Abstract
Docetaxel, recognized as a stabilizing microtubule agent, is frequently administrated as a first line treatment for prostate cancers. Due to high side effects of monotherapy, however, combinations with novel adjuvants have emerged as an alternative strategy in cancer therapy protocols. Here, we investigated the combined effects of stattic and docetaxel on the DU145 prostate cancer cell line. Cytotoxicity was evaluated by MTT assay. To understand molecular mechanisms of stattic action, apoptotic related genes including Bcl-2, Mcl-1, Survivin and Bax were evaluated by real-time RT-PCR. Alteration in the expression of pro-apoptotic Bax and anti-apoptotic Bcl-2 genes and Bax/Bcl-2 ratio were investigated via the 2ΔΔCT method. The IC50 values for docetaxel and stattic were 3.7 ± 0.9 nM and 4.6±0.8 µM, respectively. Evaluation of key gene expression levels revealed a noticeable decrease in antiapoptotic Bcl-2 and Mcl-1 along with an increase in pro-apoptotic Bax mRNA levels (p<0.05). Our results suggest that combination of a STAT3 inhibitor with doctaxel can be considered as a potent strategy for induction of apoptosis via increasing Bax mRNA expression.
Keywords: Prostate cancer cells, apoptosis, docetaxel, monotherapy, combination chemotherapy, stattic
Introduction
Cancer is recognized as second leading causes of death worldwide, headed by cardiovascular and infectious diseases (Albini, DeCensi et al., 2016). Worldwide, prostate cancer is the most prevalent cause of cancer-related death in men with 27,540 deaths, while lung cancer causing death both in men and women, is responsible for 158040 deaths yearly, as on 2015 (Siegel, Miller et al., 2015, Fabre, Grosman et al., 2016).
Nullification of apoptosis and continuous cell survival are critical step in the expansion of cancer (Fiandalo and Kyprianou 2012). Activation of prosurvival signal transcription factor STAT3, is among the most typical molecular alterations affecting cell survival and therapeutic susceptibility determined in cancers. Activation of STAT3 has been individually shown to appear in the majority of carcinomas such as lung and prostate. Constitutive activation of STAT3 is detected in the majority of prostate cancer cell lines, and established to promote cell survival, tumor development and resistance to apoptosis (Kamran, Patil et al., 2013, Yu, Lee et al., 2014).
Apoptosis is organized by Bcl-2 family members, which consists of both anti-apoptotic genes such as Bcl-2, Bcl-xl and Mcl-1 and pro-apoptotic genes such as Bad, Bak and Bax (Pirouzpanah, Sabzichi et al., 2014). Functions of the apoptotic pathways are assisted by regulation of expression of a family of pro-apoptotic and anti-apoptotic proteins, and the ratio of expressed pro and anti-apoptotic proteins has been conveyed to establish whether cells survive or sustain apoptosis (Thomas, Quinn et al., 2013, Czabotar, Lessene et al., 2014). STAT3 can upregulate transcription of antiapoptotic protein Bcl-2, that manages cell growth and survival. Extensive studies involving the investigation of Bcl-2 and Bax expression in cultured cells have demonstrated that Bcl-2 protein can be practically characterized as an apoptosis-suppressing factor whereas the Bax protein is more practically characterized as a key apoptosis-promoting factor (Park, Kundu et al., 2014, Jahanafrooz, Motameh et al., 2015). A low relative ratio of Bax to Bcl-2 has been discovered in prostate cancer, and overexpression of Bcl-2 has been established to suppress the initiation of apoptosis and promote resistance to anticancer drugs and radiation therapy. The intracellular ratio of Bax/Bcl-2 genes can strongly control the ability of a cell to react to an apoptotic signal. Then, a cell with a high Bax/Bcl-2 ratio will be more susceptible to apoptotic stimuli when compared to a similar cell type with a relatively low Bax/Bcl-2 ratio (Huang, Yang et al., 2012, Lee, Jung et al., 2012, Del Principe, Dal Bo et al., 2016).
Multiple chemotherapeutic agents have been characterized to induce apoptosis in cancer cells (Sui, Kong et al., 2014). An adverse side effect of taxan derivatives (i.e. docetaxel) is a critical challenge that limits the efficacy of cancer therapy protocols. The innovation of novel inhibitors and also an application of combination therapy strategy shed light on chemoresistance and drug toxicity of current chemotherapeutic regiment (Fizazi, Higano et al., 2013, Swain, Baselga et al., 2015). Stattic (6-Nitrobenzo[b]thiophene-1,1-dioxide) is a small molecule inhibitor of STAT3. It selectively inhibits STAT3 activation, dimerization, and nuclear translocation by preventing the binding of tyrosine-phosphorylated peptide motifs to the STAT3 SH2 domain (Pan, Zhou et al., 2013).
In this study we applied STAT3 inhibitor, stattic concomitant with docetaxel to improve the efficacy of this chemotherapeutic agent in the induction of apoptosis. Furthermore, this study exhibits for the first time that stattic in combination with docetaxel down-regulated Survivin, Bcl-2 and Mcl-1 expression, up-regulated Bax mRNA level and altered Bax/Bcl-2 gene expression ratio in A549 and DU145 cells. Our results suggest that STAT3 inhibitors can be applied as effective adjuvants to increase docetaxel-induced cell death in prostate cancer cells.
Materials and Methods
Cell culture
Human prostate cancer (DU145) cell line was obtained from National Cell Bank of Iran (Pasteur Institute, Iran). The cells were cultured under the condition of 37 °C, 5% CO2, in RPMI (Roswell Park Memorial Institute)1640 Medium (Gibco®, Invitrogen, USA) with 10% FBS (Fetal Bovine Serum) enriched with 100 units/ml streptomycin/penicillin (Sigma, St Louis, MO, USA).
Cell viability assessment using MTT assay
Cells were seeded in the 96-well plate (2000 per well) in triplicate and treated with 1 to 16 nmol/L of docetaxel and 1 to 16 µmol/L of stattic for 24, 48 and 72 hours. The media in each well was replaced with 200 µl fresh media containing 20 μL of MTT (Methylthiazolyldiphenyl-tetrazolium bromide) solution (2 mg/ml). Then; the cells were incubated for 4 hours at 37 °C. Subsequently, media/MTT mixture was removed and 200µl of DMSO (Dimethyl sulfoxide) plus 25µl of Sorenson’s glycine buffer as solubilization solution was added to each well(Sabzichi, Samadi et al., 2016, Tupal, Sabzichi et al., 2016). The absorbance at 570 nm was read after shaking for 30 minutes, employing a microplate reader (Awareness, statfax 3200, USA). Half maximal inhibitory concentration (IC50) was calculated using Graphpad prism 6.07 software.
Cell viability analysis using trypan blue staining
Trypan Blue is a “vital stain” in which dead cells were excluded from live cells. Live cells appear colorless and bright (retractile) while dead cells stain blue and are non-retractile. Cells were re-suspended with equal volumes of 0.4% trypan blue stain for 3 min. Then, the cells were counted by hemocytometer (Wang, Zhu et al., 2016). The number of cells from the untreated control represented optimal cell survival (100%) and the relative surviving cells of each treatment were calculated using the following equation:
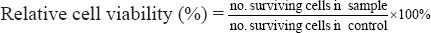
The experiments were repeated three times separately, to confirm reproducibility.
RNA extraction and reverse transcriptase-PCR (RT-PCR)
Cells and DU145 were treated with stattic (2μM), docetaxel (2nM) and the combination of them (docetaxel 1nM+stattic 1μM) and incubated for 24 h. RNA extraction of treated cells was carried out using TRI reagent (Sigma-Aldrich), according to the manufacturer’s instructions. The quality of RNA was evaluated by assessing the integrity of total RNA on agarose gel electrophoresis and the concentration of RNA was determined by measuring the absorbance at A260/A280 ratio with NanoDrop 1,000 spectrophotometer (Wilmington, DE, USA). After reverse transcription, cDNA was consequently amplified by regular PCR method with the primers presented in Table 1. The PCR products were separated using electrophoresis through 1% agarose gels containing Ethidium bromide and PCR products were visualized under UV illumination (Minaei, Sabzichi et al., 2016).
Table 1.
Primers Used for qRT-PCR Analysis
| Objective genes | Primer sequence |
|---|---|
| Bcl-2 | Forward: 5’-CATCAGGAAGGCTAGAGTTACC-3’ |
| Reverse: 5’-CAGACATTCGGAGACCACAC-3’ | |
| Mcl-1 | Forward: 5’-GGACACAAAGCCAATGGGCAGGT-3’ |
| Reverse: 5’-GCAAAAGCCAGCAGCACATTCCTGA-3’ | |
| BAX | Forward: 5’-GATGCGTCCACCAAGAAG-3’ |
| Reverse: 5’-AGTTGAAGTTGCCGTCAG-3’ | |
| Survivin | Forward: 5’-GACCACCGCATCTCTACATTC-3’ |
| Reverse: 5’-TGCTTTTTATGTTCCTCTATGGG-3’ | |
| β-actin | Forward: 5’-TGCCCATCTACGAGGGGTATG-3’ |
| Reverse: 5’-CTCCTTAATGTCACGCACGATTTC-3’ |
Quantitative real-time PCR
Cells were treated with different concentrations of docetaxel and stattic and incubated for 24 h. Total RNA was isolated from cell pellets using TRI reagent according to the protocol as described previously (Pirouzpanah, Sabzichi et al., 2014, Armat, Bakhshaiesh et al., 2016). Following reverse transcription, cDNA was subsequently amplified by quantitative real-time PCR, using QPCR Green Master with low ROX (California, USA) with primers presented in Table.1 The PCR reaction was performed using the Roche Light Cycler® (Germany).
Statistical analysis
All statistical analyses were carried out by Graphpad prism V6.07 (GraphPad Software Inc., San Diego, CA, USA). The statistical significance was determined using T-test for MTT assay and one-way ANOVA for Real Time PCR and p<0.05 was considered to be statistically significant.
Results
Anti-proliferative effects of docetaxel and stattic on DU145 cells (using MTT and Trypan Blue cell count assays)
Anti-proliferative activity of docetaxel and stattic was measured by MTT assay using DU145 cell. IC50 values for stattic and docetaxel in different concentration shown in Table 2. After 24 h incubation, MTT and Trypan Blue cell count assays indicated that DU145 cells exposed to stattic and docetaxel had significantly lower cell viability than the untreated control. In contrast, the two assays showed stattic and docetaxel treatment had a remarkable difference in cell viability compared the control.
Table 2.
IC50 Values of Docetaxel and Stattic in DU145 Cells
| Agents | Incubation time | IC50 |
|---|---|---|
| Docetaxel (nM) | 24 | 3.7 ± 0.9 |
| 48 | 2.4 ± 1.0 | |
| 72 | 1.7 ± 0.6 | |
| Stattic (μM) | 24 | 4.7 ± 0.8 |
| 48 | 2.9 ± 0.9 | |
| 72 | 1.7 ± 0.9 |
On the other hand, after 48 and 72 h treatment, MTT and Trypan Blue cell count assays demonstrated that DU145 cells exposed to stattic and docetaxel (Figure. 1 A-D) had significantly decreased cell viability compared to 24 h treatment and the untreated control (p < 0.05).
Figure 1.
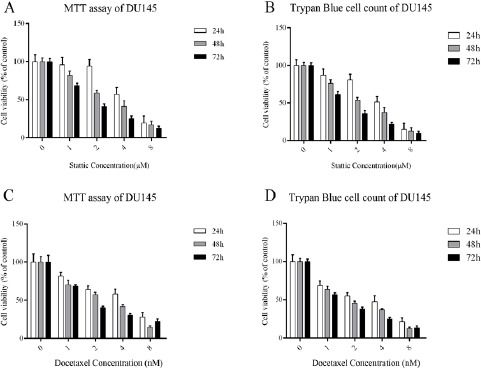
DU145 Cells Exposed to Docetaxel and Stattic Treatments for 24, 48 and 72 h Determined by: MTT (A–C); Trypan Blue Cell Count (B-D). Data are Expressed as Cell Viability (%) ± SD of Three Independent Experiments.
Semi-quantitative RT-PCR
For semi-quantitative RT-PCR, the cells were incubated with docetaxel at the concentration of 2nM, stattic 2µM (IC50 values) and their combination for 24h. Our semi-quantitative data showed a remarkable down-regulation of anti-apoptotic Mcl-1, Bcl-2 and Survivin genes and up-regulation of pro apoptotic Bax compared to single treatment sample. Results, after normalization against β-actin, are consistent with reduction of Mcl-1, Bcl-2 and Survivin and increase of Bax transcripts in DU145 cell line but in the combinatorial treatment reduction of transcripts are more considerable (Figure 2B).
Figure 2.
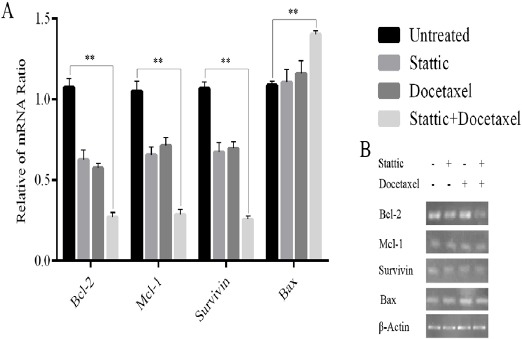
Effect of Stattic, Docetaxel and Their Combination on the Expression of Bcl-2, Survivin, Mcl-1 and Bax in DU145 cells. A. The levels of Bcl-2, Survivin, Mcl-1 and Bax mRNA expression, followed by treatment with docetaxel (2 nM), stattic (2 µM) and their combination (stattic 1 µM+docetaxel 1 nM) for 24h, were determined using real-time PCR. Mean ± SD was calculated from three independent experiments(**p<0.01). B. Levels of Bcl-2, Mcl-1, Survivin and Bax expression were analyzed. Bands representing Bcl-2, Mcl-1, Survivin and Bax are shown.
Combined effect of stattic and docetaxel on Bcl-2, Mcl-1, Survivin and Bax mRNA expression levels and Bax /Bcl-2 ratio
Real time RT-PCR was performed to quantitate levels of the transcripts. For DU145 cell line, the only concentration of 2nM docetaxel and 2µM stattic and their combination (docetaxel 1nM + stattic 1µM) were tested in parallel with untreated cells. Expression of Bcl-2, Mcl-1 and Survivin genes decreased in cells treated with docetaxel (2nM) and stattic (2µM) and remarkably decreased in combination treatment (stattic+docetaxel) after 24h incubation. On the other hand expression of Bax gene increased after treatment with the aforementioned condition in which in combinational treatment was increased more significantly (Figure 2A). Our results also revealed a considerable increase in Bax/Bcl-2 ratio in treatment by docetaxel (2nM) and stattic (2µM). The most increase in Bax/Bcl-2 ratio was seen in combinational treatment after 24h incubation (Figure 3).
Figure 3.
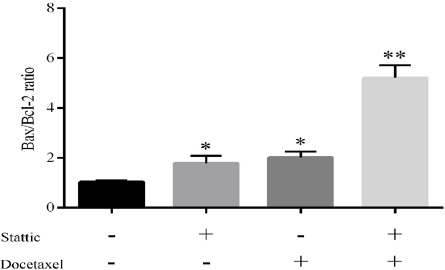
Effect of Stattic and/or Docetaxel on Bax/Bcl-2 Ratio. Bax/Bcl-2 Ratio was Calculated and Show That It was Significantly Increased in Stattic and/or Docetaxel Treated DU145 Cells (*p<0.05, **p<0.01).
Discussion
Docetaxel is the first line treatment in prostate cancer therapy protocols (Sonpavde, Matveev et al., 2012). Currently, the combination of two or more drugs is administered in the most chemotherapies regiment. This strategy reduces cytotoxicity and increase the efficacy of chemotherapeutic agents by overcoming chemoresistance (von Minckwitz 2007, Samadi, Ghanbari et al., 2014). In this investigation, we found that stattic enhances the anti-proliferative effect of docetaxel in DU145 prostate cancer cell line.
STAT3 is found constitutively active in several types of human neoplastic diseases in which contribute cancer progression and resistance to apoptosis. It has been demonstrated that cancer cells harboring anomalous STAT3 activity have elevated levels of anti-apoptotic (Mcl-1, Survivin and Bcl-2). Thus, cancer cells expressing constitutively activated STAT3 are more resistant to apoptosis (Zhang, Du et al., 2015). Over last decade, STAT3 has increasingly been noticed as a pivotal target for cancer therapy., A line of evidence introduced a variety of different STAT3 inhibitors and the mechanisms of action for these inhibitors (Saini, Naidu et al., 2016). Inhibition of STAT3 results in deregulation of downstream target genes and consequently leads to growth inhibition and apoptosis (Siveen, Sikka et al., 2014). Stattic, a selective inhibitor of STAT3, has gained great attention because of its unique pharmacological properties. More ever, previous studies showed that stattic could inhibit the function of STAT3 SH2 domain despite of STAT3 phosphorylation state (Zhang, Zhang et al., 2015). Human prostate cancer cells line, DU145, are popular for investigating the biochemical mechanism and assessing the response to chemotherapy. Therefore, we carried out this study using DU145 to investigate whether stattic enhance the anti-proliferative efficacy of docetaxel and induction of apoptosis via alteration in Bax/Bcl-2 ratio. This finding was consistent with previous studies demonstrating that sanguinarine increases the expression ratio of Bax/Bcl-2 in human colon cancer cells (Lee, Jung et al., 2012).
To study the mechanism in which stattic enhance the apoptotic effect of docetaxel, we performed some experiments on the genes expression involved in the apoptotic pathway. Real time PCR analysis revealed that in contrast to individual exposure, co-treatment of stattic and docetaxel results in a remarkable decrease in expression levels of Bcl-2, Mcl-1 and Survivin while Bax mRNA levels increased. Decreased apoptosis is a critical step in the procedure of cancer evolution (Su, Mei et al., 2013). The mitochondrial apoptosis pathway is the most significant pathway by regulating the ratio of apoptotic proteins Bcl-2 and Bax. Exclusively, Bcl-2 suppresses apoptosis partly by preventing the release of cytochrome c from mitochondria, and the initiation of apoptosis while Bax has an apoptosis-promoting effect by antagonizing Bcl-2. The balance between the expression levels of two apoptosis regulatory genes, Bcl-2 and Bax is important for cell survival and death since the increase in Bax/Bcl-2 ratio contributes to the efflux of cytochrome C from mitochondria and activates the intrinsic mitochondrial apoptotic pathway. As a result alteration in the expression of Bax and Bcl-2 expression determines cell susceptibility to apoptosis. An increased Bax/Bcl-2 ratio can activate caspase-3 and result in cell death(Renault, Teijido et al., 2013, Sharifi, Barar et al., 2015).
In prostate cancer, it is identified that apoptosis plays a role in response to chemotherapy, recommending a relationship between drug-induced apoptosis and therapeutic efficiency (Sun, Bao et al., 2013). As a result, the analysis of mitochondrial apoptotic genes may lead to introduce a key prognostic tool to predict resistance to chemotherapeutic agents. Independently, the functions of Bcl-2 and Bax have been studied widely in prostate and other cancers, providing facts of their opposing function in cell survival and apoptosis (Zhang, Bi et al., 2014).
In this study, the alteration in Bcl-2 (anti-apoptotic), Bax (pro-apoptotic) gene expression, Bax/Bcl-2 ratio, subsequent to the stimulation of apoptosis by stattic, docetaxel and their combination in DU145 cells were reported. Our results in DU145 cells demonstrated that in vitro treatments induced a decreased expression of the antiapoptotic oncogene Bcl-2, which determines an augment in Bax/Bcl-2 ratio after treatment.
Our investigation demonstrated that stattic augmented docetaxel-induced cytotoxicity and also alter expression ratio of Bax/Bcl2 in the prostate cancer cell, DU145. Our findings suggested that utilizing stattic along with docetaxel can be regarded as a novel approach to reach more effectual results in the patient with prostate cancer.
Acknowledgements
This study was financially supported by grant for PhD post graduate theses from Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- Albini A, DeCensi A, Cavalli F, Costa A. Cancer prevention and interception: a new era for chemopreventive approaches. Clin Cancer Res. 2016;17:4322–7. doi: 10.1158/1078-0432.CCR-16-0695. [DOI] [PubMed] [Google Scholar]
- Armat M, Bakhshaiesh TO, Sabzichi M, et al. The role of Six1 signaling in paclitaxel-dependent apoptosis in MCF-7 cell line. Bosn J Basic Med Sci. 2016;16:28. doi: 10.17305/bjbms.2016.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- Del Principe MI, Dal Bo M, Bittolo T, et al. Clinical significance of bax/bcl-2 ratio in chronic lymphocytic leukemia. Haematologica. 2016;101:77–85. doi: 10.3324/haematol.2015.131854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre B, Grosman H, Gonzalez D, et al. Prostate cancer, high cortisol levels and complex hormonal interaction. Asian Pac J Cancer Prev. 2016;17:3167. [PubMed] [Google Scholar]
- Fiandalo M, Kyprianou N. Caspase control: protagonists of cancer cell apoptosis. Exp Oncol. 2012;34:165. [PMC free article] [PubMed] [Google Scholar]
- Fizazi K, Higano CS, Nelson GB, et al. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2013;31:1740–47. doi: 10.1200/JCO.2012.46.4149. [DOI] [PubMed] [Google Scholar]
- Huang F, Yang Z, Yu D, et al. Sepia ink oligopeptide induces apoptosis in prostate cancer cell lines via caspase-3 activation and elevation of Bax/Bcl-2 ratio. Mar Drugs. 2012;10:2153–65. doi: 10.3390/md10102153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanafrooz Z, Motameh N, Bakhshandeh B. Comparative evaluation of silibinin effects on cell cycling and apoptosis in human breast cancer MCF-7 and T47D cell lines. Asian Pac J Cancer Prev. 2015;17:2661–65. [PubMed] [Google Scholar]
- Kamran MZ, Patil P, Gude RP. Role of STAT3 in cancer metastasis and translational advances. Biomed Res Int 2013. 2013 doi: 10.1155/2013/421821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Jung WK, Jeong MH, Yoon TR, Kim HK. Sanguinarine induces apoptosis of HT-29 human colon cancer cells via the regulation of Bax/Bcl-2 ratio and caspase-9-dependent pathway. Int J Toxicol. 2012;31:70–7. doi: 10.1177/1091581811423845. [DOI] [PubMed] [Google Scholar]
- Minaei A, Sabzichi M, Ramezani F, Hamishehkar H, Samadi N. Co-delivery with nano-quercetin enhances doxorubicin-mediated cytotoxicity against MCF-7 cells. Mol Biol Rep. 2016;43:99–105. doi: 10.1007/s11033-016-3942-x. [DOI] [PubMed] [Google Scholar]
- Pan Y, Zhou F, Zhang R, Claret FX. Stat3 inhibitor Stattic exhibits potent antitumor activity and induces chemo-and radio-sensitivity in nasopharyngeal carcinoma. PloS One. 2013;8:e54565. doi: 10.1371/journal.pone.0054565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KW, Kundu J, Chae IG, et al. Carnosol induces apoptosis through generation of ROS and inactivation of STAT3 signaling in human colon cancer HCT116 cells. Int J Oncol. 2014;44:1309–15. doi: 10.3892/ijo.2014.2281. [DOI] [PubMed] [Google Scholar]
- Pirouzpanah MB, Sabzichi M, Pirouzpanah S, Chavoshi H, Samadi N. Silibilin-induces apoptosis in breast cancer cells by modulating p53, p21, Bak and Bcl-XL pathways. Asian Pac J Cancer Prev. 2014;16:2087–92. doi: 10.7314/apjcp.2015.16.5.2087. [DOI] [PubMed] [Google Scholar]
- Renault TT, Teijido O, Antonsson B, Dejean LM, Manon S. Regulation of bax mitochondrial localization by Bcl-2 and Bcl-x L: keep your friends close but your enemies closer. Int J Biochem Cell Biol. 2013;45:64–67. doi: 10.1016/j.biocel.2012.09.022. [DOI] [PubMed] [Google Scholar]
- Sabzichi M, Samadi N, Mohammadian J, et al. Sustained release of melatonin: A novel approach in elevating efficacy of tamoxifen in breast cancer treatment. Colloids Surf B Biointerfaces. 2016;145:64–71. doi: 10.1016/j.colsurfb.2016.04.042. [DOI] [PubMed] [Google Scholar]
- Saini U, Naidu S, ElNaggar A, et al. Elevated STAT3 expression in ovarian cancer ascites promotes invasion and metastasis: a potential therapeutic target. Oncogene. 2016 doi: 10.1038/onc.2016.197. doi: 10.1038/onc.2016.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadi N, Ghanbari P, Mohseni M, et al. Combination therapy increases the efficacy of docetaxel, vinblastine and tamoxifen in cancer cells. J Cancer Res Ther. 2014;10:715. doi: 10.4103/0973-1482.139152. [DOI] [PubMed] [Google Scholar]
- Sharifi S, Barar J, Hejazi MS, Samadi N. Doxorubicin changes Bax/Bcl-xL ratio, caspase-8 and 9 in breast cancer cells. Adv Pharm Bull. 2015;5:351. doi: 10.15171/apb.2015.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- Siveen KS, Sikka S, Surana R, et al. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta 1845. 2014:136–54. doi: 10.1016/j.bbcan.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Sonpavde G, Matveev V, Burke J, et al. Randomized phase II trial of docetaxel plus prednisone in combination with placebo or AT-101, an oral small molecule Bcl-2 family antagonist, as first-line therapy for metastatic castration-resistant prostate cancer. Ann Oncol. 2012;23:1803–08. doi: 10.1093/annonc/mdr555. [DOI] [PubMed] [Google Scholar]
- Su M, Mei Y, Sinha S. Role of the crosstalk between autophagy and apoptosis in cancer. J Oncol 2013. 2013 doi: 10.1155/2013/102735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X, Kong N, Ye L, et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014;344:174–79. doi: 10.1016/j.canlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Sun X, Bao J, Nelson KC, et al. Systems modeling of anti-apoptotic pathways in prostate cancer: psychological stress triggers a synergism pattern switch in drug combination therapy. PLoS Comput Biol. 2013;9:e1003358. doi: 10.1371/journal.pcbi.1003358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–34. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Quinn BA, Das SK, et al. Targeting the Bcl-2 family for cancer therapy. Expert Opin Ther Targets. 2013;17:61–75. doi: 10.1517/14728222.2013.733001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupal A, Sabzichi M, Ramezani F, Kouhsoltani M, Hamishehkar H. Dermal delivery of doxorubicin-loaded solid lipid nanoparticles for the treatment of skin cancer. J Microencapsul. 2016;33:372–80. doi: 10.1080/02652048.2016.1200150. [DOI] [PubMed] [Google Scholar]
- von Minckwitz G. Docetaxel/anthracycline combinations for breast cancer treatment. Expert Opin Pharmacother. 2007;8:485–95. doi: 10.1517/14656566.8.4.485. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhu Y, Fang S, Li S, Liu S. Lanthanum chloride enhances cisplatin-induced apoptosis in ovarian cancer cells. Cell Mol Biol. 2016;62:1. [PubMed] [Google Scholar]
- Yu H, Lee H, Herrmann H, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14:736–46. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- Zhang J, Du J, Liu Q, Zhang Y. Down-regulation of STAT3 expression using vector-based RNA interference promotes apoptosis in Hepatocarcinoma cells. Artif Cells Nanomed Biotechnol. 2015;44:1201–5. doi: 10.3109/21691401.2015.1029628. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhang C, He J, et al. STAT3 inhibitor stattic enhances radiosensitivity in esophageal squamous cell carcinoma. Tumor Biol. 2015;36:2135–42. doi: 10.1007/s13277-014-2823-y. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bi L, Ye Y, Chen J. Formononetin induces apoptosis in PC-3 prostate cancer cells through enhancing the Bax/Bcl-2 ratios and regulating the p38/Akt pathway. Nutr Cancer. 2014;66:656–61. doi: 10.1080/01635581.2014.894098. [DOI] [PubMed] [Google Scholar]


