet and lifestyle characteristics, was obtained by direct interviews using a validated questionnaire. Logistic regression analyses were performed to assess associations between tea consumption variables and PCa risk.
Results:
The control subjects reported higher tea consumption levels in terms of cumulative exposure, frequency and quantity of tea drank than the PCa patients. After accounting for confounding factors, increasing tea consumption was found to be associated with reduced risk of PCa. The adjusted odds ratios (95% confidence intervals) were 0.52 (95% CI 0.35-0.79) and 0.30 (95% CI 0.18-0.48) for participants drinking 100-500 ml/day and > 500 ml/day, respectively, relative to those drinking < 100 ml/day. Significant inverse dose-response relationships were also observed for years of drinking and number of cups consumed daily (P <0.01).
Conclusion:
Habitual tea consumption is associated with a reduced risk of PCa in Vietnamese men.
Keywords: Case-control study, epidemiological, prostate cancer, tea drinking, Vietnam
Introduction
Globally, prostate cancer (PCa) is the second most common malignancy in men, with about one million incident cases and 300,000 deaths estimated in 2012 (Ferlay et al., 2015). It is the sixth leading cause of cancer-related deaths and imposes a large economic burden, notably in western countries (Roehrborn and Black, 2011). This cancer is also of concern in Asia, accounting for 18% and 27% of the world’s new cases and associated mortality, respectively (International Agency for Research on Cancer/World Health Organization, 2012). Given a high probability of survival for localised PCa (99% for a 5-year relative survival rate) (Siegel et al., 2016) and increasing costs of treatment and management of advanced PCa (Roehrborn and Black, 2011), it is important to develop appropriate measures for prevention.
Nutrition and diet were shown to inhibit PCa initiation and progression (Gathirua-Mwangi and Zhang, 2014; Patel, 2014), and many focus on the role of antioxidant-rich foods (Vance et al., 2013). Tea, particularly green tea, is widely consumed in Asia and it contains many bioactive compounds including catechins and polyphenols (Higdon and Frei, 2003), which have been demonstrated to suppress tumor promotion, induce tumor apoptosis and inhibit the growth of PCa cells in in vitro and in vivo (Butt et al., 2015). However, the available epidemiological evidence of its protective effects remains inconsistent (Lee et al., 2006; Zheng et al., 2011). Pooled data from a recent meta-analysis indicated a lack of association (Zhang et al., 2015), whereas two individual studies reported a positive association between black tea and PCa (Montague et al., 2012; Shafique et al., 2012). Similar to observational studies, clinical trials have yielded mixed results (Yuan, 2013). In view of the different types of tea and variations in consumption pattern between countries, further epidemiological studies are needed to elucidate the effect of tea on the PCa risk.
Vietnam, the fifth largest tea producer in the world, has a low incidence of PCa but trend is increasing over the last decade (Van Dong et al., 2014). Since the prostate-specific antigen (PSA) testing for screening for PCa is not a common practice in Vietnam, the disease is typically diagnosed at its advanced stage. Moreover, despite the popularity of tea, especially green tea, no epidemiological study of tea and PCa has been conducted in Vietnam. It is of interest whether the high consumption of tea can contribute to the low incidence of PCa in this country. Therefore, the present case-control study aimed to investigate whether habitual tea consumption has an etiological association with the risk of PCa among Vietnamese men. We hypothesize that regular tea consumption reduces PCa risk.
Materials and Methods
Study design and recruitment
This study was reported according to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) recommendations (von Elm et al., 2007). A case-control study was conducted in Ho Chi Minh City, the largest city in southern Vietnam, between January 2013 and July 2015. Cases were patients aged 64-75 years with incident, histologically confirmed PCa, who were admitted to the urology department of two hospitals in the metropolitan area. Controls were men either attending the same hospitals for treatment or residing in the same catchment area as the cases. Controls were frequency-matched to cases by 5-year age groups, using a ratio of 1.5 control subjects per case.
Potential cases were identified through histological examination of their prostate gland. All cases underwent the biopsy for the first time, and did not have any history of malignancy or severe chronic diseases, such as diabetes and stroke. These men were briefed about the study procedure and invited for an interview upon confirmation of a positive biopsy result. Eligible cases were those with a primary and final diagnosis of PCa. Of the 272 confirmed cases, 19 declined to participate, and 253 PCa patients subsequently undertook the interview within one week of diagnosis.
During the same period, community-based controls were recruited through the assistance of local commune health centers in Ho Chi Minh City. Of the 700 generally healthy male residents contacted, 429 men agreed to participate in the study. After initial screening, 62 men were excluded due to (1) malignant or severe chronic diseases; (2) refusal to undertake a PSA test; or (3) their serum PSA level exceeded 4 ng/ml. Finally, 367 eligible men consented to attend the interview, giving a response rate of 52.4%. For hospital-based controls, 120 patients who resided in the same catchment area as cases and attended the same hospitals for minor treatment, were approached after referral by their medical doctors and upon satisfying the selection criteria. A total of 83 patients consented to take part in the interview (response rate 69.1%). We further excluded 27 community-based controls and 4 hospital-based controls due to illogical or missing information, so that 419 (340 community-based and 79 hospital-based) controls, frequency matched to cases by age, were eventually available for analysis.
Interview
Informed written consent was obtained from each participant prior to the interview, which took about 40 minutes to complete. Whenever possible, the interview was conducted in a private room at the hospital or commune health center with the presence of the participant’s next-of-kin to maximize the accuracy of the information. Both interviewers and participants were blinded to the study hypothesis. Five interviewers, who followed a standardized protocol for the interview process, were experienced research assistants trained by the investigator. This study was approved by the Human Research Ethics Committee of Curtin University (approval number: HR 109/2012). Recruitment and access to medical records were permitted by the participating hospitals and local commune health centers.
Questionnaire and exposure measurements
A structured questionnaire was administered to obtain information on demographic and lifestyle characteristics (e.g. age, marital status, education level and smoking), medical history (including histological examination, PSA level and anthropometry) and dietary habits, via personal interview and medical records retrieval. Dietary intakes by the participants were assessed using a validated and reliable food frequency questionnaire specifically developed for Vietnamese older adults (Tran et al., 2013). It consisted of 109 common food and beverage items, soliciting detailed information on frequency and amount of intake. The recall period for dietary habits was set to three years before the interview. A picture booklet was shown to participants to assist their estimation of intake amount and portion size of certain food items. Their corresponding energy contents were taken from the Vietnamese Food Composition Tables (National Institute of Nutrition, 2007), and total energy intake (kcal) was calculated by summing energy intakes across individual food and beverage items consumed. In relation to tea drinking, participants reported the frequency (times per day, week, month or year) and quantity per session (number of cups), together with the type of tea consumed (green tea, black tea or oolong tea) and duration of drinking (years). Any change in tea drinking habit was also recorded.
Sample size calculation
We assumed that about 70% of Vietnamese middle-aged and older men without prostate cancer would drink tea on a daily basis (Nguyen et al., 2016) and we needed to achieve 80% power to detect an expected odds ratio of 0.6 (Zheng et al., 2011) for highest versus non/lowest tea consumption in relation to the PCa risk with 5% level of significance. For a 1:2 case-control ratio, a sample of at least 196 cases and 392 controls was required, using the “power mcc” command in Stata (StataCorp, 2015).
Statistical analysis
Characteristics between groups, especially habitual tea consumption levels, were compared using chi-square, two-sample t-test and Wilcoxon rank-sum test. Since there were no differences in demographic and lifestyle characteristics and tea consumption variables between community- and hospital-based controls, the two control groups were combined in subsequent analysis. To ascertain the association between tea drinking exposure and PCa risk, separate unconditional logistic regression analyses were performed for quantity of tea drank (< 100, 100-500, or > 500 ml per day), drinking frequency (< 1, 1-5, or > 5 cups per day), duration of drinking (< 10, 10-30, or > 30 years) and cumulative consumption (< 20, 20-60, or > 60 cup-years), with the respective lowest level being taken as the reference category. Both crude and adjusted odds ratios (OR) and associated 95% confidence intervals (CI) were presented, and tests for linear trend were conducted to assess the dose-response relationship. However, analysis by tea type was not undertaken because the majority of tea drinkers regularly drank a combination of green, black and oolong teas.
Besides tea consumption variables, independent factors included in the logistic regression models were age at interview (years), age at marriage (years), body mass index (kg/m2), alcohol consumption (g/day), total energy intake (kcal/day), education level (primary, high school, tertiary), marital status (never married or separated, married), cigarette smoking (never, former, current), lifetime physical activity (never active, active) and first-degree family history of PCa (yes, no). These variables were either established or plausible risk factors according to the literature. Additionally, subgroup analyses were conducted with respect to localised (Gleason score ≤7) and advanced (Gleason score 8-10) PCa (Humphrey, 2004). All statistical analyses were performed using Stata 14.0 (StataCorp, 2015). A P-value <0.05 was considered statistically significant.
Results
Table 1 presents characteristics of the participants by case-control status. Participants were about 69 years of age on average, but the PCa patients were married at a younger age, drank more alcohol and had significantly less energy intake before diagnosis than the controls. The two groups were also different in terms of educational level and lifetime physical activity, with the cases being less educated and active than their control counterparts. First-degree family history of PCa was reported for cases only.
Table 1.
Characteristics of Participants by Case-Control Status
| Characteristics | Cases (n = 253) | Controls (n = 419) | P value† |
|---|---|---|---|
| Age at interview (years): mean ± SD | 69.2 ± 7.6 | 68.3 ± 5.9 | 0.08 |
| Age at marriage (years): mean ± SD | 24.9 ± 4.6 | 27.3 ± 4.9 | < 0.01 |
| Body mass index (kg/m2): mean ± SD | 22.0 ± 3.0 | 21.9 ± 3.3 | 0.81 |
| Alcohol consumption (g/day): median (IQR) | 7.1 (40.9) | 1.9 (33.8) | 0.01 |
| Total energy intake (kcal/day): median (IQR) | 1,519 (113) | 1837 (875) | < 0.01 |
| Education level: n (%) | < 0.01 | ||
| Primary | 72 (28.4) | 74 (17.7) | |
| High school | 135 (53.4) | 270 (64.4) | |
| Tertiary | 46 (18.2) | 75 (17.9) | |
| Marital status: n (%) | 0.05 | ||
| Never married or separated | 11 (4.3) | 35 (8.4) | |
| Married | 242 (95.7) | 384 (91.6) | |
| Smoking habit: n (%) | 0.14 | ||
| Never | 61 (24.1) | 114 (27.2) | |
| Former | 126 (49.8) | 176 (42.0) | |
| Current | 66 (26.1) | 129 (30.8) | |
| Lifetime physical activity: n (%) | < 0.01 | ||
| Never active | 208 (82.2) | 199 (47.5) | |
| Active | 45 (17.8) | 220 (52.5) | |
| First-degree family history of prostate cancer: n (%) | 7 (2.8) | 0 (0.0) |
SD, standard deviation; IQR, interquartile range;
Based on chi-square; t-test or Wilcoxon rank-sum test between case and control groups.
Table 2 compares the tea consumption patterns between case and control groups. Although the prevalence of tea drinking was similar between the two groups, it appears that among the tea drinkers, the control participants drank twice more tea on average (both quantity and frequency), while their cumulative tea consumption (cup-years) was at least double that of the cases.
Table 2.
Comparison of Tea Consumption Levels among Tea Drinkers between Case and Control Groups
| Tea consumption variables | Cases (n = 212) Mean ± SD | Controls (n = 361) Mean ± SD | P value† |
|---|---|---|---|
| Tea drinking prevalence (%) | 83.8 | 86.2 | 0.42 |
| Quantity of tea drank (ml per day) | 192.0± 249.0 | 400.0 ± 443.0 | < 0.01 |
| Frequency of tea drinking (cups per day) | 2.4 ± 3.1 | 5.0 ± 5.5 | < 0.01 |
| Duration of tea drinking (years) | 25.3 ± 16.8 | 27.3 ± 18.9 | 0.2 |
| Cumulative tea consumption (cup-years) | 59.8 ± 101.9 | 135.7 ± 193.2 | < 0.01 |
SD, standard deviation; †Based on chi-square or t-test between case and control groups
Figure 1 summarises the results of logistic regression analyses. It is evident that increasing the level of habitual tea consumption was significantly associated with a lower risk of PCa. Compared to men drinking < 100 ml/day, the adjusted OR (95% CI) for PCa among those drinking 100-500 ml/day and > 500 ml/day were 0.52 (95% CI 0.35-0.79) and 0.30 (95% CI 0.18-0.48), respectively. Similar reductions in PCa risk were also found for drinking 1-5 cups and more than 5 cups of tea daily. Furthermore, inverse dose-response relationships were observed for drinking duration and cumulative consumption, with significant risk reductions for long-term tea drinking over 30 years or beyond 60 cup-years. Such inverse associations persisted regardless of the grade of PCa, when comparing the highest versus the lowest level of tea consumption variables, with the exception of drinking duration (Supplemental Table).
Figure 1.
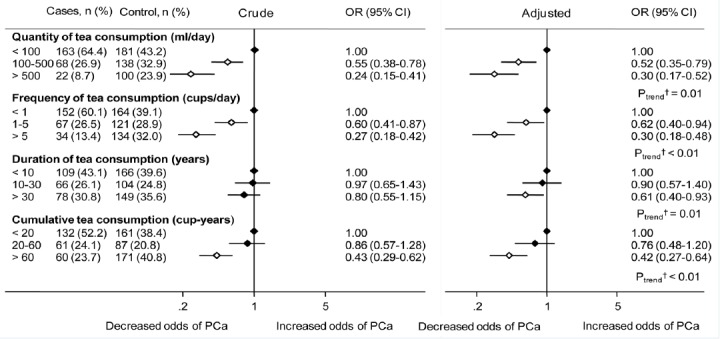
Crude and Adjusted Odds Ratios of Prostate Cancer by Metrics of Tea Consumption.
The diamonds indicate the point estimates and the bars represent the corresponding 95% confidence interval. Unfilled diamonds denote statistical significance relative to the lowest category of tea consumption. Variables adjusted for in the logistic regression analyses were age at interview (years), age at marriage (years), body mass index (kg/m2), alcohol consumption (g/day), total energy intake (kcal/day), education level (primary, high school, tertiary), marital status (never married or separated, married), smoking habit (never, former, current), lifetime physical activity (never active, active), and first-degree family history of prostate cancer (yes, no). †Based on the unconditional logistic regression model, treating exposures as continuous variables CI, confidence interval; OR, odds ratio; PCa, prostate cancer.
Discussion
The present case-control study was the first epidemiological investigation of habitual tea consumption in relation to the risk of PCa in Vietnam. Our finding of an inverse association between tea consumption level and PCa risk is consistent with previous observational studies in other countries (Jain et al., 1998; Jian et al., 2004; Kurahashi et al., 2008; Geybels et al., 2013; Fei et al., 2014). Therefore, the present study provides further evidence to support the protective effect of habitual tea drinking against the development of PCa.
On the other hand, five meta-analyses (Zheng et al., 2011; Fei et al., 2014; Lin et al., 2014; Yu et al., 2014; Zhang et al., 2015) conducted so far remained inconclusive, with the inverse association between tea drinking and PCa risk being observed among case-control studies (Zheng et al., 2011; Fei et al., 2014; Lin et al., 2014) but not from cohort studies (Zheng et al., 2011; Fei et al., 2014; Lin et al., 2014; Yu et al., 2014; Zhang et al., 2015). The lack of association in the latter may be partly due to their reliance on baseline assessment of tea drinking, despite the tea consumption level and habit can change over the life course. Alternatively, tea drinking assessed prospectively can be non-differentially misclassified, leading to a dilution of statistical association. The discrepancy between our result and some previous case-control studies may be attributed to the differences in tea types, preparation methods and drinking habits between cultures. Green tea is the most popular type of tea drank by Vietnamese men. It contains a much higher level of catechins than black tea (Astill et al., 2001; Higdon and Frei, 2003), particularly Epigallocatechin-3-gallate (EGCG), which has been shown to inhibit tumor growth and encourage apoptosis of the PCa cells in mice (Lee et al., 2008). Another possibility is the different methods of manufacture, processing and strength of tea brewed, which can affect the composition of tea infusion (Astill et al., 2001). It is conceivable that Vietnamese men typically drink hot and strong green tea brewed directly from dried tea leaves without adding sugar or milk may increase the bioavailability and antioxidant activity of tea polyphenols.
PCa has a relatively long latency period (Etzioni et al., 1998), so that it may take years of tea drinking for effective disease prevention. In fact, we found significantly lower odds of PCa among individuals who drank tea regularly for over 30 years or exceeding 60 cup-years, when compared to others with less than 10 years or 20 cup-years of drinking history, respectively. But few studies have considered the effect of cumulative exposure to tea drinking (Jian et al., 2004). Our results suggest that, besides the intensity of tea drinking, a long duration of tea consumption may be associated with a lower risk of PCa in later life.
There are plausible biological mechanisms underlying the beneficial effect of habitual tea consumption. Increased oxidative stress, inflammation and androgens have been implicated in the development of prostate tumorigenesis (Taichman et al., 2007; Hyde et al., 2012). Tea, especially green tea, is a rich source of antioxidants (Liao et al., 2001), which have been found to inhibit the initiation and progression of PCa cells (Wang et al., 2012) as well as to decrease PSA levels (Kumar et al., 2015). EGCG, the predominant component of tea polyphenols, is also known to downregulate pro-inflammatory pathway, the insulin like growth factor axis and multiple kinases (Peairs et al., 2010). In vitro experiments suggested that EGCG can deprive androgen receptor activity (Siddiqui et al., 2011). In addition, EGCG has been demonstrated to suppress tumor promotion by blocking signal transduction and oncogene expression, inducing apoptosis and cell cycle arrest, and neutralising free radicals (Johnson et al., 2010; Lee et al., 2012).
Several limitations of the present study deserve attention. First, a cause-effect relationship between tea drinking and risk of PCa cannot be established due to the retrospective cross-sectional design. Second, there are inherent biases from this observational study. Selection bias could not be avoided as participants were voluntary and not randomly selected from the population. Information bias, however, was unlikely because all participants were unaware of the study hypothesis, while the beneficial role of tea drinking against PCa has not been documented in Vietnam. Recall bias may occur if cases recalled their history of tea drinking differently from the controls. To minimise the bias and to improve the accuracy of information obtained, we employed the same well-trained interviewers to conduct direct interviews of both case and control groups using an identical protocol under similar conditions. Information about the habit of tea drinking was also sought from the participant’s next-of-kin. Moreover, a consistent inverse association with the PCa risk was evident for the various measures of tea consumption. Although all selected controls had a PSA level ≤4 ng/ml, misclassification of their case-control status would still be possible. Indeed, the resulting association should have been weakened given the low incidence of PCa in Vietnam (International Agency for Research on Cancer/World Health Organization, 2012). Third, the effects of different tea types (green, black and oolong) could not be distinguished because of the mixed drinking habits of the tea drinkers. Finally, despite all participants were recruited from the same catchment area within Ho Chi Minh City, our findings still cannot be generalisable to the entire Vietnamese population.
In conclusion, the present case-control study suggests that habitual tea consumption can lower the risk of PCa among Vietnamese men, with significant inverse dose-response observed by increasing the quantity, frequency and duration of tea drinking. Our findings add further epidemiological evidence on the benefits of regular tea consumption, especially over the long term, for potential prevention and control of this emerging chronic disease in Vietnam. However, replications of the present study in other locations and large-scale clinical trials are required to confirm the findings.
Authorship responsibility
V.D.H., principal investigator, was responsible for conceptual design, study development and implementation, as well as drafting the manuscript. A.H.L., assisted with statistical analysis and revised the manuscript. N.M.P., was involved in the discussion of results and manuscript revision. DX contributed to the study design, methodology and ethical clearance of the project. C.W.B., was the project supervisor and provided advice on all aspects of the study. All authors have read and approved the final version of the manuscript for publication.
Acknowledgments
The authors are indebted to the study participants who agreed to be interviewed. Thanks are also due to the medical and nursing staff of the participating hospitals for their assistance in patient recruitment. The research was financially supported by the researchers’ institution. The first author gratefully acknowledges the PhD scholarship from Curtin University to conduct this study.
References
- Astill C, Birch MR, Dacombe C, et al. Factors affecting the caffeine and polyphenol contents of black and green tea infusions. J Agric Food Chem. 2001;49:5340–7. doi: 10.1021/jf010759+. [DOI] [PubMed] [Google Scholar]
- Butt MS, Ahmad RS, Sultan MT, et al. Green tea and anticancer perspectives: updates from last decade. Crit Rev Food Sci Nutr. 2015;55:792–805. doi: 10.1080/10408398.2012.680205. [DOI] [PubMed] [Google Scholar]
- Etzioni R, Cha R, Feuer EJ, et al. Asymptomatic incidence and duration of prostate cancer. Am J Epidemiol. 1998;148:775–85. doi: 10.1093/oxfordjournals.aje.a009698. [DOI] [PubMed] [Google Scholar]
- Fei X, Shen Y, Li X, et al. The association of tea consumption and the risk and progression of prostate cancer: a meta-analysis. Int J Clin Exp Med. 2014;7:3881–91. [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Gathirua-Mwangi WG, Zhang J. Dietary factors and risk for advanced prostate cancer. Eur J Cancer Prev. 2014;23:96–109. doi: 10.1097/CEJ.0b013e3283647394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geybels MS, Neuhouser ML, Stanford JL. Associations of tea and coffee consumption with prostate cancer risk. Cancer Causes Control. 2013;24:941–8. doi: 10.1007/s10552-013-0170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- Humphrey PA. Gleason grading and prognostic factors in carcinoma of the prostate. Mod Pathol. 2004;17:292–306. doi: 10.1038/modpathol.3800054. [DOI] [PubMed] [Google Scholar]
- Hyde Z, Flicker L, McCaul KA, et al. Associations between testosterone levels and incident prostate, lung, and colorectal cancer. A population-based study. Cancer Epidemiol Biomarkers Prev. 2012;21:1319–29. doi: 10.1158/1055-9965.EPI-12-0129. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer/World Health Organization. 2012. GLOBOCAN. 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012 [Online] [Accessed 15 April 2016]. Available: http://globocan.iarc.fr/
- Jain MG, Hislop GT, Howe GR, et al. Alcohol and other beverage use and prostate cancer risk among Canadian men. Int J Cancer. 1998;78:707–11. doi: 10.1002/(sici)1097-0215(19981209)78:6<707::aid-ijc7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Jian L, Xie LP, Lee AH, et al. Protective effect of green tea against prostate cancer: A case-control study in southeast China. Int J Cancer. 2004;108:130–5. doi: 10.1002/ijc.11550. [DOI] [PubMed] [Google Scholar]
- Johnson JJ, Bailey HH, Mukhtar H. Green tea polyphenols for prostate cancer chemoprevention: a translational perspective. Phytomedicine. 2010;17:3–13. doi: 10.1016/j.phymed.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NB, Pow-Sang J, Egan KM, et al. Randomized, Placebo-Controlled Trial of Green Tea Catechins for Prostate Cancer Prevention. Cancer Prev Res (Phila) 2015;8:879–87. doi: 10.1158/1940-6207.CAPR-14-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi N, Sasazuki S, Iwasaki M, et al. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71–7. doi: 10.1093/aje/kwm249. [DOI] [PubMed] [Google Scholar]
- Lee AH, Fraser ML, Meng X, et al. Protective effects of green tea against prostate cancer. Expert Rev Anticancer Ther. 2006;6:507–13. doi: 10.1586/14737140.6.4.507. [DOI] [PubMed] [Google Scholar]
- Lee SC, Chan WK, Lee TW, et al. Effect of a prodrug of the green tea polyphenol (-)-epigallocatechin-3-gallate on the growth of androgen-independent prostate cancer in vivo. Nutr Cancer. 2008;60:483–91. doi: 10.1080/01635580801947674. [DOI] [PubMed] [Google Scholar]
- Lee YH, Kwak J, Choi HK, et al. EGCG suppresses prostate cancer cell growth modulating acetylation of androgen receptor by anti-histone acetyltransferase activity. Int J Mol Med. 2012;30:69–74. doi: 10.3892/ijmm.2012.966. [DOI] [PubMed] [Google Scholar]
- Liao S, Kao YH, Hiipakka RA. Green tea: biochemical and biological basis for health benefits. Vitam Horm. 2001;62:1–94. doi: 10.1016/s0083-6729(01)62001-6. [DOI] [PubMed] [Google Scholar]
- Lin YW, Hu ZH, Wang X, et al. Tea consumption and prostate cancer: an updated meta-analysis. World J Surg Oncol. 2014;12:38. doi: 10.1186/1477-7819-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague JA, Butler LM, Wu AH, et al. Green and black tea intake in relation to prostate cancer risk among Singapore Chinese. Cancer Causes Control. 2012;23:1635–41. doi: 10.1007/s10552-012-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Nutrition. Vietnamese Food Composition Table. Hanoi: Medical Publishing House; 2007. [Google Scholar]
- Nguyen CT, Pham NM, Tran DV, et al. Lifestyle and diet in relation to risk of type 2 diabetes in Vietnam: a hospital-based case-control study. Springerplus. 2016;5:687. doi: 10.1186/s40064-016-2313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel VH. Nutrition and prostate cancer: an overview. Expert Rev Anticancer Ther. 2014;14:1295–304. doi: 10.1586/14737140.2014.972946. [DOI] [PubMed] [Google Scholar]
- Peairs A, Dai R, Gan L, et al. Epigallocatechin-3-gallate (EGCG) attenuates inflammation in MRL/lpr mouse mesangial cells. Cell Mol Immunol. 2010;7:123–32. doi: 10.1038/cmi.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrborn CG, Black LK. The economic burden of prostate cancer. BJU Int. 2011;108:806–13. doi: 10.1111/j.1464-410X.2011.10365.x. [DOI] [PubMed] [Google Scholar]
- Shafique K, McLoone P, Qureshi K, et al. Tea consumption and the risk of overall and grade specific prostate cancer: a large prospective cohort study of Scottish men. Nutr Cancer. 2012;64:790–7. doi: 10.1080/01635581.2012.690063. [DOI] [PubMed] [Google Scholar]
- Siddiqui IA, Asim M, Hafeez BB, et al. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J. 2011;25:1198–207. doi: 10.1096/fj.10-167924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Taichman RS, Loberg RD, Mehra R, et al. The evolving biology and treatment of prostate cancer. J Clin Invest. 2007;117:2351–61. doi: 10.1172/JCI31791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran DV, Hoang DV, Nguyen CT, et al. Validity and reliability of a food frequency questionnaire to assess habitual dietary intake in Northern Vietnam. Vietnam J Public Health. 2013;1:57–64. [Google Scholar]
- Van Dong H, Lee AH, Nga NH, et al. Epidemiology and prevention of prostate cancer in Vietnam. Asian Pac J Cancer Prev. 2014;15:9747–51. doi: 10.7314/apjcp.2014.15.22.9747. [DOI] [PubMed] [Google Scholar]
- Vance TM, Su J, Fontham ET, et al. Dietary antioxidants and prostate cancer: a review. Nutr Cancer. 2013;65:793–801. doi: 10.1080/01635581.2013.806672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–8. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Heber D, Henning SM. Quercetin increased the antiproliferative activity of green tea polyphenol (-)-epigallocatechin gallate in prostate cancer cells. Nutr Cancer. 2012;64:580–7. doi: 10.1080/01635581.2012.661514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Jin Z, Jiang H, et al. Tea consumption and the risk of five major cancers: a dose-response meta-analysis of prospective studies. BMC Cancer. 2014;14:197. doi: 10.1186/1471-2407-14-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr. 2013;98:1676–81. doi: 10.3945/ajcn.113.058271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YF, Xu Q, Lu J, et al. Tea consumption and the incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Eur J Cancer Prev. 2015;24:353–62. doi: 10.1097/CEJ.0000000000000094. [DOI] [PubMed] [Google Scholar]
- Zheng J, Yang B, Huang T, et al. Green tea and black tea consumption and prostate cancer risk: an exploratory meta-analysis of observational studies. Nutr Cancer. 2011;63:663–72. doi: 10.1080/01635581.2011.570895. [DOI] [PubMed] [Google Scholar]


