Abstract
Breast cancer is the fifth most common cause of death among women worldwide. Resistance to cisplatin is a main challenge in its treatment. Our present aim was to prepare nanoniosomated cisplatin and examine its efficacy in vitro using the BT-20 cell line. Niosome nanoparticles containing cisplatin were prepared by reverse-phase evaporation and characterized by dynamic light scattering (DLS), scanning electron microscopy (SEM), spectrophotometry and MTT assay. The size and zeta potential of the nanodrug were estimated as 489.3 ± 23.66 nm and 23.4 ± 2.1 mV, respectively. Drug encapsuies confirmed appropriate retention of particles. Nanoparticles also increased the cytotoxic effects of cisplatin by 1.5 times compared to the standard drug. Findings of our study suggest that niosome nanoparticles are good carriers for cisplatin delivery to breast cancer cells.
Keywords: Breast cancer, cisplatin, niosome nanoparticles, MTT, cytotoxicity
Introduction
Breast cancer is the most common diagnosed malignancy and main cause of cancer related death among women in the world (Babaei et al., 2014). Although intensive advances in chemotherapy of breast cancer were achieved, mortality rates of disease have kept fairly unchanged over the three past decades (Bayindir et al., 2015). Primary response to chemotherapeutics and subsequent resistance as relapse and metastatic spread to pivotal organs such as lung, liver and bone have considered the main failure of chemotherapy (Blanco et al., 2014). Use of nanotechnology materials fordeliveryof chemotherapeutics is a promising approach since they increase the tumor penetration of drugs, enhance the tumor targeting and simultaneously decrease the drugs side effects (Hosseinian et al., 2016).
Cisplatin haswidely used chemotherapeutic agent against various types of malignancies including breast cancer (Kanamala et al., 2016). The drug has affected cancer cells through binding to DNA molecule and consequently induction of apoptosis and necrosis (Kowapradit et al., 2012). However cisplatin usage has associated with various side effects such as nephrotoxicity and neurotoxicity(Li et al., 2015).
Niosomes are non-ionic surfactant vesicles, considering as carrier for drug delivery. They have capabilityto directly target the tumor cells. Therefore, enhance efficacy and simultaneously decrease the side effects are achieved. In addition they are able to encapsulate the lipophilic and amphiphilic drugs. Carriers in nanoscale size are used for drug delivery since they couldpenetrate into biological barriers, drug preservation and favorable release of drugs (Mokhtari et al., 2012).
We here prepared cisplatin loaded pegylated niosomal nanoparticles by thin film hydration technique. Polyethylene glycol (PEG) wasused since it improves the stability of particles (Mokhtari et al., 2012). Nanoparticles containing drug were evaluated by light microscopy and Scanning Electron Microscopy (SEM). Inaddition, they were characterized in terms ofsize, zeta potential, drug loading and encapsulation efficiency and drug retention capability. Afterthat, nanodrug efficacy as cytotoxicity effects on breast cancer cell line BT-20 was evaluated by MTT assay.
Materials and Methods
Span60 and cholesterol were provided by Sigma Company (USA). Polyethylene glycol 3,350 and cisplatin werepurchased from Kimyagaran Emrooz Chemical Ind. (Iran) andkoCak Farma Company(Turkey), respectively. Human breast cancer cell line BT-20 was supplied by the cell bank of Pasteur Institute of Iran. Distilled water was used throughout the study. All materials were analytical grade.
Preparation of niosome nanoparticles containing cisplatin
Niosomes containing cisplatin were prepared by thin film hydration technique. Briefly, Span 60, Cholesterol and PEG were dissolved in ethanol 96%. The solvent was evaporated using rotary evaporator (Heidolph, Germany) in 90 rpm and 45 °C. The thin film formed at the bottom of round bottom flask was then hydrated by Phosphate Buffer Saline (PBS, pH 7.4) containing cisplatin (1 mg/ml) and stirred (150 rpm, 30 min). The final concentration of Span, Cholesterol, PEG and Cisplatin were calculated 7, 4, 1 and 3.3 mm respectively. To provide smaller and more homogenized particles, they were sonicated using bath sonication (60 HZ. Bandelin Sonorex Digitec) for 10 min. blank nanoparticles were prepared with the abovementioned method without the drug.
Measurement of size and zeta potential of nanoparticles
Size and zeta potential of nanoparticles were calculated using Zetasizer (Nano ZS3600, Malvern Instruments, UK). Briefly, suspension of nanoparticles was diluted and the absorption was estimated in 630 nm by spectrophotometer. The suspension was then introducedto Zetasizer.
Morphological evaluation of nanoparticles
They were thenlyophilized (Edwards High Vacuum, Manor Royal, Crawley, Sussex, England) and evaluateby SEM microscopy (KYKY-EM3200, China).
Drug loading and encapsulation efficiency
To this purpose, suspension of nanoparticles containing drug was centrifuged (21,000 rpm, 30 min and 4 °C) and the supernatant was obtained. The concentration of drug into supernatant was calculated using standard curve at 320 nm by spectrophotometer and then the drug loading and encapsulation efficiency were estimated by following formulae:
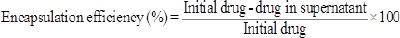
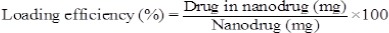
Drug release from nanoparticles
To estimate the drug retention capability of nanoparticles, sediment of nanodrug was obtained using centrifugation process (21000 rpm, 30 min, and 4°C). The sediment of nanodrug containing 2 mg cisplatin was resuspended into fresh PBS and poured into dialysis bag (Cut off; 12,000 Da Sigma), immersed into 50 ml PBS (pH7.4) and stirred (100 rpm, 37°C). At the predetermined time intervals, 2 ml of samples was withdrawn and replaced with fresh one. The cumulative release rate of drug was estimated and the relative curve was plotted.
Cytotoxicity effects of niosomal nanoparticles containing cisplatin
MTT assay was used to evaluate the cytotoxicity effects of cisplatin loaded niosomal nanoparticles. Briefly, BT-20 cell line was cultured in RPMI 1640 culture medium containing 10% FetalBovine Serum (FBS) supplemented with streptomycin/penicillin antibiotics in the concentration of 0.1 and 0.06 mg/ml respectively. Cells were cultured at37°C in a humidified incubatorcontaining 5% CO2. Once the confluency reached to 80%, cells were trypsinized and cultured into 96 well plate atthe density of 104 per well. After 24 h, the culture media was replaced with one containing cisplatin in the standard or encapsulated form. Following to 48h incubation, MTT solution (0.5 mg/ml) was substituted with culture media and left for 3h. The MTT was removed and isopropanol was added to dissolve the formazan crystals. Theabsorbance of the dissolved violet formazan crystals was read at 570 nm, using ELISA Reader device (BioTek Instruments, VT, USA). Cell viability was calculated by below formula:
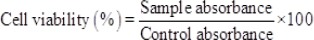
In addition, IC50 of nanodrug and standard drug was estimated by Pharm software.
Statistical analysis
SPSS software version 19was used to analyze the data. P-values<0.05 was considered significance.
Results
Nanoparticles characterization
Size and zeta potential of nanoparticles containing drug were estimated489.3 ± 23.66 nm (Figure 1) and -23.4 ± 2.1 mV, respectively.
Figure 1.
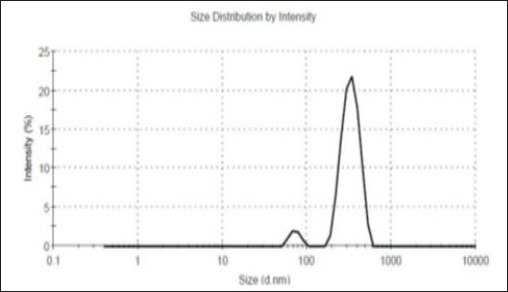
The Size of Nanoparticles
Morphological evaluation
In addition, there was no crystallization in the suspension of nanoparticles containing cisplatin. SEM evaluation also showed crystals shapes of particles (Figure 2).
Figure 2.

SEM Image of Cisplatin Loaded Niosomal Nanoparticles
Cisplatin encapsulation and loading efficiency
Calculating the drug concentration in the supernatant provide the possibility to estimate the cisplatin encapsulation and loading efficiencythat were41±2 and 2.3±0.15 percent, respectively.
Drug release from nanoparticles
The results of drug release from nanoparticles showed a sustained release pattern. However, a burst release was observed in the first hour of study, in which 32% of total release was occurred in this time. Nevertheless, at the end of study, 25% of encapsulated drug was released after 48 hours (Figure 3).
Figure 3.
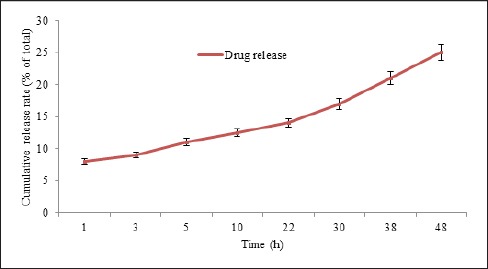
Cisplatin Release from Pegylated Niosomal Nanoparticle Versus Time
Cytotoxicity effects of niosomal nanoparticles containing cisplatin
MTT results were shown the dose dependent cytotoxicity of standard and encapsulated cisplatin. However, the cytotoxicity effects were much more when the drug becomes encapsulated into nanoparticles (Figure 4). IC50 values for nanodrug and standard drug were estimated 92 and 135 µM, respectively. In other word, nanoparticle increased the drug cytotoxicity by 65%.
Figure 4.
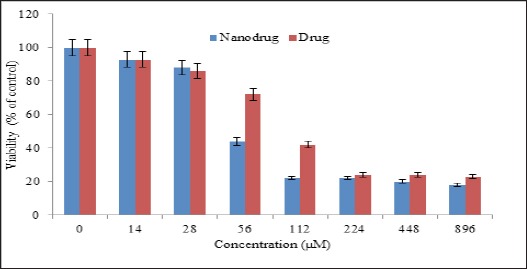
The Cytotoxicity Effects of Nanoniosomal Cisplatin and Free Cisplatin on the Human Breast Carcinoma Cell Lines BT-20. Results Are Expressed as Mean±5% Values Obtained from Three Independent Experiments
Discussion
Chemotherapy is a mainstay to treating various cancers. However it has suffered many challenges such as poor tumor selectivity and multidrug resistance (MDR). Targeted drug delivery via nanotechnology materials have afforded a new approach to solve the shortcomings (Mujoriya et al., 2012). Niosome nanoparticles are regarded as promising drug delivery system since they can act as reservoir for drugs and control drug release through changing their compositions (Ostad et al., 2010). There are various formulations of niosome containing chemotherapeutics in the literature (Pawar et al., 2016; Rajput et al., 2015). Rajput et al., (2015) provided a pH-sensitive multilamellar gold niosome containing both Akt-siRNA and thymoquinone and evaluated its efficacy against tamoxifen-resistant and Akt-overexpressing MCF7 breast cancer cells. The results of study showed the effective capability of formulation to knockdown Akt and thus sensitizing cells to thymoquinone (Pawar et al., 2016). In another study N-lauryl glucosamine (NLG) was used as a targeting ligand for doxorubicin loaded niosomal nanoparticles. The size of particles and entrapment efficiency were calculated 150 nm and 90%, respectively. In vivo pharmacokinetics study showed targeted nanoparticles have long circulation time with improved bioavailability that significantly decreased clearance and volume of distribution compared to doxorubicin solution and non-targeted niosomes (Redig et al., 2013). Drug delivery recommended in pharmaceutical biotechnology. In this regard, nanocarriers can present promising results. Niosomal nanoparticles are one of the potential nanocarriers (Pawar et al., 2016). MujoriyaR et al., (2012) studied atargeted niosomal formulation for ketoprofen delivery. The result of study showed targeted drug delivery not only increases the efficacy but simultaneously decreases the adverse effects of ketoprofen. According to the results, theyconcluded niosomal vesicles could be recruited as an appropriate delivery system (Shahbazian et al., 2015). In another study, studied on the preparation and physicochemical properties of niosomes containing aceclofenac. Aceclofenac is a medicine with poor healing properties and short biological half-life. The purpose of their study was production and also increasing the efficiency of a formulation of aceclofenac, to improve its bioavailability. In this study, the effect of different compounds such as non-ionic surfactant and cholesterol on encapsulation efficiency, nanoparticle size and drug delivery was evaluated. The results indicated that in the presence of these surfactants, the rate of drug release was increased in all formulations, and also by increasing the concentration of surfactant, the efficiency of drug retention was increased, as well (Rajput et al., 2015). In this study, we could successfully load the cisplatin on niosomal nanoparticles. The cytotoxicity of free cisplatin drug and its niosomal formulation on human breast carcinoma cell line was evaluated by MTT method and the physicochemical properties of the produced nanoparticles were examined.
The produced nanoparticles in this research were of good quality in terms of size and zeta potential. Also the produced nanodrug had less IC50 than the free drug, in terms of cytotoxicity. This shows that niosomal form of cisplatin is more efficient in killing breast carcinoma cells.
The reason for its better performance can be related to the role of niosomal nanocarrier in increasing the half-life of the drug in bloodstream, and also to its protective role. Although the loading was low in this study, but since the nanoparticles were prepared in the presence of polyethylene glycol, then they have the potential of remaining hidden. This allows them to remain longer in the bloodstream, and as a result, increase the efficiency of the drug (Shahbazian et al., 2015).
To study the pattern of drug release, dialysis tubing method was used. Release process of cisplatin containing nanoparticles included rapid diffusion in initial phase, then diffusion phase became slower. According to the obtained results, it can be suggested that most of drug release was occurred at the first 4 hours.
However, further studies are needed in order to produce a better cisplatin containing nanoparticle which has the maximum loading and efficiency, so that it can be used as a better candidate in treatment of breast cancer or other cancers. In this regard, using folate ligands, dextran or monoclonal antibodies are recommended.
Acknowledgment
This work was a part of MSc thesis that was performed in the pilot nanobiotechnology department of Pasteur Institute of Iran that hereby thanks to all colleagues.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Babaei M, Ardjmand M, Akbarzadeh A, et al. Efficacy comparison of nanoniosomal and pegylated nanoniosomal Cisplatin on A172 cell line. Tissue Eng Regen Med. 2014;11:350–54. [Google Scholar]
- Bayindir ZS, Besikci A, Yuksel N. Paclitaxel-loaded niosomes for intravenous administration: pharmacokineticsand tissue distribution in rats. Turk J Med Sci. 2015;45:1403–12. [PubMed] [Google Scholar]
- Blanco E, Ferrari M. Emerging nanotherapeutic strategies in breast cancer. The Breast. 2014;23:10–18. doi: 10.1016/j.breast.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Hosseinian S, Khajavi Rad A, Hadjzadeh M, et al. The protective effect of Nigella sativa against cisplatin-induced nephrotoxicity in rats. Avicenna J Phytomed. 2016;6:44–54. [PMC free article] [PubMed] [Google Scholar]
- Kanamala M, Wilson WR, Yang M, Palmer BD, Wu Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials. 2016;85:152–67. doi: 10.1016/j.biomaterials.2016.01.061. [DOI] [PubMed] [Google Scholar]
- Kowapradit J, Apirakaramwong A, Ngawhirunpat T, et al. Methylated N-(4-N, N-dimethylaminobenzyl) chitosan coated liposomes for oral protein drug delivery. Eur J Pharm Sci. 2012;47:359–66. doi: 10.1016/j.ejps.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Li M, Tang Z, Zhang Y, et al. Targeted delivery of cisplatin by LHRH-peptide conjugated dextran nanoparticles suppresses breast cancer growth and metastasis. Acta biomaterialia. 2015;18:132–43. doi: 10.1016/j.actbio.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Mokhtari MJ, Akbarzadeh A, Hashemi M, et al. Cisplatin induces down regulation of Bcl2 in T47D Breast cancer cell line. Adv Stud Biol. 2012;4:19–25. [Google Scholar]
- Mokhtari MJ, Akbarzadeh A, Hashemi M, et al. Cisplatin Induces Up-Regulation of KAI1, a Metastasis Suppressor Gene, in MCF-7 Breast Cancer Cell Line. Trop J Pharm Res. 2012;11:523–29. [Google Scholar]
- Mujoriya R, Babu Bodla R. Design and development of niosomal delivery system for Ketoprofen. 2012. available at http://hdl.net/123456789/97,2012 .
- Ostad SN, Dehnad S, Esmail Nazari Z, et al. Cytotoxic activities of silver nanoparticles and silver ions in parent and tamoxifen-resistant T47D human breast cancer cells and their combination effects with tamoxifen against resistant cells. Avicenna J Med Biotechnol. 2010;2:187–96. [PMC free article] [PubMed] [Google Scholar]
- Pawar S, Shevalkar G, Vavia P. Glucosamine anchored doxorubicin loaded targeted nano-niosomes: pharmacokinetic, toxicity and pharmacodynamic evaluation. J Drug Target. 2016;24:730–43. doi: 10.3109/1061186X.2016.1154560. [DOI] [PubMed] [Google Scholar]
- Redig AJ, McAllister SS. Breast cancer as a systemic disease: a view of metastasis. J Intern Med. 2013;274:113–26. doi: 10.1111/joim.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput S, Puvvada N, Kumar BNP, et al. Overcoming akt induced therapeutic resistance in breast cancer through siRNA and thymoquinone encapsulated multilamellar gold niosomes. Mol Pharmaceutics. 2015;12:4214–25. doi: 10.1021/acs.molpharmaceut.5b00692. [DOI] [PubMed] [Google Scholar]
- Shahbazian S, Akbarzadeh A, Torabi S, Omidi M. Anti-cancer activity of pegylated liposomal trans-anethole on breast cancer cell lines MCF-7 and T47D. Biotechnol Lett. 2015;37:1355–9. doi: 10.1007/s10529-015-1813-5. [DOI] [PubMed] [Google Scholar]


