Abstract
Carotenoids are the main tomato components, especially lycopene. Lycopene is more bioavailable in tomato processed products than in raw tomatos, since formation of lycopene cis-isomers during food processing and storage may increase its biological activity. In the current study, we evaluated the influence of lycopene extracts (5 mg / mL) from different tomato-based food products (paste, sauce, extract and ketchup) on cell viability and apoptosis on primary human prostate cancer cells (PCa cels) for 96h. Using MTT assay, we observed a significant decrease on primary PCa cell viability upon treatment with lycopene extracted from either 4 tomato-based food products. Flow cytometeric analysis revealed that lycopene from tomato extract and tomato sauce promoted up to fifty-fold increase on the proportion of apoptotic cells, when compared to the control group. Using real time PCR assay, we found that lycopene promoted an upregulation of TP53 and Bax transcript expression and also downregulation of Bcl-2 expression in PCa cells. In conclusion, our data demostrate that cis-lycopene promoted a significant inhibition on primary PCa cell viability, as well as an increase on their apoptotic rates, evidencing that cis-lycopene contained in tomato sauce and extract cain mainly modulate of primary human prostate cancer cell survival.
Keywords: Tomato-based food products, lycopene, prostate cancer, bioactive compounds, apoptosis, TP53
Introduction
Epidemiologic evidence indicates that high consumption of fruit and vegetables reduces the risk of chronic pathologies, such as cancer and cardiovascular disease (Rissanen et al., 2003; Jian et al., 2005). For instance, consumption of four or five servings of tomato products per week has been associated with a 40% lower risk of prostate cancer in U.S. men (Giovannucci et al., 1995). The most abundant phytonutrients in tomatoes are the carotenoids, with lycopene being the most prominent one. The mechanisms by which lycopene reduces prostate cancer risk are still unclear. Lycopene has been shown to inhibit proliferation in several tumor cell lines (Chen et al., 2001). However, the poor absorption of carotenoids by laboratory rodents has severely hampered the use of animal models in cancer prevention studies to evaluate the efficacy and mechanism of action of lycopene (Lee et al., 1999).
More than 80% of the dietary lycopene (a carotenoid without provitamin A activity) intake comes from the consumption of tomato products, including raw tomatoes, tomato juice and tomato sauces (USDA, 2003). Numerous studies have demonstrated an inverse association between dietary lycopene intake and prostate cancer risk (Mills et al., 1989; Giovannucci et al., 2002). Notably, higher consumption of tomato products, estimated dietary lycopene intake, and circulating lycopene concentrations are inversely associated with prostate cancer risk in several human cohort studies (Wan et al., 2014). Laboratory studies have provided evidence that tomato and lycopene may suppress oxidative damage, modulate intracellular signaling resulting in reduced proliferation, and enhance sensitivity to apoptosis, among other mechanisms (Tan et al., 2010). Some reports have been suggesting that tomato or lycopene intake may modulate testosterone production, serum concentrations, and metabolism, and may impact gene expression in human prostate cancer cells.
Notably, cooked lycopene or consumed in oil media, such as tomato paste, tomato sauce, or pizza, appear to be optimal for the efficient absorption of lycopene (Kirsh et al., 2006). Therefore, its biological effecacy may vary according to the specific food source and the preparation method. In order to better comprehend how lycopene can decrease prostate cancer risk, in this study we evaluated the putative effects of 4 kinds tomato-based food products on prostate cancer (PCa) cell behaviour, especially on modulating cell viability and apoptosis.
Materials and Methods
Cell culture reagents
Dulbecco’s cell culture medium (DMEM) and bovine serum albumin were obtained from Sigma, and fetal bovine serum (FBS) from Laborclin (São Paulo, Brazil). Cell culture flasks and cell scrapers were obtained from Nunc (Roskilde, Denmark). All chemicals were of analytical grade.
Samples and Lycopene extraction
Ketchup, tomato sauce, tomato extract and tomato paste were purchased in the local supermarket (Rio de Janeiro, Brazil). Lycopene extracts were obtained using ethanol as solvent (Nunes and Mercadante, 2004). Lycopene has been identified and quantified by high-performance liquid chromatography. The lycopene extracts described above were submitted to the lyophilization process. The material obtained from this process was milled to obtain a “flour” and stored in amber bottles in -18ºC until assay procedures in PCa primary cell cultures.
Isolation and Characterization of Primary Cell Cultures
PCa cells were obtained from fragments of prostate tissues obtained from cancer cases submitted to radical prostatectomy. Participants provided their written consent to participate in this study upon signature on the Consent Term established. The procedures were approved by the Ethics Committee of the Clementino Fraga Filho University Hospital, Federal University of Rio de Janeiro, Protocol-CAAE0029.0.197.000-05.
Tissues were cut in fragments of 1–3 mm3 that were grown in 24-well plates containing DMEM supplemented with 10% FBS and 1 μL/mL penicillin (Sigma). The medium was changed every 2 days. Cells were trypsinized and transferred to 25 mm2 culture dishes. After six passages, a homogeneous cell population was obtained. The cells were immunocytochemically characterized as follows. Cells were washed twice with PBS and fixed with 4% paraformaldehyde-PBS (Sigma) for 10 min. After fixation, they were washed with PBS and incubated in a 50 nM NH4Cl for 30 min. The nonspecific antibody binding was blocked with PBS/BSA 5%, and the primary antibodies were incubated overnight. We used antibodies against cytokeratin 5 (Cell Marque) and alpha-methylacyl CoA racemase (P504S-Cell Marque) in order to characterize primary PCa cells. After incubation with primary antibodies, cells were washed with PBS and incubated for 2 additional hours with either goat antimouse Alexa 488 or goat antirabbit Alexa 488/546 secondary antibodies (Invitrogen). Cell nuclei were stained with DAPI (Santa Cruz Biotechnology). Finally, cells were washed in distilled water and mounted on histological slides with N-propylgallate (Sigma). Images were captured using a confocal microscopy (Olympus IX81) and a Hamamatsu OrcaR2 digital camera.
Cell culture experiments
Primary PCa cells were plated in 25 cm2 cell culture flasks at a density of 1.0×106 cells/flask, and maintained in DMEM supplemented with 10% fetal bovine serum (FBS) and 2 g/L HEPES buffer, pH 7.4, under a 5% CO2 atmosphere. Cell passages were done twice a week and performed by trypsinization when reaching 70-80% of confluence. For each experiment, primary PCa cells were seeded at a density of 104 cells/cm2 in 6- and 96-multiwell plates for apoptosis and cell viability analyses, respectively. Lycopene extracts previously tested for solubility of the products in water and the final concentration obtained was 5 mg / mL. Lycopene extracts from the different tomato-based food products were then added to the plates. Untreated cells (controls) were included on each plate. Cells were then incubated for 96 hours with daily medium replacement.
Cell viability assays
The status of cancer cell line viability was determined by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; thiazolyl blue) assay (Sigma, New York, USA) as first described by Mosmann (1983). The cellular proliferation inhibition rate (CPIR) was calculated using the following formula: CPIR = (1–average absorbance value of experimental group/average absorbance value of control group) × 100%.
Apoptosis assays
Cells were resuspended in 400 μL of binding buffer containing 5 μL of annexin V FITC and 5 μL propidium iodide (Apoptosis Detection Kit II, BDBiosciences) for 15 min at room temperature. Annexin V binding was evaluated by flow cytometry (FACScalibur, BD Biosciences), and after acquisition of 20,000 events the data were analyzed in Cell Quest software.
Quantitative Real time PCR
Total RNA from PCa cells was extracted using RNeasy Mini Kit (Qiagen), according to the manufacturer’s instructions. RNA yield and quality were determined by a spectrophotometer NanoDrop ND-1000 V3.2 (Nanodrop Technologies, Wilmington, DE). Equal amounts (1 μg) of RNA from cells were reverse transcribed using cDNA Synthesis kit “Superscript II First-Strand Synthesis System for RT-PCR” (Invitrogen) and Oligo (dT) primer (Invitrogen). The cDNA was used as a template for subsequent real-time polymerase chain reaction (RT-PCR). Quantitative real time PCR was performed in a CFX96 Real Time System (BIORAD) C1000 Thermal Cycler using SYBRGreen systems (Applied Biosystems, Grand Island, NY) following the manufacturer’s instructions. The transcript TP53 and Bcl-2 expression levels was normalized using GAPDH as the constitutive gene. The following primers were used in real time PCR assays for these genes: TP53 (forward: 5’TAACAGTTCCTGCATGGGCGGC-3’; reverse: 5’AGGACAGGCACAAACACGCACC-3’), Bcl-2 (forward: 5’-CTGCACCTGACGCCCTTCACC-3’; reverse:
5’-CACATGACCCCACCGAACTCAAAGA-3’) and GAPDH
(forward: 5’GGTGTCGCTGTTGAAGTCAGAG-3’;
reverse: 5’GGTGTCGCTGTTGAAGTCAGAG-3’). To evaluate the quality of the RT-PCR products, melt curve analyses were conducted after each assay. Relative expression was determined using the ∆∆CT method.
Statistical analysis
The presented data are mean values ± standard error of three independent experiments done in duplicate (n = 6). Statistical comparisons were carried out by analysis of variance and post hoc Tukey’s test using Graph Pad Prism 5.0 and Statistical 6.0 program. The differences were considered significant when P<0.05.
Results
Primary PCa cells characterization
A primary PCa homogeneous cell population was obtained from PCa tissue (Figure 1A). These cells were characterized by the expression of cytokeratin 5 (CK5), a marker of basal proliferating cells in the prostate epithelium (Figure 1B). They were also positive for alpha-methylacyl CoA racemase (Figure 1C), which is considered to be a useful marker for neoplastic transformation in the prostate (Iwasa et al., 2007).
Figure 1.
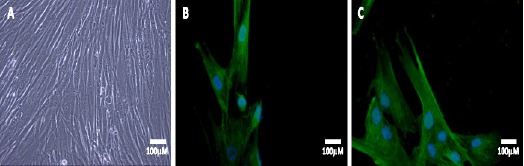
Characterization of Primary PCa Established Cells. Phase-contrast micrograph of isolated primary prostate cancer cells (A). Primary PCa cells were stained for CK5 (B) and racemase (C). Cell nuclei were stained with DAPI
Total lycopene content on tomato-base products food
Figure 2 shows total lycopene content in tomato-based products. The results showed that the average content of lycopene in tomato paste was 75.00 μg / g, 160.36 μg / g in tomato sauce, 141.71 μg / g in ketchup and 80.99 μg / g in tomato extract. The total lycopene content was statistically different between samples of tomato extract, ketchup and tomato sauce. However, total lycopene content obtained for tomato paste was not statistically different from the values presented by the tomato extract. According Barber and Barber (2002) and Waliszewski and Blasco (2010) although ketchup has high concentrations of lycopene, when evaluated ratio total carotenoids vs cis-lycopene isomers, tomato extract has a lower ratio and therefore higher cis-lycopene isomers content.
Figure 2.
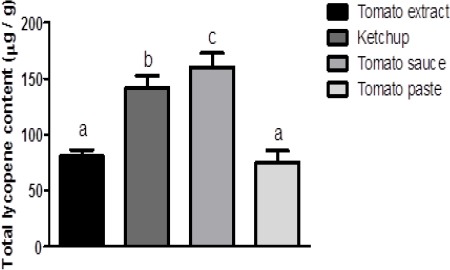
Total Lycopene Content On Tomato-Base Products Food Legend: The data is expressed as means± standard errors. Small different letters indicate significant differences (P < 0.05) among tomato-based food products
Lycopene contained in the 4 tomato products inhibit primary PCa cell viability
Primary isolated PCa cells demonstrated normal growth features which was expected when using standard in vitro conditions.
PCa cell plating was followed by 24 h recovery, and cells were subsequently incubated with 5 mg / mL of lycopene extracts for 96 h. Using MTT assay, we observed a decrease of cell viability in PCa cells after treatment with all 4 tomato-based food extracts. Lycopene from tomato paste promoted an average inhibition of 53,6% after 96 h of treatment. The effect observed with lycopene from tomato extract was a decrease of 39,7% on PCa cell viability. While lycopene from tomato sauce promoted an average inhibition of 44,1%. And lycopene from ketchup reduced cell viability around 51,1%. (Figure 3). No statistical significant difference has been observed among the 4 tomato-based food products.
Figure 3.
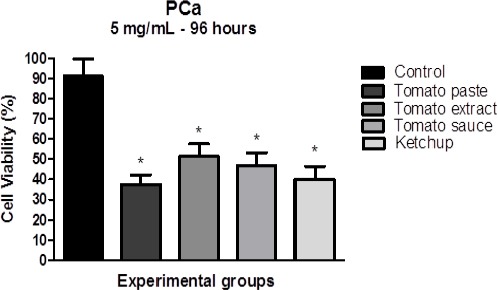
Effect of Tomato Paste, Tomato Extract, Tomato Sauce and Ketchup on PCa Cell Viability After 96 H Exposure. The results are expressed as means ± standard error. Significant differences between untreated cells (control) and those treated with tomato extracts (5 mg / mL) were compared by Tukey’s multiple comparison post-hoc test. *: Significant vs. control at P < 0.05.
Lycopene contained in the 4 tomato products induce PCa cell apoptosis
Quantification of apoptotic rates can be a useful measure of cancer cell kinetics (Table 1 and Figure 4), once alterations on the balance between proliferation and apoptosis are associated with cancer. We evaluated the effect of lycopene from all 4 tomato-based products, after 96 h of incubation on different stages of cellular death in PCa cells. Table 1 shows the percentage of viable, early apoptotic, late apoptotic and necrotic cells treated with tomato paste, tomato extract, tomato sauce and ketchup (5 mg / mL). Lycopene obtained from tomato paste, tomato extracts and tomato sauce significantly promoted apoptosis in PCa cells, with an average of 51.07-fold increase on apoptotic rates (early more late apoptosis), when compared to control (untreated cells) cells. While lycopene from tomato paste promoted a 40.7-fold increase on apoptotic rates (early more late apoptosis), the ketchup had the lowest response achieved in late apoptosis stage.
Table 1.
Effect of Tomato-Based Products on Stages of Cell Death Process in Primary PCa Cells after 96 h Treatment.
| Cell Type | Stages of Cell Death | Untreated Cells (Control) | Tomato paste | Tomato extract | Tomato sauce | Ketchup |
|---|---|---|---|---|---|---|
| 5 mg/mL | 5 mg/mL | 5 mg/mL | 5 mg/mL | |||
| PCa | Viable cells | 96.1 ± 0.8a | 44.2 ± 2.2b | 40.1 ± 2.3b | 40.2 ± 2.1b | 76.9 ± 0.9c |
| Early apoptosis | 0.8 ± 0.2a | 13.2 ± 1.7b | 9.02 ± 0.3c | 6.9 ± 0.4d | 2.9 ± 0.3e | |
| Late apoptosis | 0.7 ± 0.02a | 40.7 ± 0.4b | 49.7 ± 1.7 | 52.4 ± 1.6c | 19.1 ± 0.5d | |
| Necrotic cells | 3.2 ± 0.1a | 1.8 ± 0.1b | 1.4 ± 0.3b | 0.7 ± 0.1c | 1.1 ± 0.2d |
Legend, The data is expressed as means± standard errors; Small different letters, indicate significant differences (P < 0.05) among treatment concentration vs. control group within the same line.
Figure 4.
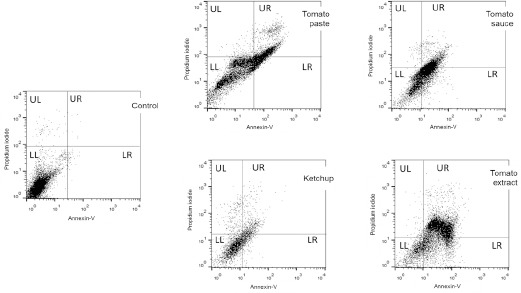
Effect of Tomato Paste, Tomato Extract, Tomato Sauce and Ketchup on PCa Programmed Cell Death After 96 H Exposure. Legend: The flow cytometric analyzes are shown according to tomato-based products. Control: Untreated cells; LL (lower left): living cells; UL (upper left): necrotic cells; LR (lower right): early apoptosis cells; UR (upper right): late apoptosis cells
Tomato-based products modulate the expression transcriotional levels of TP53, Bax and Bcl-2
In order to investigate putative molecular mechanisms by which lycopene modulate primary PCa cell viability and apoptotic cell death, we then analyzed the expression profile of two apoptosis-related genes (Figure 5). In primary PCa cells, lycopene treatment promoted a significant upregulation of TP53 and Bax transcript levels and a downregulation of Bcl-2 transcript. Lycopene from tomato extract promoted an average 52.9-fold increase for Bax while ketchup and tomato paste promoted an up-regulation around 2.2-fold increase and tomato sauce demonstrated lower effect with an up-regulation around 1.2-fold increase for this gene. In Bax transcript analysis there were no statistical differences between lycopene from tomato paste and ketchup. Ketchup and tomato extract promoted an up-regulation around 5.7-fold for TP53 gene. Whereas lycopene from tomato paste demonstrated an average 16.8-fold increase in TP53 transcript levels. Lycopene from tomato sauce had a lower effect with an up-regulation around 1.9-fold increase for TP53. There were no statistical differences between lycopene from tomato extract and ketchup. For Bcl-2 transcript levels only lycopene from tomato paste was statistically different from the other products used in this study. Lycopene from tomato paste promoted an average 1.2-fold increase for Bcl-2 gene. While lycopene obtained from tomato extract, tomato sauce and ketchup promoted an average 1.0-fold increase in Bcl-2 transcript levels. There were no statistical differences between these products.
Figure 5.
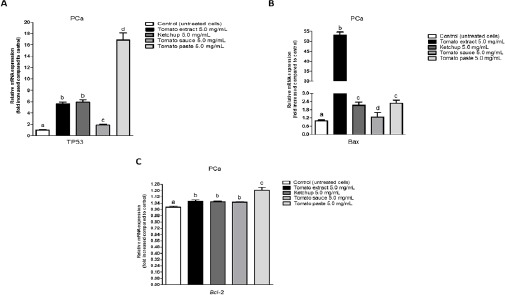
Tomato-Based Products Modulate The Expression Of TP53, Bax And Bcl-2. Quantitative real-time PCR of TP53, Bax and Bcl-2 transcript levels has been performed after 96 h treatement with tomato-based products. Transcript levels RNA GAPDH gene were used as internal control. The data is expressed as means± standard errors. Small different letters indicate significant differences (P < 0.05) among treatment concentration vs. control group.
Discussion
Prostate cancer is a common form of cancer with high incidence worldwide, ant it is a common cause of death due to cancer in both developed and non-developed countries. The interest on lycopene-rich diets and supplements for the prevention or therapy of prostate cancer has much increased during the last years (Iwasa et al., 2013). Epidemiological studies support the possibility that lycopene can reduce cancer risk. An increase in dietary consumption of lycopene was associated with decreased prostate cancer development (Holzapfel et al., 2013).
A PCa homogeneous population cell was obtained from primary PCA tissue. These cells were characterized by expression of cytokeratin 5 (CK5), a marker of basal proliferating cells in the prostate epithelium. They were also positive for alpha-methylacyl CoA racemase, which is considered to be a useful marker for neoplastic transformation in the prostate (Molinie et al., 2004). Fichtenbaum et al. (2012) demonstrated that the novel combinations of CK7 (red)/CK5 (brown) and p53 (brown)/CK5 (red) consistently highlighted basal cells surrounding in situ urothelial carcinoma in the prostate. The use of two immunohistochemical markers in a double-stain allowed for clear discrimination of in situ urothelial carcinoma from invasive urothelial carcinoma. Every case of in situ urothelial carcinoma expressed both CK7 and p53 with these double-stains. CK5 had variable reactivity in the invasive tumor and did not show circumferential staining around invasive tumor nests. All invasive urothelial carcinoma especimens were positive for CK7 and more than 80% expressed p53. Therefore, the use of CK7 (red)/CK5 (brown) or p53 (brown)/CK5 (red) is recommended to aid in the interpretation of foci of urothelial carcinoma equivocal for invasion in the prostate. This phenotype was not studied in this paper, however we demonstrated a positive labeling for CK5 in prostate cancer cells analyzed.
In our experiment PCa cells showed a higher inhibition of viability in elevated levels of lycopene obtained all tomato-based food products used in this study. Diagnosis as well as treatment of cancer is often preceded by years of cancer progression. At presentation, many patients may already have metastases. Identification of dietary components that can intercept the process in its early stages and knowledge of the concentration that is likely to be effective is therefore valuable.
Tomato products consumed in oil, such as pizza (7.5 g fat per serving), spaghetti/tomato sauce (14.6 g), and lasagna (23.8 g), are particularly bioavailable lycopene sources, due to greater intestinal absorption in association with fat. Heating processes enhance lycopene bioavailability by rupture of plant cell walls (Gartner et al., 2013; Nambiar and Singh, 2013) and transformation from the trans- to cis-isomer, which is more readily absorbed in the gut (Stahl and Sies, 1992). Lycopene in fresh tomatoes occurs almost entirely in the trans-form. In the prostate, 80% to 90% of lycopene reaching it is in the cis-form (Shi and LeMaguer, 2000). However, little is known about the formation, distribution, and biological relevance of the cis isomers of carotenoids in human tissues. Bioavailability is an important factor that can dictate the efficacy of bioactive dietary components. Only bioavailable fractions of dietary compounds can be accessible to the target cells and/or tissues, and subsequently be bioactive to these cells and/or tissues. All lycopene extracts from the different tomato-based food products were measured by HPLC and the results showed that the lycopene cis-isomers in all tomato products tested were higher than the all-trans isomers (data not shown).
The phytochemical lycopene may decrease the incidence of prostate cancer and has shown a therapeutic role in men with prostate cancer (Clinton et al., 1996; Kucuk et al., 2001). A significant increase in serum lycopene (Ansari and Gupta, 2003; Clark et al., 2006) and reduction in prostate specific antigen and tumor size was shown with daily lycopene supplements or intake of lycopene-rich foods (e.g., tomato products) in men during various stages of prostate cancer (Bowen et al., 2002). We demonstrated a significant increase in apoptosis of prostate cells, and this was indeed observed in the apoptotic cell quantification, suggesting that another mechanism may be involved. It was reported that tomato-base products are able to act as an anti-tumor agent by arresting cell proliferation and inducing apoptosis.
The tumor suppressor p53 protein plays an important role in DNA repair, cell cycle arrest, and apoptosis (Tomkova et al., 2008). Protein 53 usually present inside the cell, but is very unstable because it is bound to Mdm2 (murine doble minute 2), which functions as a marker for degradation of p53. But with an injury to genetic material, specific enzymes act to separate Mdm2 from p53. Separated from its marker, p53 does not degrade and remains in the cell, thus increasing its concentration in order to stimulate the synthesis of p21, which binds to CDK2 and Cyclin E thereby inhibiting tha action of this complex, blocking inactivation of retinoblastoma protein (pRb) and thus promotes cell cycle arrest in G1 phase so that the damaged DNA can be repaired (Borges-Osório & Robinson, 2013). An alternative of acting p53 to unrepaired damage, if the pathaway with the pRb protein is not intact, is the induction of apoptosis (Moll and Petrenko, 2003).
Through transcription-dependent pathways, p53 functions as a transactivator to up-regulate downstream proapoptotic genes, such as Bax, and/or functions as a repressor to down-regulate antiapoptotic genes, such as Bcl-2, promoting apoptosis (Soares et al., 2013). Thus, loss of TP53 in human tumors might account for the aberrant expression of Bcl-2 protein in many types of cancer. According to the data presented in this study, we observed an increase in TP53 gene expression-induced for all the products analyzed, contributing to the understanding of the results obtained in the quantification of cells in apoptosis.
However, the apoptotic process is also regulated by the mitochondria, involving different types of molecules: those that inhibit apoptosis as the Bcl-2 protein, or those that induce this process as the Bax protein. Thus, homeostasis is maintained by the balance between the amount of pro and anti-apoptotic proteins (Mita et al., 2006). Bcl-2 is the key cell apoptosis inhibitory protein. While normal human prostatic secretory epithelial cells do not express Bcl-2, immunohistochemical studies on neoplastic human prostate tissues demonstrated an elevated expression of this apoptosis inhibitory protein (Colombel et al., 1993). The increase of Bcl-2 expression has been also described in patients with locally spread or metastatic forms of prostate cancer treated using hormone ablation therapy (Green and Kroemer, 2009). The low levels of Bcl-2 expression obtained in this study may play a key role in understanding the process of cell death. In this study, in PCa cells, all tomato products promoted greater expression of Bax protein, compared to Bcl-2 expression. Thus, the Bax / Bcl-2 imbalance induced apoptosis on prostate cancer cells.
Many investigators have reported growth inhibition with all-trans lycopene in numerous prostate cancer cell lines. The effect appears to involve cell cycle arrest and apoptosis, and it occurs at physiological concentrations. The treatment of LNCaP cells with physiologically attainable concentrations of all-trans lycopene (0.3-3.0 μM) significantly reduced mitochondrial transmembrane potential, induced the release of mitochondrial cytochrome c, and increased annexin V binding, compatible with induction of apoptosis (Hantz et al.,2005). All-trans lycopene also induced apoptosis in PC-3 cells, downregulating the expression of cyclin D1 and Bcl-2 and upregulating the expression of Bax, restraining consequently the cell proliferation (Wang and Zhang, 2007). Soares et al., (2013) demonstrated that all-trans lycopene promoted apoptosis in prostate cancer (PCa) cells with an average 1.35-fold increase after 48 h treatment, reaching the maximum 2.25-fold increase after 96 h, at the highest lycopene concentration (10 μM). It also modified the equilibrium of Bcl-2/Bax expression.
Although the comparative bioavailability values of lycopene from different tomato products are unknown, lycopene obtained from processed tomato products appears to have greater bioavailability than that found in fresh tomatoes. The release of lycopene from the food matrix, due to cooking, the presence of dietary lipids and the heat-induced isomerization, from the trans- to cis-lycopene conformation favor the increase of bioavailability of this compound.
It is critical to recognize that current evidence on dietary intake and serum lycopene concentrations reflect the consumption of tomatoes and tomato products, rather than purified lycopene supplements. The pharmacokinetic properties of lycopene remain poorly understood, therefore further studies on the bioavailability, pharmacology and biology of this carotenoid are clearly warranted. Until more definitive data on the specific benefits of lycopene conformation variations are available, the recommendations should emphasize the health benefits of diets rich in a variety of fruits and vegetables, including tomatoes and tomato products.
Lycopenes from 4 tomato-based food products inhibit cell viability, increases apoptosis and regulate expression transcriptional levels of TP53, Bax and Bcl-2 in human PCa cells at the dose used here. However, the effect achieved varied according to the product employed. This study opens up a number of prospects including research and search through in vivo tests, the influence of lycopene in carcinogenesis, and in several cellular mechanisms of disease control.
References
- Ansari M, Gupta N. A comparison of lycopene and orchidectomy vs orchidectomy alone in the management of advanced prostate cancer. BJU Int. 2003;92:375–8. doi: 10.1046/j.1464-410x.2003.04370.x. [DOI] [PubMed] [Google Scholar]
- Barber NJ, Barber J. Lycopene and prostate cancer. Prostate Cancer Prostatic Dis. 2002;5:6–12. doi: 10.1038/sj.pcan.4500560. [DOI] [PubMed] [Google Scholar]
- Borges-Osório MR, Robinson WM. Genética Humana. 3rd ed. Artmed: Porto Alegre – RS; 2013. [Google Scholar]
- Bowen P, Chen L, Stacewicz-Sapuntzakis M, et al. Tomato sauce supplementation and prostate cancer: Lycopene accumulation and modulation of biomarkers of carcinogenesis. Exp Biol Med. 2002;227:886–93. doi: 10.1177/153537020222701008. [DOI] [PubMed] [Google Scholar]
- Chen L, Stacewicz-Sapuntzakis M, Duncan C, et al. Oxidative DNA damage in prostate cancer patients consuming tomato sauce-based entrees as a whole-food intervention. J Natl Cancer Inst. 2001;93:1872–79. doi: 10.1093/jnci/93.24.1872. [DOI] [PubMed] [Google Scholar]
- Clark P, Hall C, Borden L, Jr, et al. Phase I-II prospective dose-escalating trial of lycopene in patients with biochemical relapse of prostate cancer after definitive local therapy. Urology. 2006;67:1257–61. doi: 10.1016/j.urology.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Clinton S, Emenhiser C, Schwartz S, et al. Cis-trans lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiol Biomarkers Prev. 1996;5:823–33. [PubMed] [Google Scholar]
- Colombel M, Symmans F, Gil S, et al. Detection of the apoptosis-suppressing oncoprotein bc1-2 in hormone-refractory human prostate cancers. Am J Pathol. 1993;143:390–400. [PMC free article] [PubMed] [Google Scholar]
- Fichtenbaum EJ, Marsh WL, Jr, Zynger DL. CK5, CK5/6, and double-stains CK7/CK5 and p53/CK5 discriminate in situ vs invasive urothelial cancer in the prostate. Am J Clin Pathol. 2012;138:190–97. doi: 10.1309/AJCP5ZC4GQVNWTYR. [DOI] [PubMed] [Google Scholar]
- Gartner C, Stahl W, Sies H. Lycopene is more bioavailable from tomato paste than from fresh tomatoes. Am J Clin Nutr. 1997;66:116–22. doi: 10.1093/ajcn/66.1.116. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Rimm E, Liu Y, Stampfer M, Willet W. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002;94:391–8. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Ascherio A, Rimm E, et al. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. 1995;87:1767–76. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- Green D, Kroemer G. Cytoplasmic functions of the tumour supressor p53. Nature. 2009;458:1127–30. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantz HL, Young LF, Martin KR. Physiologically attainable concentrations of lycopene induce mitochondrial apoptosis in LNCaP human prostate cancer cells. Exp Biol Med. 2005;230:171–9. doi: 10.1177/153537020523000303. [DOI] [PubMed] [Google Scholar]
- Holzapfel NP, Holzapfel BM, Champ S, et al. The potential role of lycopene for the prevention and therapy of prostate cancer: from molecular mechanisms to clinical evidence. Int J Mol Sci. 2013;14:14620–46. doi: 10.3390/ijms140714620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa Y, Mizokami A, Miwa S, Koshida K, Namiki M. Establishment and characterization of androgen-independent human prostate cancer cell lines, LN-REC4 and LNCaP-SF, from LNCaP. Int J Urol. 2007;14:233–9. doi: 10.1111/j.1442-2042.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- Jian L, Du C-J, Lee A, Binns C. Do dietary lycopene and other carotenoids protect against prostate cancer? Int J Cancer. 2005;113:1010–14. doi: 10.1002/ijc.20667. [DOI] [PubMed] [Google Scholar]
- Kirsh V, Mayne S, Peters U, et al. A prospective study of lycopene and tomato product intake and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:92–8. doi: 10.1158/1055-9965.EPI-05-0563. [DOI] [PubMed] [Google Scholar]
- Kucuk O, Sarkar F, Sakr W, et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol Biomarkers Prev. 2001;10:861–8. [PubMed] [Google Scholar]
- Lee C, Boileau A, Boileau T, et al. Review of animal models in carotenoid research. J Nutr. 1999;129:2271–77. doi: 10.1093/jn/129.12.2271. [DOI] [PubMed] [Google Scholar]
- Mills P, Beeson L, Phillips R, Fraser G. Cohort study of diet, lifestyle, and prostate-cancer in Adventist men. Cancer. 1989;64:598–604. doi: 10.1002/1097-0142(19890801)64:3<598::aid-cncr2820640306>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Mita MM, Mita AC, Tolcheret AW. Apoptosis: mechanisms and implications for cancer therapeutics. Target Oncol. 2006;1:197–214. [Google Scholar]
- Molinie V, Hervé JM, Lebret T, et al. Value of the antibody cocktail anti p63 þanti p504s for the diagnosis of prostatic cancer. Ann Pathol. 2004;24:6–16. doi: 10.1016/s0242-6498(04)93902-8. [DOI] [PubMed] [Google Scholar]
- Moll UM, Petrenko O. The MDM2-p53 Interaction. Mol Cancer Res. 2003;1:1001–8. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nambiar D, Singh R. Advances in prostate cancer chemoprevention: a translational perspective. Nutr Cancer. 2013;65:12–25. doi: 10.1080/01635581.2013.785006. [DOI] [PubMed] [Google Scholar]
- Nunes IL, Mercadante AZ. Obtenção de cristais de licopeno a partir de descarte de tomate. Ciênc Tecnol Aliment Campinas. 2004;24:440–47. [Google Scholar]
- Rissanen T, Voutilainen S, Nyyssönen K, et al. Serum lycopene concentration and carotid atherosclerosis: the kuopio ischemic heart disease risk factor study. Am J Clin Nutr. 2003;77:133–8. doi: 10.1093/ajcn/77.1.133. [DOI] [PubMed] [Google Scholar]
- Shi J, LeMaguer M. Lycopene in tomatoes: chemical and physical properties affected by food processing. Crit Rev Food Sci Nutr. 2000;40:1–42. doi: 10.1080/10408690091189275. [DOI] [PubMed] [Google Scholar]
- Soares N, Teodoro A, Oliveira F, et al. Influence of lycopene on cell viability, cell cycle, and apoptosis of human prostate cancer and benign hyperplastic cells. Nutr Cancer. 2013;65:1076–85. doi: 10.1080/01635581.2013.812225. [DOI] [PubMed] [Google Scholar]
- Stahl W, Sies H. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J Nutr. 1992;122:2161–6. doi: 10.1093/jn/122.11.2161. [DOI] [PubMed] [Google Scholar]
- Tan H-L, Thomas-Ahner J, Grainger E, et al. Tomato-based food products for prostate cancer prevention: what have we learned? Cancer metastasis Rev. 2010;29:553–68. doi: 10.1007/s10555-010-9246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkova K, Tomka M, Zajac V. Contribution of p53, p63, and p73 to the developmental diseases and cancer. Neoplasma. 2008;55:177–81. [PubMed] [Google Scholar]
- USDA - United States Department of Agriculture. US Food supply database. Beltsville (MD): Center for nutrition and policy promotion; 2003. Available from: http://www.ars.usda.gov/ba/bhnrc/ndl . [Google Scholar]
- Waliszewski KN, Blasco G. Propriedades nutraceúticas del licopeno. Salud Publ Mex. 2010;52:254–65. doi: 10.1590/s0036-36342010000300010. [DOI] [PubMed] [Google Scholar]
- Wan L, Tan H-L, Thomas-Ahner J, et al. Dietary tomato and lycopene impact androgen signaling- and carcinogenesis-related gene expression during early TRAMP prostate carcinogenesis. Cancer Prev Res (Phila) 2014;7:1228–39. doi: 10.1158/1940-6207.CAPR-14-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Zhang L. Effect of lycopene on proliferation and cell cycle of hormone refractory prostate cancer PC-3 cell line. J Hyg Res. 2007;36:575–8. [PubMed] [Google Scholar]


