Abstract
Long non-coding RNAs (lncRNAs) are a novel class of non-protein coding RNAs that are involved in a wide variety of biological processes. There are limited data regarding the impact of lnc-LAMC2-1:1 rs2147578 as well as CASC8 rs10505477 T>C polymorphisms on cancer development. Here we examined for the first time whether rs2147578 and rs10505477 polymorphisms are associated with childhood acute lymphoblastic leukemia (ALL) in a total of 110 cases and 120 healthy controls. Genotyping was achieved by the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. The rs2147578 variant increased the risk of ALL in codominant (OR=4.33, 95%CI=2.00-9.37, p<0.0001, CG vs CC, and OR=5.81, 95%CI=2.30-14.69, p=0.0002, GG vs CC), dominant (OR=4.63, 95%CI=2.18-9.86, p<0.0001, CG+GG vs CC), overdominant (OR=1.74, 95%CI=1.02-2.97, p=0.0444, CG vs CC+GG) and allele (OR=1.91, 95%CI=1.32-2.77, p=0.0008, G vs C) inheritance models tested. No significant association was found between the CASC8 rs10505477 T>C variant and risk of childhood ALL. In conclusion, the present study revealed that the lnc-LAMC2-1:1 rs2147578 polymorphism may be a risk factor for developing childhood ALL. Further studies with larger sample sizes with different ethnicities are now required to confirm our findings.
Keywords: Long non-coding RNA, lnc-LAMC2-1:1, CASC8, acute lymphoblastic leukemia, Polymorphism
Introduction
Acute lymphoblastic leukemia (ALL) is the most common type of malignancy diagnosed in children and constitutes about 75% of pediatric acute leukemias (Siegel et al., 2013). Though the etiology of ALL is not completely understood, a great number of genes have been shown to be associated with the development of childhood ALL (Hasani et al., 2014; Bahari et al., 2016a; Bahari et al., 2016b; Tong et al., 2016).
Non-coding RNAs (ncRNAs) divided into microRNAs (~22 nucleotides) and long non-coding RNAs (lncRNAs) which are longer that 200 nucleotides and do not serve as templates for proteins (Rinn and Chang, 2012). LncRNAs as an emerging kind of non-coding RNA have gained extensive attention for their biological regulatory functions (Kung et al., 2013). It has been proposed that lncRNAs can control gene expression at diverse levels including chromatin modification (Gupta et al., 2010), transcription, and post-transcriptional processing (Tripathi et al., 2010; Qi and Du, 2013) and are having many kinds of functions in different physiological as well as pathological processes such as tumorigenesis (Shang et al., 2016). Single nucleotide polymorphisms (SNP) can affect gene expression or protein function. Cumulative evidence shows that SNPs in some lncRNAs are linked to carcinogenesis and chemotherapy response (Shen et al., 2014; Kang et al., 2015; Gong et al., 2016b; Li et al., 2016; Shang et al., 2016).
The rs2147578 polymorphism of lnc-LAMC2-1:1 is located on chromosome 1 at position 183107699 (Gong et al., 2016a). There is only one study regarding the association of this variant and the risk of cancer. For the first time, Gong et al (Gong et al., 2016a) showed that lnc-LAMC2-1:1 rs2147578 polymorphism significantly increased the risk of colorectal cancer.
Cancer susceptibility candidate 8 (CASC8) gene is a lncRNA mapped on chromosome 8 (8q24.21) (Ma et al., 2015). The rs10505477 variant, located in the intron of lncRNA CASC8 gene, has been indicated to be associated with colorectal cancer (CRC) risk (Tomlinson et al., 2007; Zanke et al., 2007; He et al., 2011) and the prognosis of gastric cancer (Ma et al., 2015).
In the present study, for the first time, we examined the impact of lnc-LAMC2-1:1 rs2147578 and CASC8 rs10505477 T>C on risk of childhood ALL in a sample of Iranian population.
Materials and Methods
Patients
The current case-control study included 110 children diagnosed with ALL and 120 age- and sex-matched healthy children in Zahedan, southeast Iran. The study design and enrolment process have been described previously (Hasani et al., 2014; Bahari et al., 2016a; Bahari et al., 2016b; Bahari et al., 2016c; Hashemi et al., 2016). The local Ethics Committee of Zahedan University of Medical Sciences approved the project and informed consent was taken from parents of all participants. Extraction of genomic DNA from whole blood was done by using salting out method (Hashemi et al., 2013).
Genotyping
We designed polymerase chain reaction restriction fragment lengths polymorphism (PCR–RFLP) method for detection the variants. For lnc-LAMC2-1:1 rs2147578 polymorphisms, the sequence of forward and reverse primers were 5’- CACGAACTTGTGGTTACTTGCTCAC-3’ and 5’-ATCCAAACCAACATCCACCCC-3’, respectively. Into each 0.20 ml PCR reaction tube, 1 μl genomic DNA (~100 ng/ml), 1 μl (10 μM) forward and reverse primers, 10 μl 2X Prime Taq Premix (Genet Bio, Korea), and 7 μl ddH2O were added. The PCR conditions were as follows: 6 min preheating at 95°C, 30 cycles of 95°C for 30s, 68°C for 30s, and 72 °C for 30s followed by a final extension step for 5 min at 72 °C. Then, 10 μl of amplified product was digested by TaaI restriction enzyme (Fermentas) according to the manufacturer’s procedure. The C allele digested and produced two fragments (154-bp and 82-bp), while G allele was undigested (236-bp fragment) (Figure 1).
Figure 1.
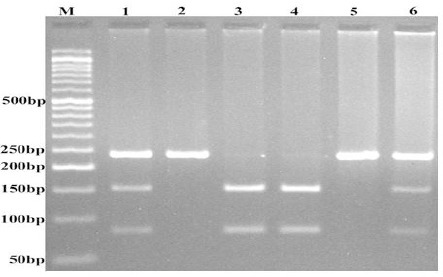
Photograph of Electrophoresis Pattern of the PCR-RFLP Method for Detection of lnc-LAMC2-1:1 rs2147578 C>G polymorphism.
C allele digested by TaaI restriction enzyeme and produces 152 and 82 bp fragments and the G allele undigested (236 bp). M: DNA marker; Lanes 1 and 6: CG; Lanes 2 and 5: GG; Lanes 3 and 4: CC.
Regarding CASC8 rs10505477 T>C variant, we designed mismatch PCR–RFLP. The forward and reverse primers were 5’- GGAAGAATTTAAAGGAGAGCAGGGA -3’ and 5’-CTTTGCCCCTTTTCTAAATCTTCATCTgC -3’, respectively. The PCR conditions were as follows: 6 min preheating at 95°C, 30 cycles of 95°C for 30s, 60°C for 30s, and 72 °C for 30 s followed by a final extension step for 5 min at 72 °C. Then, 10 μl of amplified product was digested by PstI restriction enzyme (Fermentas) according to the manufacturer’s procedure. The C allele digested and produced two fragments (200-bp and 28-bp), while T allele was undigested (228-bp fragment) (Figure 2).
Figure 2.
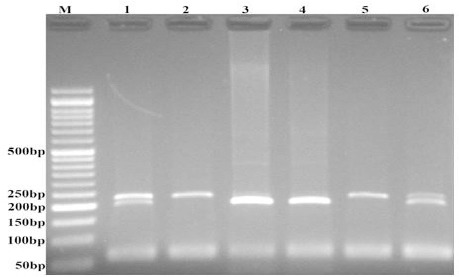
Photograph of Electrophoresis Pattern of the PCR-RFLP Method for Detection of CASC8 rs10505477 T>C.
C allele digested by PstI restriction enzyeme and produces 200 and 28 bp fragments, while the T allele undigested (228 bp). M: DNA marker; Lanes 1 and 6: TC; Lanes 2 and 5: TT; Lanes 3 and 4: CC
For quality control, repeated analyses were done for 20% randomly selected samples and the finding showed 100% concordance.
Statistical analysis
Data were summarized using frequencies and percentages for categorical variables and means and standard deviations for continuous variables. Statistical analysis of the data was done by statistical package SPSS 22 software. The categorical and continuous data were analyzed using χ2 and t-test, respectively. Individual SNP associations with childhood ALL risk were calculated using unconditional logistic regression analyses, in which ORs and 95% CIs were estimated. Hardy- Weinberg equilibrium (HWE) was calculated by χ2 test. The statistical level of significance was defined as P<0.05.
Results
A total of 230 subjects including 110 confirmed childhood ALL (65 male, 45 female; age 6.0 ± 3.9 years) and 120 unrelated healthy children (57 male, 63 female; age 5.6 ± 2.1 years) were evaluated. No significant difference was found between the groups regarding sex and age (p=0.087 and p=0.369, respectively).
The genotype and allele distributions of lnc-LAMC2-1:1 rs2147578 C>G polymorphism in ALL and healthy children are displayed in table 1. The finding revealed that rs2147578 variant increased the risk of ALL in codominant (OR=4.33, 95%CI=2.00-9.37, p<0.001, CG vs CC; OR=5.81, 95%CI=2.30-14.69, p<0.001, GG vs CC), dominant (OR=4.63, 95%CI=2.18-9.86, p<0.001, CG+GG vs CC), and overdominant (OR=1.74, 95%CI=1.0-3.0, p=0.044, CG vs CC+GG) inheritance models tested. The G allele was associated with increased risk of childhood ALL (OR=1.9, 95%CI=1.3-2.8 p <0.001) compared to C allele.
Table 1.
Association of lnc-LAMC2-1:1 rs2147578 and CASC8 rs10505477 T>C Polymorphism and the Risk of ALL
| Polymorphism | Case n (%) | Control n (%) | OR (95%CI) | p |
|---|---|---|---|---|
| lnc-LAMC2-1:1 rs2147578 | ||||
| Codominant | ||||
| CC | 10 (9.1) | 38 (31.7) | 1.0 | - |
| CG | 74 (67.3) | 65 (54.2) | 4.3 (2.0-9.4) | <0.001 |
| GG | 26 (23.6) | 17 (14.1) | 5.8 (2.3-14.7) | <0.001 |
| Dominant | ||||
| CC | 10 (9.1) | 38 (31.7) | 1.0 | - |
| CG+GG | 100 (90.9) | 82 (68.3) | 4.6 (2.2-9.9) | <0.001 |
| Recessive | ||||
| CC+CG | 84 (76.4) | 103 (85.9) | 1.0 | - |
| GG | 26 (23.6) | 17 (14.1) | 1.9 (0.9-3.7) | 0.089 |
| Overdominant | ||||
| CC+GG | 36 (32.7) | 55 (45.8) | 1 | - |
| CG | 74 (67.3) | 65 (54.2) | 1.7 (1.0-3.0) | 0.044 |
| Allele | ||||
| C | 94 (42.7) | 141 (58.7) | 1.0 | - |
| G | 126 (57.3) | 99 (41.3) | 1.9 (1.3-2.8) | <0.001 |
| CASC8 rs10505477 T>C | ||||
| Codominant | ||||
| TT | 40 (36.4) | 35 (29.2) | 1.0 | - |
| TC | 43 (39.1) | 56 (46.6) | 0.7 (0.4-1.2) | 0.222 |
| CC | 27 (24.5) | 29 (24.2) | 0.8 (0.4-1.6) | 0.599 |
| Dominant | ||||
| TT | 40 (36.4) | 35 (29.2) | 1.0 | - |
| TC+CC | 70 (63.6) | 85 (70.8) | 0.7 (0.4-1.2) | 0.265 |
| Recessive | ||||
| TT+TC | 83 (75.5) | 91 (75.8) | 1.0 | - |
| CC | 27 (24.5) | 29 (24.2) | 1.0 (0.6-1.9) | 0.976 |
| Overdominant | ||||
| TT+CC | 67 (60.9) | 64 (53.4) | 1.0 | - |
| TC | 43 (39.1) | 56 (46.6) | 0.7 (0.4-1.2) | 0.287 |
| Allele | ||||
| T | 123 (60.4) | 126 (52.5) | 1.0 | - |
| C | 97 (39.6) | 114 (47.5) | 0.9 (0.6-1.3) | 0.512 |
Our findings showed that CASC8 rs10505477 T>C variant may not be associated with the risk of childhood ALL in any inheritance models tested (table 1).
Association between the lnc-LAMC2-1:1 rs2147578 C>G and CASC8 rs10505477 T>C polymorphisms with the patients’ clinical characteristics were evaluated. As shown in Table 2, a significant association between rs2147578 C>G and platelet count was found (p=0.023).
Table 2.
Association of lnc-LAMC2-1:1 rs2147578 Polymorphism with Demographic and Clinical Features of Patients
| Factors | lnc-LAMC2-1: | 1 rs2147578 | C>G | p | CASC8 | rs10505477 | T>C | p |
|---|---|---|---|---|---|---|---|---|
| CC | CG | GG | TT | TC | CC | |||
| Sex | 0.513 | 0.189 | ||||||
| Male | 7.0 | 41.0 | 17.0 | 21.0 | 30.0. | 14.0 | ||
| Female | 3.0 | 33.0 | 9.0 | 19.0 | 13.0 | 13.0 | ||
| Age at diagnosis (Years) | 4.9±2.1 | 5.7±3.6 | 7.2±4.9 | 0.161 | 5.9±4.5 | 6.50±3.53 | 5.3±3.4 | 0.469 |
| WBC (×106/mL) | 36.1±29.5 | 33.1±45.1 | 55.1±71.7 | 0.177 | 35.9±40.9 | 46.23±64.21 | 30.8±44.3 | 0.43 |
| Hemoglobin (g/dL) | 7.4±2.3 | 7.1±2.4 | 7.6±1.6 | 0.948 | 7.1±2.2 | 7.43±2.43 | 7.0±2.0 | 0.714 |
| Platelet (×106/mL) | 43.8±44.1 | 65.0±53.0 | 35.9±31.6 | 0.023 | 54.9±50.5 | 53.54±54.67 | 62.4±39.0 | 0.754 |
| Organomegally | 0.418 | 0.816 | ||||||
| Positive | 9.0 | 69.0 | 22.0 | 36.0 | 40.0 | 24.0 | ||
| Negative | 1.0 | 5.0 | 4.0 | 4.0 | 3.0 | 3.0 | ||
| Lymphadenopathy | 0.066 | 0.9 | ||||||
| Positive | 9.0 | 54.0 | 14.0 | 27.0 | 31.0 | 19.0 | ||
| Negative | 1.0 | 20.0 | 12.0 | 13.0 | 12.0 | 8.0 | ||
| Cerebrospinal fluid involvement | 0.527 | 0.114 | ||||||
| Positive | 0.0 | 6.0 | 3.0 | 3.0 | 6.0 | 0.0 | ||
| Negative | 10.0 | 68.0 | 23.0 | 37.0 | 37.0 | 26.0 |
The genotype distribution of the lnc-LAMC2-1:1 rs2147578 C>G and CASC8 rs10505477 T>C polymorphisms in control group were consistent with the HWE (χ2=1.66, P=0.198 and χ2=0.496, P=0.481, respectively).
Discussion
Although the functional roles of lncRNAs remained mostly elusive, increasing evidence shows that up to 90% of the non-coding transcripts in the human genome have significant and diverse biological roles (Geisler and Coller, 2013).
It has been proposed that gene expression or protein function is affected by SNPs. Growing evidence validates that SNPs in some lncRNAs are related to tumorigenesis and chemotherapy response (Shen et al., 2014; Kang et al., 2015; Gong et al., 2016b; Li et al., 2016; Ronchetti et al., 2016; Shang et al., 2016). Abnormal expression of lncRNAs in various cancers (Morris, 2009; Han et al., 2012; Hauptman and Glavac, 2013; Rodriguez-Malave et al., 2015; Ricciuti et al., 2016) proposed the potentially tumor suppressor or oncogenic role (Emmrich et al., 2014; Yang et al., 2014; Morlando et al., 2015; Sun et al., 2015; Xing et al., 2015).
It has been shown that overexpression of lncRNA BALR-2 led to increased cell growth and resistance to prednisone treatment in B-acute lymphoblastic leukemia (B-ALL) (Fernando et al., 2015). Zeng et al.,(2015) showed that lncRNA PVT1 is upregulated in acute promyelocytic leukemia (APL) cells. LncRNA HOTAIR is upregulated in acute myeloid leukemia (AML) and highly expressed HOTAIR significantly associated with a poor clinicopathological prognostic stratification (Hao and Shao, 2015).
The lnc-LAMC2-1:1 locates on chromosome 1, overlaps with the protein-coding gene LAMC1 and is close to LAMC2. There is only one report concerning the impact of lnc-LAMC2-1:1 rs2147578 variant on cancer risk. For the first time, Gong et al (Gong et al., 2016a) showed that CG and GG genotypes of the rs2147578 variant of lnc-LAMC2-1:1 significantly increased the risk for colorectal cancer (CRC) compared to the rs2147578 CC genotype. Bioinformatics analyses revealed that rs2147578 is located in the transcript of lnc-LAMC2-1:1 and could influence the binding of lnc-LAMC2-1:1/miR-128-3p (Gong et al., 2016a). In the current study we inspected the impact of lnc-LAMC2-1:1 rs2147578 C>G on risk of childhood ALL. Our finding showed that CG, GG and CG+GG genotypes significantly increased the risk of childhood ALL risk. Furtheremore, the rs2147578 G allele significantly increased the risk of childhood ALL compared with the C allele.
Current evidence proposed that CASC8 rs10505477 variant is related to developing of numerous cancers including CRC (Li et al., 2015; Yao et al., 2015), gastric cancer (Zhou et al., 2014; Ma et al., 2015) and ovarian cancer (Ghoussaini et al., 2008).
It has been proposed that CASC8 rs10505477 variant could be used to determine the response and toxicity of platinum-based chemotherapy in lung cancer patients (Hu et al., 2016). In the present study, for the first time we evaluate the possible association between rs10505477 variant and risk of childhood ALL. The findings did not support an association between CASC8 rs10505477 T>C variant and risk/protection of childhood ALL risk.
In summary, the findings of this study proposed that lnc-LAMC2-1:1 rs2147578 C>G polymorphism significantly increased the risk of childhood ALL in a sample of Iranian population. Further studies with larger sample sizes and different ethnicities are needed to confirm our findings.
Acknowledgements
This paper was funded as a research grant from the Deputy for Research, Zahedan University of Medical Sciences.
Conflicts of interest
All of the authors declare that there are no conflicts of interest.
References
- Bahari G, Hashemi M, Naderi M, et al. Association of SHMT1 gene polymorphisms with the risk of childhood acute lymphoblastic leukemia in a sample of Iranian population. Cell Mol Biol (Noisy-le-grand) 2016a;62:45–51. [PubMed] [Google Scholar]
- Bahari G, Hashemi M, Naderi M, et al. IKZF1 gene polymorphisms increased the risk of childhood acute lymphoblastic leukemia in an Iranian population. Tumour Biol. 2016b;37:9579–86. doi: 10.1007/s13277-016-4853-0. [DOI] [PubMed] [Google Scholar]
- Bahari G, Hashemi M, Naderi M, et al. TET2 Promoter DNA Methylation and Expression in Childhood Acute Lymphoblastic Leukemia. Asian Pac J Cancer Prev. 2016c;17:3959–62. [PubMed] [Google Scholar]
- Emmrich S, Streltsov A, Schmidt F, et al. LincRNAs MONC and MIR100HG act as oncogenes in acute megakaryoblastic leukemia. Mol Cancer. 2014;13:171. doi: 10.1186/1476-4598-13-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando TR, Rodriguez-Malave NI, Waters EV, et al. LncRNA Expression Discriminates Karyotype and Predicts Survival in B-Lymphoblastic Leukemia. Mol Cancer Res. 2015;13:839–51. doi: 10.1158/1541-7786.MCR-15-0006-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Coller J. RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol. 2013;14:699–712. doi: 10.1038/nrm3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoussaini M, Song H, Koessler T, et al. Multiple loci with different cancer specificities within the 8q24 gene desert. J Natl Cancer Inst. 2008;100:962–6. doi: 10.1093/jnci/djn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Tian J, Lou J, et al. A functional polymorphism in lnc-LAMC2-1: 1 confers risk of colorectal cancer by affecting miRNA binding. Carcinogenesis. 2016a;37:443–51. doi: 10.1093/carcin/bgw024. [DOI] [PubMed] [Google Scholar]
- Gong WJ, Yin JY, Li XP, et al. Association of well-characterized lung cancer lncRNA polymorphisms with lung cancer susceptibility and platinum-based chemotherapy response. Tumour Biol. 2016b;37:8349–58. doi: 10.1007/s13277-015-4497-5. [DOI] [PubMed] [Google Scholar]
- Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–6. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Zhang K, Shi Z, et al. LncRNA pro file of glioblastoma reveals the potential role of lncRNAs in contributing to glioblastoma pathogenesis. Int J Oncol. 2012;40:2004–12. doi: 10.3892/ijo.2012.1413. [DOI] [PubMed] [Google Scholar]
- Hao S, Shao Z. HOTAIR is upregulated in acute myeloid leukemia and that indicates a poor prognosis. Int J Clin Exp Pathol. 2015;8:7223–8. [PMC free article] [PubMed] [Google Scholar]
- Hasani SS, Hashemi M, Eskandari-Nasab E, et al. A functional polymorphism in the miR-146a gene is associated with the risk of childhood acute lymphoblastic leukemia: a preliminary report. Tumour Biol. 2014;35:219–25. doi: 10.1007/s13277-013-1027-1. [DOI] [PubMed] [Google Scholar]
- Hashemi M, Bahari G, Naderi M, et al. Pri-miR-34b/c rs4938723 polymorphism is associated with the risk of childhood acute lymphoblastic leukemia. Cancer Genet. 2016;209:493–6. doi: 10.1016/j.cancergen.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Hashemi M, Hanafi Bojd H, Eskandari Nasab E, et al. Association of Adiponectin rs1501299 and rs266729 Gene Polymorphisms With Nonalcoholic Fatty Liver Disease. Hepat Mon. 2013;13:e9527. doi: 10.5812/hepatmon.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptman N, Glavac D. Long non-coding RNA in cancer. Int J Mol Sci. 2013;14:4655–69. doi: 10.3390/ijms14034655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Wilkens LR, Stram DO, et al. Generalizability and epidemiologic characterization of eleven colorectal cancer GWAS hits in multiple populations. Cancer Epidemiol Biomarkers Prev. 2011;20:70–81. doi: 10.1158/1055-9965.EPI-10-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Chen SH, Lv QL, et al. Clinical significance of long non-coding RNA CASC8 rs10505477 polymorphism in lung cancer susceptibility, platinum-based chemotherapy response, and toxicity. Int J Environ Res Public Health. 2016;13:545. doi: 10.3390/ijerph13060545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Sang Y, Gu H, et al. Long noncoding RNAs POLR2E rs3787016 C/T and HULC rs7763881 A/C polymorphisms are associated with decreased risk of esophageal cancer. Tumour Biol. 2015;36:6401–8. doi: 10.1007/s13277-015-3328-z. [DOI] [PubMed] [Google Scholar]
- Kung JT, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics. 2013;193:651–69. doi: 10.1534/genetics.112.146704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Jia F, Bai P, et al. Association between polymorphisms in long non-coding RNA PRNCR1 in 8q24 and risk of gastric cancer. Tumour Biol. 2016;37:299–303. doi: 10.1007/s13277-015-3750-2. [DOI] [PubMed] [Google Scholar]
- Li L, Lv L, Liang Y, et al. Association of 8q23-24 region (8q23.3 loci and 8q24.21 loci) with susceptibility to colorectal cancer: a systematic and updated meta-analysis. Int J Clin Exp Med. 2015;8:21001–13. [PMC free article] [PubMed] [Google Scholar]
- Ma G, Gu D, Lv C, et al. Genetic variant in 8q24 is associated with prognosis for gastric cancer in a Chinese population. J Gastroenterol Hepatol. 2015;30:689–95. doi: 10.1111/jgh.12801. [DOI] [PubMed] [Google Scholar]
- Morlando M, Ballarino M, Fatica A. Long Non-Coding RNAs: New Players in Hematopoiesis and Leukemia. Front Med (Lausanne) 2015;2:23. doi: 10.3389/fmed.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KV. Long antisense non-coding RNAs function to direct epigenetic complexes that regulate transcription in human cells. Epigenetics. 2009;4:296–301. doi: 10.4161/epi.4.5.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi P, Du X. The long non-coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod Pathol. 2013;26:155–65. doi: 10.1038/modpathol.2012.160. [DOI] [PubMed] [Google Scholar]
- Ricciuti B, Mencaroni C, Paglialunga L, et al. Long noncoding RNAs: new insights into non-small cell lung cancer biology, diagnosis and therapy. Med Oncol. 2016;33:18. doi: 10.1007/s12032-016-0731-2. [DOI] [PubMed] [Google Scholar]
- Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–66. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Malave NI, Fernando TR, Patel PC, et al. BALR-6 regulates cell growth and cell survival in B-lymphoblastic leukemia. Mol Cancer. 2015;14:214. doi: 10.1186/s12943-015-0485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchetti D, Agnelli L, Taiana E, et al. Distinct lncRNA transcriptional fingerprints characterize progressive stages of multiple myeloma. Oncotarget. 2016;7:14814–30. doi: 10.18632/oncotarget.7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang C, Guo Y, Zhang H, et al. Long noncoding RNA HOTAIR is a prognostic biomarker and inhibits chemosensitivity to doxorubicin in bladder transitional cell carcinoma. Cancer Chemother Pharmacol. 2016;77:507–13. doi: 10.1007/s00280-016-2964-3. [DOI] [PubMed] [Google Scholar]
- Shen L, Du M, Wang C, et al. Clinical significance of POU5F1P1 rs10505477 polymorphism in Chinese gastric cancer patients receving cisplatin-based chemotherapy after surgical resection. Int J Mol Sci. 2014;15:12764–77. doi: 10.3390/ijms150712764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Sun QL, Zhao CP, Wang TY, et al. Expression profile analysis of long non-coding RNA associated with vincristine resistance in colon cancer cells by next-generation sequencing. Gene. 2015;572:79–86. doi: 10.1016/j.gene.2015.06.087. [DOI] [PubMed] [Google Scholar]
- Tomlinson I, Webb E, Carvajal-Carmona L, et al. A genome-wide association scan of tag SNPs identifies a susceptibility variant for colorectal cancer at 8q24.21. Nat Genet. 2007;39:984–8. doi: 10.1038/ng2085. [DOI] [PubMed] [Google Scholar]
- Tong N, Chu H, Wang M, et al. Pri-miR-34b/c rs4938723 polymorphism contributes to acute lymphoblastic leukemia susceptibility in Chinese children. Leuk Lymphoma. 2016;57:1436–41. doi: 10.3109/10428194.2015.1092528. [DOI] [PubMed] [Google Scholar]
- Tripathi V, Ellis JD, Shen Z, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–38. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing CY, Hu XQ, Xie FY, et al. Long non-coding RNA HOTAIR modulates c-KIT expression through sponging miR-193a in acute myeloid leukemia. FEBS Lett. 2015;589:1981–7. doi: 10.1016/j.febslet.2015.04.061. [DOI] [PubMed] [Google Scholar]
- Yang X, Song JH, Cheng Y, et al. Long non-coding RNA HNF1A-AS1 regulates proliferation and migration in oesophageal adenocarcinoma cells. Gut. 2014;63:881–90. doi: 10.1136/gutjnl-2013-305266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K, Hua L, Wei L, et al. Correlation Between CASC8, SMAD7 Polymorphisms and the Susceptibility to Colorectal Cancer: An Updated Meta-Analysis Based on GWAS Results. Medicine (Baltimore) 2015;94:e1884. doi: 10.1097/MD.0000000000001884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanke BW, Greenwood CM, Rangrej J, et al. Genome-wide association scan identifies a colorectal cancer susceptibility locus on chromosome 8q24. Nat Genet. 2007;39:989–94. doi: 10.1038/ng2089. [DOI] [PubMed] [Google Scholar]
- Zeng C, Yu X, Lai J, et al. Overexpression of the long non-coding RNA PVT1 is correlated with leukemic cell proliferation in acute promyelocytic leukemia. J Hematol Oncol. 2015;8:126. doi: 10.1186/s13045-015-0223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CP, Pan HZ, Li FX, et al. Association analysis of colorectal cancer susceptibility variants with gastric cancer in a Chinese Han population. Genet Mol Res. 2014;13:3673–80. doi: 10.4238/2014.May.9.10. [DOI] [PubMed] [Google Scholar]


