Abstract
Background:
Patients with recurrent or progressive lung cancer experience a significant symptom burden, negatively affecting quality of life and reducing life expectancy. Thoracic re-irradiation can be used for palliative treatment to relieve symptoms or as a curative treatment.
Methods:
Using patient charts, we identified and reviewed 28 cases that had received palliative thoracic re-irradiation for recurrent lung cancer.
Results:
Before re-irradiation, 32% of patients had stage III non-small cell lung cancer and six had small cell lung cancer. The median interval between treatments was 18.7 months. Median follow-up was 31.2 months from the initial radiotherapy and 5 months after re-irradiation. A better performance status before re-irradiation (<80 vs >80, p=0.09) and a lower overlap 90% isodose (<70 vs >70, p=0.09) showed trends toward improved survival. Grade 1-2 toxicity from re-irradiation was recorded in 12/28 patients, and no grade 3 or 4 acute toxicity was encountered.
Conclusion:
The role of palliative treatment in survival is not clear but it can provide symptomatic relief in patients, with no high grade toxicity. Further studies with greater patient numbers and longer follow-up times should facilitate determination of the role of this treatment in toxicity and effects on survival.
Keywords: Re-irradiation, locally recurrent lung cancer, quality of life
Introduction
Lung cancer is the most common cancer in the worldwide and is the leading cause of cancer death (Jemal et al., 2010). Due to advances in staging and earlier detection, use of combined treatment modalities and advances in radiation therapy (RT), patients with lung cancer are living longer (Malyezzi et al., 2013). Despite the curative intent chemotherapy (CT) and RT, five year rate of loco-regional recurrence is 30% in non-small cell lung cancer (NSCLC) (Auperin et al., 2010) and local failure occurs in 36% of patients with small cell lung cancer(SCLC) (Turrisi et al., 1999). Patients with recurrent or progressive lung cancer experience significant symptom burden, negatively affecting quality of life and short life expectancy (Hopwood et al., 1995; Kramer et al., 2004; Cetingoz et al., 2009; Jeremic et al., 2011).
Reirradiation (re-RT) with or without CT became more popular with the advent of new techniques in RT in many cancer types treatment (Dizman et al., 2014). Thoracic re-RT can be used for palliative treatment, mediastinal lymph node recurrence, to relieve symptoms, delay consequences of tumor growth or as a curative treatment for new primary lung cancers (Wu et al, 2003; Griffioen et al., 2014; Drodge et al., 2014; Kruser et al., 2014). Although improved RT techniques, uncertainty persists about the tolerance of mediastinal organs. Because of the toxicity concerns, the possibility of tumor radio-resistance and lack of robust evidence, thoracic re-RT has been limited (Paltinnikov et al., 2005).
We retrospectively analyzed our institutional experience to determine prognostic factors, clinical outcomes and survival after thoracic re-RT with external beam RT for locally recurrent lung cancer.
Materials and Methods
The design of the present study was approved by the Ethical Committee and Institutional Review Board of Necmettin Erbakan University Faculty of Medicine, where the study was conducted.
For this retrospective study we identified 28 adult patients in our center database that had received their second course of conventionally fractionated thoracic RT for recurrent lung cancer in our department between June 2010 and June 2015.
Using the patients charts, the fallowing data was obtained: sex, age, Karnofsky performance status (KPS) before first and second course RT, tumor histology and stages at the time of first and second RT, using of CT, planning tumor volume (PTV) of first and second treatment (cc), percentage of overlap PTV, RT doses of first and second course RT, cumulative biologically equivalent dose (BED), percentage of overlap 50% and 90% isodose, time between RT courses, adverse effects, symptomatic response to RT and survival times.
Thoracic recurrence was defined as documented radiographic findings and represent recurrence by interpreting radiologists. Local and distant progression was defined using clinical symptoms and imaging reports. Local recurrence was defined as a recurrence in the high-dose treatment volume and a distant recurrence as all recurrences outside this volume. A tumor was defined as a recurrence if it relapsed within 2 years of initial treatment. Prior to re-RT, patients are reviewed at a multidisciplinary tumor board. If chemotherapy is used, this is usually given sequentially, before re-RT.
Reirradiation was delivered by three dimensional conformal radiotherapy (3D- CRT) using lineer accelator (Simens, Primus). Conventional fractionation was defined median 30 Gy (24-30 Gy) for re-RT. To form the clinical target volume (CTV) 10 mm added in all directions of gross tumor volume (GTV) and to expand the CTV 5 mm to form the PTV. No elective nodal irradiation was utilized in the re-RT setting in any patient.
The overlap was described with two metrics. First, PTV overlap was the degree of physical overlap of the PTV, which consists of the tumor, pathological lymph nodes and a margin for microscopic disease in the RT process. The volume of intersection of the first and second PTV was defined as a percentage of the second PTV. Second, dosimetric overlap was the overlap between 90% and 50% of the prescribed dose in each treatment plan (90% and 50% isodoses respectively). The volume of intersection of the 90% or 50% isodoses in the first and second treatment were determined and then expressed as a percentage of the 90% and 50% isodoses from the second treatment (Figure 1; A, B, C). All of the patients received the initial treatment in our center. Normalized tumor doses in 2 Gy fractions using α/β ratio of 10 were calculated for all radiation courses (initial, re-RT, and cumulative). The cumulative BED was calculated by the addition of the BED of the first and second courses RT.
Figure 1.
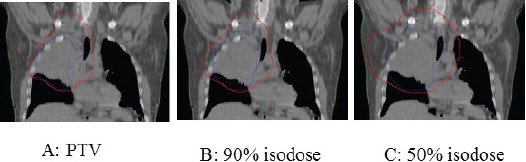
A:Overlap of Planning Target Volumes (PTV), B: The Isodoses Lines Representing 90%, C: The Isodoses Lines Representing 50%
Toxicities were scored with Common Terminology Criteria for Adverse Events version 4.0, with acute toxicity occurring within 3 months of treatment.
Follow-up was completed at October, 2016. The interval between the first and second course RT was the number of months between the start of the first and second RT. Overall survival after re-RT was the time between the first day of re-RT and last control or death from any cause.
Median overall survival (OS) and progression free survival (PFS) in the whole cohort were computed using Kaplan–Meier analysis. Median follow-up was calculated using the reverse Kaplan–Meier method. Median times between first day of diagnosis and known local progression and distant metastasis were calculated within the subgroup with documented local
progression and distant- metastasis, respectively. Kaplan–Meier analyses were performed to assess whether characteristics of the primary tumor and the re-RT (performance status, stage ≥III versus <III and type of radiotherapy [radiotherapy alone, sequential or concurrent CRT]) were associated with OS. Cox regression was used to assess whether age, percentage overlap in isodose (50% and 90%) of the two tumors were associated with OS. Statistical analyses were performed with SPSS version 18.0.1 (SPSS Inc., Chicago, IL, USA) and p-value of <0.05 was considered statistically significant.
Results
Twenty eight patients were treated for clinical and radiologic progressive disease. 25 (89%) patients were men, 3 (11%) patients were women. Staging prior to re-RT, all patients had thorax and abdominal computed tomography (CT), included a whole body 18F-fluorodeoxyglucose positron emission tomography (FDG PET) scan in 8 (29%) patients and a magnetic resonance imaging (MRI) of the brain in12 (49%). The majority of patients (50%) had stage III NSCLC, 7 (25%) patients had stage IV NSCLC at the time of initial treatment. Before the start of re-RT, 9 (32%) patients were stage III NSCLC, 12 patients (42.8%) had stage IV NSCLC disease. Five patients had limited stage SCLC and 1 patient had extensive stage SCLC at the time of initial treatment and re-RT. All SCLC patients were had mediastinal involvement at diagnosis. Initially, 5 had limited stage SCLC and were treated with definitive RT and cisplatin- etoposide chemotherapy. One extensive stage SCLC patient was treated with palliative RT for superior vena cava syndrome. Median KPS before re- RT was 70 (range: 50-100).
Before the thoracic re-RT, 18 NSCLC patients (75%) and all of SCLC patients (100%) had received CT for recurrent disease. Four patients received only RT with a median total dose of 30 Gy (range: 24-30). The median dose of the initial RT was 57 Gy (range: 30-66) given in a median of 32 fractions (range: 10–33). The median BED for the two treatments combined with 2Gy/fraction was 86 Gy (range: 64-98 Gy).
The median percentage of PTV overlap volume was 73.8% (range: 3.2–98%). Of the 28 patients, only 2 did not exhibit overlap of either the 50% or 90% isodoses. Of the remaining 26 patients, 25 had an overlap that included at least a part of the mediastinum. The median of percentage of dosimetric overlap volume for 50% and 90% of the prescribed dose was 75.1% (range: 0–96) and 67.5% (range: 0–97) respectively.
The median time to progression was 19.2 months (range: 2-53.2 months, SCLC: 23.3 months, NSCLC: 17.7 months) (Figure 2). The median interval between first and second treatment for all patients was 18.7 months (range: 6-51 months, SCLC: 27.1 months, NSCLC: 16.9 months). Median follow-up was 31.2 months (range: 7.7-66.7 months, SCLC: 33.6 months, NSCLC: 30.6 months) from the initial RT and 5 months (range: 2-20, SCLC: 1.3 month, NSCLC: 4.9 months) after re-RT (Figure 3 and 4). At the time of last review, 2/28 patients were still alive (1 SCLC, 1 NSCLC patients).
Figure 2.
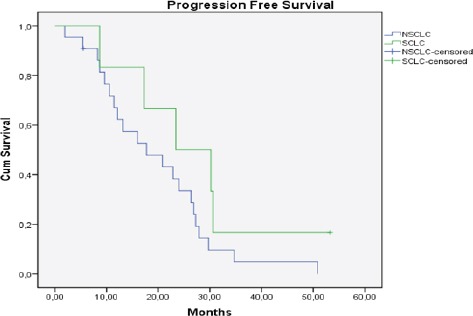
Kaplan Meier Curve of Progression Free Survival for All Patients (n=28), (NSCLC: Non Small Cell Lung Cancer, SCLC: Small Cell Lung Cancer)
Figure 3.
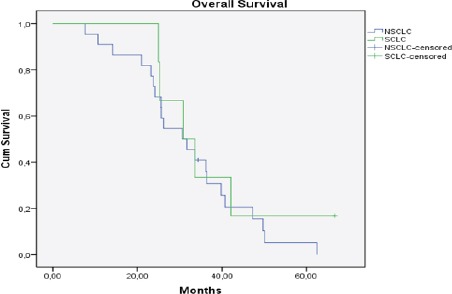
Kaplan Meier Curve of Overall Survival for All Patients (n=28), (NSCLC: Non Small Cell Lung Cancer, SCLC: Small Cell Lung Cancer)
Figure 4.
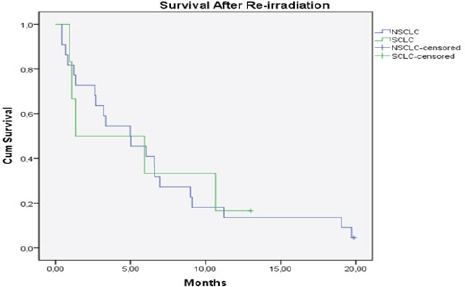
Kaplan Meier Curve of Survival After re-İrradiationfor All Patints (n=28), (NSCLC: Non Small Cell Lung Cancer, SCLC: Small Cell Lung Cancer)
At the time of control two months after re-RT, 21 (75%) patients regressed, 5 (18%) patients were stable. Only 2 (7%) patients were progressed, 1 patient had brain metastasis and 1 patient had bone metastasis after re-RT.
Of all characteristics considered, KPS before the re-RT (<80 vs>80, p=0.09) and overlap 90% isodose (<70 vs >70, p=0.09) showed a trend toward improved survival after thoracic re-RT. Kaplan–Meier analysis did not show significant associations between OS and tumor stage, RT approach (RT alone or CT-RT), interval between first and second course RT or PTV volume. Cox regression did not show significant associations between OS and age or dosimetric overlap 50%. Acute toxicity from re-RT was minimal and was recorded in 12/28 patients, consisting mainly of grade 1–2 fatigue or anorexia (n = 5), mucositis (n = 1), or esophagitis (n = 6). Grade 3 or 4 acute toxicity was not recorded. Of the 28 patients, a total of 26 were deceased at time of analysis.
Discussion
This is a retrospective analysis on the use of thoracic re-RT in a series of 28 patients with recurrent, clinically and radiological progressive lung cancer. Patients treated with conventionally fractionated palliative dose re-RT. The aim of this study was to report prognostic factors and outcomes in NSCLC and SCLC patients receiving thoracic re-RT.
Indications for re-RT can be sorted into four groups: 1; emergent and symptomatic group, e.g. superior vena cava occlusion, 2; symptomatic but not emergent group, e.g. dyspnea, 3; asymptomatic but with radiological progressive disease and 4; asymptomatic but impending serious event, e.g. airway obstruction (Drodge et al., 2014). In many studies, at least 95% patients (Kramer et al., 2004; Cetingoz et al., 2009; Jackson and Ball, 1987) and in one study just over three- quarter patients (Paltinnikov et al., 2005) were symptomatic at the time of re-RT and all patients were treated with palliative intent. In our study more than 90% patients were symptomatic and we treated with palliative dose re-RT.
Median OS was from 5 to 7 months in most reported thoracic re-RT series (Kramer et al., 2004; Gressen et al., 2000; Ebara et al., 2007; Jackson and Ball, 1987; Montebelo et al., 1993; Tada et al., 2005). Kruser et al. (2014) was reported median survival as 4.2 months, 5.1 months for NSCLC patients and 3.1 months for SCLC patients treated with thoracic re-RT. Recurrent SCLC should be considered separately because of aggressive with a dismal prognosis, especially with symptomatic. In 2 studies, high-dose re-RT was applied in carefully selected patients and median OS were 14 to 15 months (Wu et al., 2003; Okomoto et al., 2002). In our study, all patients were classified as having recurrent disease and median OS after re-RT was 4.9 months for NSCLC and 1.3 months for SCLC.
From the available studies in the literature, a better performance score before re-RT and a longer retreatment interval appear to correlate with improved survival (Cetingoz et al., 2009; Kruser et al., 2014; Tada et al., 2005; Green et al., 1982). However, with a median of 18.7 months between treatments, we did not see a significant difference in OS between patients with a re-RT interval above or below the median.
The relationship between PTV, PTV overlap and OS is uncertain in lung cancer and this observation is correlative with studies of re-RT of head and neck cancer (Chen et al., 2011). Griffioen et al., (2014) was reported that a smaller PTV size was associated with significantly better OS and EFS for high dose thoracic re-RT for lung cancer. However, 90% and 50% isodose volumes did no correlate with outcomes. In our study we used palliative dose re-RT and did not find any relationship between PTV size and OS.
Allowing for retrospective methodology, the reported high grade toxicity after conventionally fractionated re-RT is relatively low. However, in the study of Trakul et al. (2012) reports high rates of grade 4–5 toxicity specifically includes stereotactic re-RT for central lung tumors and metastases In the study by McAvoy et al., (2013) using proton radiotherapy for recurrent NSCLC, they reported 3/28 cases of grade 4 toxicity, and found a trend toward higher pulmonary toxicity in patients with central tumors (p = 0.08) but no grade 5 toxicity has been observed. Based on our follow-up notes and data, acute toxicity from re-RT was minimal and was recorded in 12/28 patients, consisting mainly of grade 1–2 fatigue or anorexia (n = 5), mucositis (n = 1), or esophagitis (n = 6). Grade 3 or 4 acute toxicity was not recorded.
There are some limitations in our study. First limitation was that, this study was based on retrospective data. The number of patients was small, they were selected patients who were received only palliative dose RT for recurrent tumor and the duration of follow-up for some was short and relatively small number of patients alive at a longer follow-up. The patient population was heterogeneous regarding pathology and stage. These factors could make statistical analysis less reliable, limit certain conclusions and statistical power and raises the possibility that other important prognostic factors could be missed. We have tried to limit the heterogeneity of our study by only selecting patients who received palliative dose conventional thoracic re-RT and focused on sub-group analyses.
Radiotherapy techniques and technology have evolved over time, high dose conventional re-RT can deliver significant survival, especially in patients with smaller PTV’s. However, normal tissue recovery in high dose treatments and the relationship with disease and patient factors is unclear. Overall toxicity appears acceptable, but the risks seem to increase. The role of palliative treatments on survival is not clear but it can provide symptomatic relief in patients with no high grade or non-acceptable toxicities. Re-RT in recurrent lung cancer with a curative or palliative dose should be decided on a case-by-case basis, by a multidisciplinary tumor board. Further studies with more patient numbers and longer follow-up times can help to determine the role of this treatment on toxicity analyses and effect on survival.
Consent
Patient gave informed consent.
Conflict of Interests
The authors state they have no conflict of interests.
References
- Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–90. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- Cetingoz R, Arican-Alicikus Z, Nur-Demiral A, et al. Is re-irradiation effective in symptomatic local recurrence of non-small cell lung cancer patients?. A single institution experience and review of the literature. J BUON. 2009;14:33–40. [PubMed] [Google Scholar]
- Chen AM, Phillips TL, Lee NY. Practical considerations in the re-irradiation of recurrent and second primary head-and-neck cancer: who, why, how, and how much? Int J Radiat Oncol Biol Phys. 2011;81:1211–9. doi: 10.1016/j.ijrobp.2011.06.1998. [DOI] [PubMed] [Google Scholar]
- Dizman A, Breuneval MC, Inan GA, et al. Reirradiation with robotic stereotactic body radiotherapy for recurrent nasopharyngeal carcinoma. Asian Pac J Cancer Prev. 2014;15:3561–6. doi: 10.7314/apjcp.2014.15.8.3561. [DOI] [PubMed] [Google Scholar]
- Drodge CS, Ghosh S, Fairchild A. Thoracic reirradiation for lung cancer: a literature review and practical guide. Ann Palliat Med. 2014;3:75–91. doi: 10.3978/j.issn.2224-5820.2014.03.04. [DOI] [PubMed] [Google Scholar]
- Ebara T, Tanio N, Etoh T, et al. Palliative re-irradiation for in-field recurrence after definitive radiotherapy in patients with primary lung cancer. Anticancer Res. 2007;27:531–4. [PubMed] [Google Scholar]
- Green N, Melbye RW. Lung cancer: retreatment of local recurrence after definitive irradiation. Cancer. 1982;49:865–8. doi: 10.1002/1097-0142(19820301)49:5<865::aid-cncr2820490507>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Gressen EL, Werner-Wasik M, Cohn J, et al. Thoracic reirradiation for symptomatic relief after prior radiotherapeutic management for lung cancer. Am J Clin Oncol. 2000;23:160–3. doi: 10.1097/00000421-200004000-00011. [DOI] [PubMed] [Google Scholar]
- Griffioen GH, Dahele M, de Haan PF, et al. High-dose, conventionally fractionated thoracic reirradiation for lung tumors. Lung Cancer. 2014;8:356–62. doi: 10.1016/j.lungcan.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Hopwood P, Stephens RJ. Symptoms at presentation for treatment in patients with lung cancer: implications for the evaluation of palliative treatment. The Medical Research Council (MRC) Lung Cancer Working Party. Br J Cancer. 1995;71:633–6. doi: 10.1038/bjc.1995.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MA, Ball DL. Palliative retreatment of locally-recurrent lung cancer after radical radiotherapy. Med J Aust. 1987;147:391–4. doi: 10.5694/j.1326-5377.1987.tb133559.x. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, et al. Cancer statistics 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Jeremić B, Videtic GM. Chest reirradiation with external beam radiotherapy for locally recurrent non-small cell lung cancer: a review. Int J Radiat Oncol Biol Phys. 2011;80:969–77. doi: 10.1016/j.ijrobp.2011.01.069. [DOI] [PubMed] [Google Scholar]
- Kramer GW, Gans S, Ullmann E, et al. Hypofractionated external beam radiotherapy as retreatment for symptomatic non-small cell lung carcinoma: an effective treatment? Int J Radiat Oncol Biol Phys. 2004;58:1388–93. doi: 10.1016/j.ijrobp.2003.09.087. [DOI] [PubMed] [Google Scholar]
- Kruser TJ, McCabe BP, Mehta MP, et al. Reirradiation for locoregionally recurrent lung cancer: outcomes in small cell and non-small cell lung carcinoma. Am J Clin Oncol. 2014;37:70–6. doi: 10.1097/COC.0b013e31826b9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvezzi M, Bertuccio P, Levi F, et al. European cancer mortality predictions for the year 2013. Ann Oncol. 2013;24:792–800. doi: 10.1093/annonc/mdt010. [DOI] [PubMed] [Google Scholar]
- McAvoy SA, Ciura KT, Rineer JM, et al. Feasibility of proton beam therapy for reirradiation of locoregionally recurrent non-small cell lung cancer. Radiother Oncol. 2013;109:38–44. doi: 10.1016/j.radonc.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Montebello JF, Aron BS, Manatunga AK, et al. The reirradiation of recurrent bronchogenic carcinoma with external beam irradiation. Am J Clin Oncol. 1993;16:482–8. doi: 10.1097/00000421-199312000-00004. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Murakami M, Yoden E, et al. Reirradiation for locally recurrent lung cancer previously treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2002;52:390–6. doi: 10.1016/s0360-3016(01)02644-x. [DOI] [PubMed] [Google Scholar]
- Poltinnikov IM, Fallon K, Xiao Y, et al. Combination of longitudinal and circumferential three-dimensional esophageal dose distribution predicts acute esophagitis in hypofractionatedreirradiation of patients with non-small cell lung cancer treated in stereotactic body frame. Int J Radiat Oncol Biol Phys. 2005;62:652–8. doi: 10.1016/j.ijrobp.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Turrisi AT, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–71. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- Tada T, Fukuda H, Matsui K, et al. Non-small-cell lung cancer: reirradiation for loco-regional relapse previously treated with radiation therapy. Int J Clin Oncol. 2005;10:247–50. doi: 10.1007/s10147-005-0501-1. [DOI] [PubMed] [Google Scholar]
- Trakul N, Harris JP, Le QT, et al. Stereotactic ablative radiotherapy for reirradiation of locally recurrent lung tumors. J Thorac Oncol. 2012;7:1462–5. doi: 10.1097/JTO.0b013e31825f22ce. [DOI] [PubMed] [Google Scholar]
- Wu KL, Jiang GL, Qian H, et al. Three-dimensional conformal radiotherapy for locoregionally recurrent lung carcinoma after external beam irradiation: a prospective phase I-II clinical trial. Int J Radiat Oncol Biol Phys. 2003;57:1345–50. doi: 10.1016/s0360-3016(03)00768-5. [DOI] [PubMed] [Google Scholar]


