Abstract
Background:
The serum level of gastrin-releasing peptide precursor (proGRP) is generally? elevated in patients with small cell lung cancer (SCLC). However, the diagnostic sensitivity and specificity of serum proGRP in SCLC cases remains controversial. The study aimed to assess the diagnostic value of this biomarker by meta-analysis.
Materials and Methods:
The Cochrane, Clinical trials, Pubmed, Web of Science and Embase databases were searched and diagnostic values were calculated or extracted. Statistical analysis was accomplished with RevMan 5.3 and STATA 12.0 software.
Results:
A total of 27 studies with 7268 participants were included. The pooled sensitivity, specificity, PLR, NLR and DOR were 0.754 (95% CI: 0.700-0.802), 0.945 (95% CI: 0.916-0.965), 13.804 (95% CI: 9.096-20.948), 0.260 (95% CI: 0.213-0.317) and 53.101 (95% CI: 34.327-82.145) respectively. The AUC was 0.910 (95% CI: 0.880-0.930). Significant publication bias was not found (P =0.622).
Conclusions:
The meta-analysis indicated that serum proGRP is indeed a useful biomarker with goodsensitivity and high specificity for diagnosis of SCLC. Therefore proGRP can be expected to be widely applied in the clinic for identification of lung cancer patients.
Keywords: proGRP, diagnosis, SCLC, meta-analysis
Introduction
Lung cancer is one of the most common malignant tumor in China and even in the world, and it is also one of malignant tumors with the highest mortality (Chen et al., 2016; Siegel et al., 2016). According to histological classification, it can be divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) (Nordlund et al., 2012). Although SCLC accounts for only 15-20% of lung cancer, it is an invasive and rapidly growing tumor (Jackman and Johnson, 2005). When majority of patients were diagnosed with SCLC, the tumor metastasis to regional lymph nodes or distant organs has occurred. Therefore, early diagnosis is particularly important and will be expected to bring favorable survival for patients because SCLC is highly sensitive to chemotherapy and radiotherapy (Shibayama et al., 2001). Accordingly, many researchers stated that sensitive and specific tumor makers would provide available information for early diagnosis and therapy of SCLC.
In the early stages, Neuron specific enolase (NSE) was a tumor marker of SCLC and used to diagnose diseases preferentially, but it has low positive rates in patients with LD-SCLC as clinical data shown (Carney et al., 1982; Shibayama et al., 1992) and always elevated in neuroendocrine neoplasm. So high specificity biomarker in SCLC got the attention of researchers and was expected to be found. Currently, the gastrin-releasing peptide precursor (proGRP) has been widely researched in lung cancer. Many studies have shown that the serum level of proGRP was always elevated in patients with SCLC. Some reported that proGRP had the highest diagnostic sensitivity in SCLC followed by NSE (47% versus 45%) corresponding to a specificity of 95% (Stieber et al., 1999). While other demonstrated that the proGRP was a specific tumor marker of SCLC, with a 97% specificity and a 76% sensitivity (Miyake et al., 1994). Because of these inconsistent findings, whether proGRP is a good tumor marker for diagnosis of SCLC hasn’t reached consensus yet and been used universally in clinical detection.
Therefore, increasing new studies to expand sample size and using the power statistical software (STATA12.0) to analyze data, we conducted this meta-analysis to assess the potential diagnostic value of serum proGRP as biomarker for SCLC patients.
Materials and Methods
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (http://www.prismastatement.org/statement.htm). Review protocol can be acquired on the website http://www.crd.york.ac.uk/PROSPERO/ with registration number CRD42014010777.
Literature retrieval
All literatures about serum level of proGRP in the diagnosis of SCLC were searched in the Cochrane, Clinical trials, Pubmed, Web of Science and Embase databases. The key search words were: small cell lung cancer/SCLC, gastrin-releasing peptide precursor/proGRP, biomarker.
Selection criteria
These literatures from databases were first screened by titles and abstracts, and then full-text articles of qualified studies were achieved for further review. Two authors reviewed all records independently and eventually reached consensus.
We selected eligible studies according to the following inclusion criteria: 1) SCLC patients should be diagnosed by histopathology as experimental group; the participants with no SCLC were regarded as control group, including NSCLC, benign lung disease, healthy people, etc. 2) Reference standard: serum proGRP assay was the diagnosis tool; 3) enough data to constitute the diagnostic 2×2 table. Those studies were excluded according to the following criteria: 1) the serum proGRP was not used to diagnose SCLC; 2) insufficient data to constitute the diagnostic 2×2 table.
Data extraction
We extracted the information from each study: author’s name, year of publication, country of origin, histological type, detection method and cut-off of the serum proGRP, source of reagent, true positive (TP), false positive (FP), false negative (FN) and true negative (TN).
Quality assessment
Methodological quality of eligible studies was evaluated by quality assessment of diagnostic accuracy studies 2 (QUADAS-2) (Whiting et al., 2011). It is composed of 4 key domains (patient selection, reference standard, index test, flow and timing) which is designed to judge the quality of primary diagnostic accuracy studies. The QUADAS-2 plot about risk of bias and applicability concerns summary was produced by Review Manager 5.3 software.
Statistical analysis
The effect of threshold were calculated by the Spearman correlation and P value < 0.05 suggested significant threshold effect using Meta-DiSc 1.4 software.
The statistical analysis were performed by means of the STATA 12.0 software with the MIDAS module. The “Bivariate Binomial Mixed Model” was chosen as the default format to analyze the data in this software. The overall sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR) and corresponding 95% CI (95% CI) were calculated by those values (TP, FP, FN and TN). The summary receiver operative (SROC) curve was generated and the area under the curve (AUC) was calculated. Q test and the inconsistency index (I2) were used to measure the heterogeneity caused by non-threshold effect. I2 value ≥ 50% and P value ≤ 0.05 suggested significant heterogeneity which was caused by non-threshold effect. Sub-group analyses were performed to seek for the source of heterogeneity. Publication bias was examined by the Deek’s funnel plot and P value < 0.05 indicated the existence of publication bias.
Results
Characteristics of eligible studies
The general information of included articles was shown in Table 1. After searching the databases, 27 (Miyake et al., 1994; Aoyagi et al., 1995; Yamaguchi et al., 1995; Takada et al., 1996; Okusaka et al., 1997; Goto et al., 1998; Stieber et al., 1999; Sunaga et al., 1999; Lamy et al., 2000; Yang et al., 2000; Shibayama et al., 2001; Li et al., 2003; Oremek and Sapoutzis, 2003; Molina et al., 2004; Molina et al., 2005; Shi et al., 2005; Oremek et al., 2007; Wojcik et al., 2008; Molina et al., 2009; Nisman et al., 2009; Zhang et al., 2009; Holdenrieder et al., 2010; Wang et al., 2010; Nordlund et al., 2012; Dilmaghani-Marand and Oremek, 2013; Zeng et al., 2016) studies with 7268 participants met the inclusion criteria and finally were adopted in this meta-analysis. The publication year was range from 1994 to 2016 with smallest patient number of 90 and largest patient number of 802. Seven articles were come from China mainland, nine from Japan, four from Germany, three from Spain and one from France, Israel, Poland and Norway each. The serum level of proGRP was detected by ELISA (enzyme-linked immunosorbent assay) among 23 studies. The cut-off value of serum proGRP was range from 33 to 101 ng/L among 24 studies and 3 studies were not reported about it. SCLC patients were 2062 and the control group were 5206. The values of TP, FP, FN and TN were extracted or calculated for each study.
Table 1.
The General Characteristics of Eligible 27 Studies
| Studies | Region | Assay | Cut off | SCLC | Control | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|
| Zeng 2016 | China | ECLIA | 73.5 | 31 | 131 | 23 | 7 | 8 | 124 |
| Li 2003 | China | ELISA | 40.0 | 30 | 60 | 22 | 1 | 8 | 59 |
| Shi 2005 | China | ELISA | NR | 45 | 125 | 35 | 50 | 10 | 75 |
| Yang(a) 2000 | China | ELISA | 40.0 | 30 | 60 | 22 | 1 | 8 | 59 |
| Yang(b) 2005 | China | ELISA | 46.0 | 63 | 81 | 46 | 9 | 17 | 72 |
| Zhang 2009 | China | ELISA | 46.0 | 83 | 125 | 60 | 3 | 23 | 122 |
| Wang 2010 | China | ELISA | 50 | 21 | 115 | 14 | 2 | 7 | 113 |
| Yoshio 1994 | Japan | RIA | NR | 140 | 658 | 107 | 14 | 33 | 644 |
| Ken 1995 | Japan | ELISA | 50.0 | 127 | 351 | 80 | 4 | 47 | 347 |
| Katsumi 1995 | Japan | ELISA | 45.1 | 25 | 287 | 18 | 2 | 7 | 285 |
| Takada 1996 | Japan | ELISA | 33.8 | 101 | 114 | 63 | 6 | 38 | 108 |
| Takuji 1997 | Japan | ELISA | 46.0 | 44 | 77 | 31 | 7 | 13 | 70 |
| Koichi 1997 | Japan | ELISA | 46.0 | 206 | 544 | 140 | 23 | 66 | 521 |
| Stieber 1999 | Japan | ELISA | 38.3 | 87 | 74 | 41 | 4 | 46 | 70 |
| Sunaga 1999 | Japan | ELISA | 46.0 | 48 | 79 | 36 | 5 | 12 | 74 |
| Shibayama 2000 | Japan | ELISA | 49.0 | 114 | 142 | 74 | 5 | 40 | 137 |
| Oremek(a) 2003 | Germany | ELISA | 87.0 | 80 | 129 | 80 | 67 | 0 | 62 |
| Oremek(b) 2007 | Germany | SLT-S | 87.0 | 80 | 209 | 67 | 10 | 13 | 199 |
| Stefan 2010 | Germany | ELISA | NR | 53 | 128 | 32 | 6 | 21 | 122 |
| Bijan 2013 | Germany | EIA | 101.0 | 50 | 90 | 50 | 4 | 0 | 86 |
| Molina(a) 2004 | Spain | ELISA | 50.0 | 41 | 122 | 32 | 32 | 9 | 90 |
| Molina(b) 2005 | Spain | ELISA | 50.0 | 73 | 568 | 47 | 93 | 26 | 475 |
| Molina(c) 2009 | Spain | ELISA | 50.0 | 175 | 627 | 134 | 79 | 41 | 548 |
| Lamy 2000 | France | ELISA | 53.0 | 146 | 59 | 117 | 2 | 29 | 57 |
| Nisman 2009 | Israel | ELISA | 48.0 | 37 | 88 | 28 | 2 | 9 | 86 |
| Wojcik 2008 | Poland | ELISA | 49.0 | 83 | 34 | 68 | 3 | 15 | 31 |
| Marianne 2012 | Norway | ELISA | 330 | 49 | 129 | 40 | 6 | 9 | 123 |
ECLIA, electrochemiluminescence assays; RIA, radioimmunoassay; SLT-S, SLT-Spectra photometer; EIA, enzyme-immunological assay; NR, not reported.
Diagnosis sensitivity and specificity
The total results of meta-analysis were listed in Table 2, including overall and subgroup analysis. After evaluating no significant threshold effect with Spearman correlation coefficient and Visual assessment of ROC plane as follow, the sensitivity and specificity were calculated by Bivariate Binomial Mixed Model with the MIDAS module of STATA 12.0. Against control group, the pooled sensitivity and specificity of proGRP for the diagnosis of SCLC were 0.754 (95% CI: 0.700-0.802) and 0.945 (95% CI: 0.916-0.965) respectively (Figure 1).
Table 2.
The Total Results of Meta-Analysis
| Sensitivity | Specificity | PLR | NLR | DOR | AUC | |
|---|---|---|---|---|---|---|
| Overall | 0.754 (0.700-0.802) | 0.945 (0.916-0.965) | 13.804 (9.096-20.948) | 0.260 (0.213-0.317) | 53.101 (34.327-82.145) | 0.9 (0.88-0.93) |
| Country | ||||||
| China | 0.731 (0.676-0.780) | 0.952 (0.872-0.983) | 15.395 (5.580-42.478) | 0.282 (0.234-0.340) | 54.589 (19.102-156.004) | 0.8 (0.73-0.80) |
| Japan | 0.664 (0.606-0.717) | 0.968 (0.948-0.980) | 20.513 (12.494-33.67) | 0.347 (0.294-0.411) | 59.040 (32.721-106.527) | 0.9 (0.86-0.91) |
| other | 0.846 (0.724-0.921) | 0.911 (0.837-0.953) | 9.476 (5.246-17.118) | 0.169 (0.092-0.310) | 56.215 (25.140-125.702) | 0.9 (0.92-0.96) |
| Detection Method | ||||||
| ELISA | 0.731 (0.677-0.779) | 0.941 (0.904-0.964) | 12.434 (7.814-19.785) | 0.286 (0.240-0.341) | 43.483 (27.692-68.280) | 0.9 (0.85-0.91) |
| Other | 0.878 (0.666-0.963) | 0.964 (0.943-0.977) | 24.197 (15.56-37.631) | 0.127 (0.041-0.338) | 191.188 (55.95-653.365) | 1.0 (0.96-0.99) |
Figure 1.
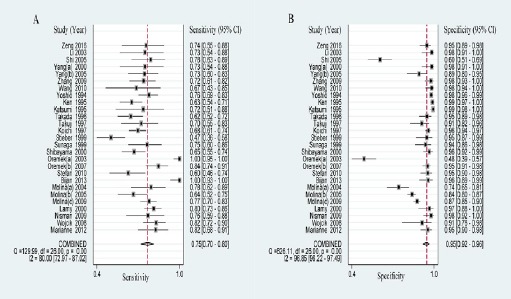
Forest Plots Of Sensitivity And Specificity Of Progrp
Pooled likelihood ratio & diagnosis odds ratio
The PLR is defined as sensitivity/(1-specificity) and the NLR is defined as (1-sensitivity)/specificity. PLR>5.0 and NLR< 0.2 were considered as clinically useful test. DOR (namely PLR/NLR) is a measure which combined sensitivity and specificity (Glas et al., 2003). In this meta-analysis, the results of PLR and NLR were 13.804 (95% CI: 9.096-20.948) and 0.260 (95% CI: 0.213-0.317) respectively (Figure 2). The DOR was 53.101 (95% CI: 34.327-82.145) (Supplementary Figure 1).
Figure 2.
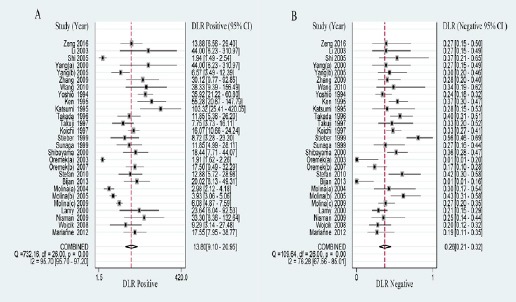
Forest Plots Of Positive And Negative Likelihood Ratio Of Progrp
Supplementary Figure 1.
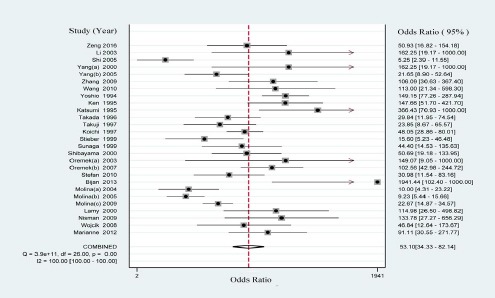
Forest Plot of Diagnosis Odds Ratio of proGRP
Area under the SROC cure and Fagan’s nomogram
The SROC curve and Fagan plot were drawn and calculated by STATA12.0 software. In this meta-analysis, the pooled area under the SROC curve was 0.910 (95% CI: 0.880-0.930), suggesting that proGRP had high diagnostic accuracy (Figure 3A). The visual presentation of diagnostic performance were assessed by Fagan plot. The posterior probability of proGRP was 78% which was higher than the prior probability of 20% (Figure 3B).
Figure 3.
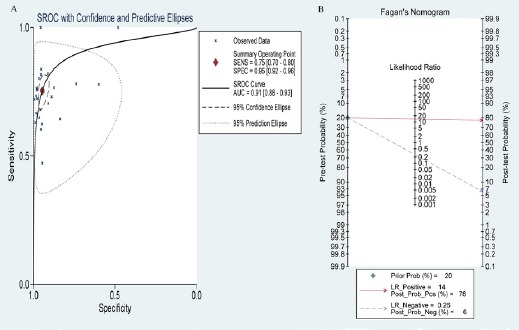
The Characteristics of SROC Curve and Fagan Plot of proGRP
Threshold effect and heterogeneity
Threshold effect is an important source of heterogeneity between studies. The Spearman correlation coefficient was 0.108 and the P value was 0.591 (P >0.05), which indicated no significant threshold effect. In addition, Visual assessment of ROC plane also showed that there was not significant threshold effect (Figure 4A). In the figure 4B, there was a regression line going through the origin. Six studies outside these 95% boundaries were regarded as outliers, suggesting that majority of those studies were homogeneous. Nevertheless, as shown in the forest plots of these values (sensitivity, specificity, PLR, NLR and DOR), the heterogeneity of pooled results existed obviously with I2 value ≥ 50% and P value ≤ 0.05. The subgroup analysis of China, Japan and ELISA detection method was measured to seek for the source of heterogeneity (Table 2). Unfortunately, both of them didn’t contributed to the heterogeneity.
Figure 4.
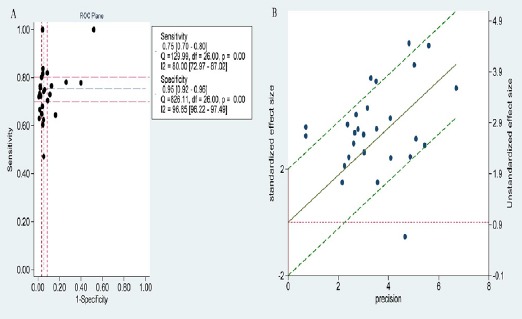
The ROC Plot and Radial Plot of Logit-tpr of proGRP
Publication bias and Quality assessment
The Deek’s funnel plot was used to evaluate the publication bias. As shown, the funnel plot and P value 0.622 (>0.05) indicated that there wasn’t publication bias (Figure 5). The results of quality assessment displayed that risks of bias were largely unclear in aspect of patient selection, index text and reference standard. However, the corresponding applicability concerns were low (Supplementary Figure 2).
Figure 5.
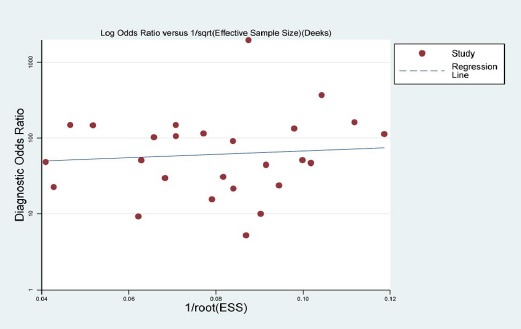
Deek’s Funnel Plot of Publication Bias
Supplementary Figure 2.
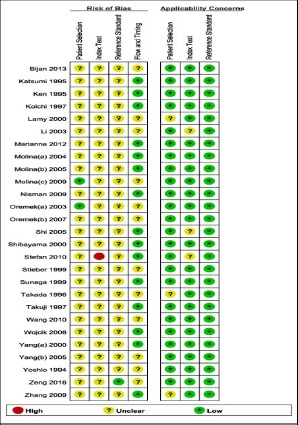
Summary of QUADAS-2 Plot
Discussion
Gastrin-releasing peptide (GRP) is a gut hormone which is isolated originally from procine stomach (McDonald et al., 1978) and well known to be secreted from SCLC cells(Yamaguchi et al., 1985; Yamaguchi et al., 1986). The proGRP is a more stable precursor of GRP, and related research points it is specific for SCLC (Miyake et al., 1994). In serum, it was elevated in SCLC patients with limited disease as well as those with extensive disease at nearly the same frequency (Oremek and Sapoutzis, 2003). As a tumor marker, compared with NSE for diagnosis of SCLC, the sensitivity of proGRP was significantly higher and ROC analysis confirmed the superiority of proGRP (Shibayama et al., 2001; Oremek and Sapoutzis, 2003; Molina et al., 2009).
In order to evaluate the diagnostic value of serum proGRP for SCLC patients, we finally adopted 7,268 subjects. After evaluating no significant threshold effect with Spearman correlation coefficient and Visual assessment of ROC plane, the pooled diagnostic sensitivity and specificity were 0.754 (95% CI: 0.700-0.802) and 0.945 (95% CI: 0.916-0.965). In view of research on this aspect, there were two articles that one was a systematic review with 6,759 participants and another was a meta-analysis with 5146 participants (Tang et al., 2011; Yang et al., 2011). In their results, the pooled sensitivity (95% CI) were 0.72 (0.70-0.75) and 0.716 (0.688-0.743) respectively; the pooled specificity (95% CI) were 0.93 (0.92-0.94) and 0.921 (0.909-0.932) respectively. Look at the comparison, our result owned higher sensitivity and specificity, and serum proGRP was confirmed once again that it was good biomarker to distinguish SCLC patients from participants without suffering from this disease. Additionally, it was noteworthy that this systematic review results above were affected with relatively poor study quality, while another study only referred to a quality assessment in methodology. For this meta-analysis, the supplementary Figure 2 showed that the quality of literatures were not high.
In the study, the overall PLR and NLR were 13.8 (>5.0) and 0.3 (nearly 0.2) respectively, reflecting the authenticity of diagnostic tests well. The AUC in pooling studies by means of the ROC curve was 0.9 (95% CI: 0.880-0.930). A perfect test is the AUC close to 1 and a poor test has the AUC close to 0.5. Likewise, the previous two studies displayed that the AUC were 0.9 and 0.9 respectively. Obviously, the 0.910 AUC was close to 1 which was slightly lower than 0.9226. Both high AUC (0.9) and DOR (53.1) suggested an overall high diagnostic accuracy of serum proGRP. In addition, the 20% prior probability and posterior 78% probability of proGRP presented the clinical utility of proGRP.
Although 27 studies were almost homogeneous and not significant threshold effect, our analytical results showed obvious heterogeneity (P value < 0.05). Hence, we performed the heterogeneity test by means of subgroup analysis such as country and detection method. As a result, both of them didn’t contribute to this heterogeneity. On a cautionary note, objects region, assay method, study size, specimen storage temperature and cut-off value were analysed as possible sources of heterogeneity in a meta-analysis mentioned above. Consistent with our findings, there were no statistical difference in p-values. However, the systematic review pointed out that inter-laboratory study conduction or reagent variations led to high heterogeneity across all studies with ELISA. Actually, many aspects including clinical difference, methodological design and statistical choice were possible sources of heterogeneity. For instance, these subjects in our study were quantitative diversity of SCLC patients who were limited or extensive stage in each study. Furthermore, considering the mutability of morbidity about SCLC, we didn’t calculate the positive predictive value and the negative predictive value.
Besides playing a role in diagnosis, some research reported that proGRP was useful predictor of response to chemotherapy and survival in patients with SCLC (Huang et al., 2016). Recently, there were some studies about plasma proGRP which was a highly specific marker for neuroendocrine (NE) lung tumors and has the highest sensitivity in the most common high grade NE lung malignancy. With a sensitivity of 84% and specificity of 96.3% at a cut off of 140 pg /ml, the robustness of plasma ProGRP as a marker for the diagnosis of SCLC was demonstrated by the results of the ROC analysis in the cross-validated model of SCLC vs NSCLC (Hye-Ran et al., 2011; Nisman et al., 2016). What’s more, the combination of proGRP and NSE was also researched to diagnose SCLC patients and more better compared to individual tumor marker (Molina et al., 2016). Furthermore, a study reported the combination of HE4 with proGRP and NSE provided an efficient tool to differential diagnosis NSCLC from SCLC (Zeng et al., 2016). However, it is important to define the thresholds of marker kinetics to improve the interpretation according to the individual level.
During recent years, circulating tumor DNA (ctDNA) in the blood was detected from the majority of tumor patients eligible for companion diagnosis. The combined use of well-established protein-based tumor biomarkers and new ctDNA-based biomarkers will be implied as new diagnostic strategies in the future (Holdenrieder, 2016). To explore the biological function of proGRP and its potential mechanism, there was a finding that proGRP in SCLC tissues and cell lines was overexpressed and associated with cell proliferation and progression (Gong et al., 2016). Therefore, we speculate it is promising to combine the proGRP and specific ctDNA to diagnose lung cancer.
In a word, although doing the similar research work, we expanded sample size and used power STATA 12.0 software to analyze the data. In summary, the results of meta-analysis are nearly consistent with the previous studies, and it further suggests that the serum proGRP is a valuable biomarker with better sensitivity and high specificity for adjunctive diagnosis of SCLC. The proGRP is expected to be widely applied to clinical for lung cancer patients as a useful biomarker.
Statement conflict of Interest
The authors have declared that they have no conflict of interests.
Acknowledgments
This work was supported by NSFC (Natural Science Foundation of China) (81360351), Start-Up Fund for Doctor of Zunyi Medical University and the Department of Science and Technology of Guizhou Province (Grant No. Qian Ke He SY[2013] 3003). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Aoyagi K, Miyake Y, Urakami K, et al. Enzyme immunoassay of immunoreactive progastrin-releasing peptide (31-98) as tumor marker for small-cell lung carcinoma: development and evaluation. Clin Chem. 1995;41:537–43. [PubMed] [Google Scholar]
- Carney D, Ihde D, Cohen M, et al. Serum neuron-specific enolase: a marker for disease extent and response to therapy of small-cell lung cancer. Lancet. 1982;319:583–5. doi: 10.1016/s0140-6736(82)91748-2. [DOI] [PubMed] [Google Scholar]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- Dilmaghani-Marand BB, Oremek GM. Tumor markers in patients with benign and malignant pulmonary diseases;competitive evaluation of ProGRP, NSE and Cyfra 21-1. Recent Pat Biomark. 2013;3:145–52. [Google Scholar]
- Glas AS, Lijmer JG, Prins MH, et al. The diagnostic odds ratio: a single indicator of test performance. JCE. 2003;56:1129–35. doi: 10.1016/s0895-4356(03)00177-x. [DOI] [PubMed] [Google Scholar]
- Gong Z, Lu R, Xie S, et al. Overexpression of pro-gastrin releasing peptide promotes the cell proliferation and progression in small cell lung cancer. Biochem Biophys Res Commun. 2016;479:312–8. doi: 10.1016/j.bbrc.2016.09.066. [DOI] [PubMed] [Google Scholar]
- Goto K, Kodama T, Hojo F, et al. Clinicopathologic characteristics of patients with nonsmall cell lung carcinoma with elevated serum progastrin-releasing peptide levels. Cancer. 1998;82:1056–61. doi: 10.1002/(sici)1097-0142(19980315)82:6<1056::aid-cncr7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Holdenrieder S. Biomarkers along the continuum of care in lung cancer. Scand J Clin Lab Invest Suppl. 2016;245:40–5. doi: 10.1080/00365513.2016.1208446. [DOI] [PubMed] [Google Scholar]
- Holdenrieder S, Von Pawel J, Duell T, et al. Clinical relevance of Thymidine Kinase for the diagnosis, therapy monitoring and prognosis of non-οperable lung cancer. Anticancer Res. 2010;30:1855–62. [PubMed] [Google Scholar]
- Huang Z, Xu D, Zhang F, et al. Pro-gastrin-releasing peptide and neuron-specific enolase: useful predictors of response to chemotherapy and survival in patients with small cell lung cancer. Clin Transl Oncol. 2016;18:1019–25. doi: 10.1007/s12094-015-1479-4. [DOI] [PubMed] [Google Scholar]
- Hye-Ran K, In-Jae O, Myung-Geun S, et al. Plasma proGRP concentration is sensitive and specific for discriminating small cell lung cancer from nonmalignant conditions or non-small cell lung cancer. J Korean Med Sci. 2011;26:625–30. doi: 10.3346/jkms.2011.26.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005;366:1385–96. doi: 10.1016/S0140-6736(05)67569-1. [DOI] [PubMed] [Google Scholar]
- Lamy PJ, Grenier J, Kramar A, et al. Pro-gastrin-releasing peptide, neuron specific enolase and chromogranin A as serum markers of small cell lung cancer. Lung Cancer. 2000;29:197–203. doi: 10.1016/s0169-5002(00)00113-6. [DOI] [PubMed] [Google Scholar]
- Li A, Yang J, Li X, et al. Diagnostic value of serum ProGRP31-98 in patients with small-cell lung cancer. AJXJTU. 2003;15:44–6. [Google Scholar]
- McDonald T, Nilsson G, Vagne M, et al. A gastrin releasing peptide from the porcine nonantral gastric tissue. Gut. 1978;19:767–74. doi: 10.1136/gut.19.9.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Y, Kodama T, Yamaguchi K. Pro-gastrin-releasing peptide(31-98) is a specific tumor marker in patients with small cell lung carcinoma. Cancer Res. 1994;54:2136–40. [PubMed] [Google Scholar]
- Molina R, Auge JM, Bosch X, et al. Usefulness of serum tumor markers, including progastrin-releasing peptide, in patients with lung cancer: Correlation with histology. Tumor Biol. 2009;30:121–9. doi: 10.1159/000224628. [DOI] [PubMed] [Google Scholar]
- Molina R, Auge JM, Filella X, et al. Pro-gastrin-releasing peptide (ProGRP) in patients with benign and malignant diseases: Comparison with CEA, SCC, CYFRA 21-1 and NSE in patients with lung cancer. Anticancer Res. 2005;25:1773–8. [PubMed] [Google Scholar]
- Molina R, Filella X, Auge JM. ProGRP: A new biomarker for small cell lung cancer. Clin Biochem. 2004;37:505–11. doi: 10.1016/j.clinbiochem.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Molina R, Marrades RM, Auge JM, et al. Assessment of a combined panel of six serum tumor markers for lung cancer. Am J Respir Crit Care Med. 2016;193:427–37. doi: 10.1164/rccm.201404-0603OC. [DOI] [PubMed] [Google Scholar]
- Nisman B, Biran H, Ramu N, et al. The diagnostic and prognostic value of ProGRP in lung cancer. Anticancer Res. 2009;29:4827–32. [PubMed] [Google Scholar]
- Nisman B, Nechushtan H, Biran H, et al. New ARCHITECT plasma pro-gastrin-releasing peptide assay for diagnosing and monitoring small-cell lung cancer. Br J Cancer. 2016;114:469–76. doi: 10.1038/bjc.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlund MS, Stieber P, Brustugun OT, et al. Characteristics and clinical validity of two immunoassays for ProGRP. Tumor Biol. 2012;33:1105–13. doi: 10.1007/s13277-012-0351-1. [DOI] [PubMed] [Google Scholar]
- Okusaka T, Eguchi K, Kasai T, et al. Serum levels of pro-gastrin-releasing peptide for follow-up of patients with small cell lung cancer. Clin Cancer Res. 1997;3:123–7. [PubMed] [Google Scholar]
- Oremek G, Sauer-Eppel H, Bruzdziak T. Value of tumour and inflammatory markers in lung cancer. Anticancer Res. 2007;27:1911–5. [PubMed] [Google Scholar]
- Oremek GM, Sapoutzis N. Pro-gastrin-releasing peptide (Pro-GRP), a tumor marker for small cell lung cancer. Anticancer Res. 2003;23:895–8. [PubMed] [Google Scholar]
- Shi GL, Hu XL, Yue SD, et al. The value of serum tumor marker in the diagnosis of lung cancer. Zhonghua zhong liu za zhi. 2005;27:299–301. [PubMed] [Google Scholar]
- Shibayama T, Ohnoshi T, Ueoka H, et al. Serum neuron specific enolase (NSE) levels in patients with non small cell lung cancer. Nihon Kyobu Shikkan Gakkai Zasshi. 1992;30:1097–102. [PubMed] [Google Scholar]
- Shibayama T, Ueoka H, Nishii K, et al. Complementary roles of pro-gastrin-releasing peptide (ProGRP) and neuron specific enolase (NSE) in diagnosis and prognosis of small-cell lung cancer (SCLC) Lung Cancer. 2001;32:61–9. doi: 10.1016/s0169-5002(00)00205-1. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Stieber P, Dienemann H, Schalhorn A, et al. Pro-gastrin-releasing peptide (ProGRP) - A useful marker in small cell lung carcinomas. Anticancer Res. 1999;19:2673–8. [PubMed] [Google Scholar]
- Sunaga N, Tsuchiya S, Minato K, et al. Serum pro-gastrin-releasing peptide is a useful marker for treatment monitoring and survival in small-cell lung cancer. Oncology. 1999;57:143–8. doi: 10.1159/000012022. [DOI] [PubMed] [Google Scholar]
- Takada M, Kusunoki Y, Masuda N, et al. Pro-gastrin-releasing peptide(31-98) as a tumour marker of small-cell lung cancer: Comparative evaluation with neuron-specific enolase. Br J Cancer. 1996;73:1227–32. doi: 10.1038/bjc.1996.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Zhang X, Zhang Z, et al. Diagnostic value of tumor marker pro-gastrin-releasing peptide in patients with small cell lung cancer: a systematic review. Chin Med J. 2011;124:1563–8. [PubMed] [Google Scholar]
- Wang J, Shi G, Zhang S, et al. Clinical value of serum TPS, CEA, pro-GRP and CYFRA21-1 in patients with lung cancer. Zhongguo Fei Ai Za Zhi. 2010;13:500–5. doi: 10.3779/j.issn.1009-3419.2010.05.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- Wojcik E, Sas-Korczynska B, Tarapacz J, et al. ProGRP, NSE and CYFRA 21-1 in lung cancer patients. Nowotwory. 2008;58:231–7. [Google Scholar]
- Yamaguchi K, Abe K, Adachi I, et al. Peptide hormone production in primary lung tumors. Recent Results Cancer Res. 1985;99:107–16. doi: 10.1007/978-3-642-82533-0_12. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Abe K, Adachi I, et al. Peptide hormone production in small cell lung carcinomas with particular reference to gastrin-releasing peptide. Jpn J Clin Oncol. 1986;16:235–41. [PubMed] [Google Scholar]
- Yamaguchi K, Aoyagi K, Urakami Ki, et al. Enzyme-linked Immunosorbent Assay of pro-gastrin-releasing peptide for small cell lung cancer patients in comparison with neuron-specific enolase measurement. Jpn J Cancer Res. 1995;86:698–705. doi: 10.1111/j.1349-7006.1995.tb02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H-j, Gu Y, Chen C, et al. Diagnostic value of pro-gastrin-releasing peptide for small cell lung cancer: a meta-analysis. Clin Chem Lab Med. 2011;49:1039–46. doi: 10.1515/CCLM.2011.161. [DOI] [PubMed] [Google Scholar]
- Yang J, Li R, Li A. Clinical significance of ProGRP31-98 in patients with small-cell lung cancer. Zhonghua zhong liu za zhi. 2000;22:216–8. [PubMed] [Google Scholar]
- Zeng Q, Liu M, Zhou N, et al. Serum human epididymis protein 4 (HE4) may be a better tumor marker in early lung cancer. Clin Chim Acta. 2016;455:102–6. doi: 10.1016/j.cca.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Zhang ZP, Chen MS, Ren LF, et al. The clinical value of combined measurement of serum ProGRP and TPS in diagnosing small-cell lung cancer. J Mod Oncol. 2009;17:1678–81. [Google Scholar]


