Abstract
Background:
Selecting the cut-off point for the fecal immunochemical test (FIT) for colorectal cancer (CRC) screening programs is of prime importance. The balance between the test performance for detecting advanced neoplasia and the available colonoscopy resources should be considered. We aimed to identify the optimal cut-off of FIT for advanced neoplasia in order to minimize colonoscopy burden.
Methods:
We conducted a multi-center study in 6 hospitals from diverse regions of Thailand. Asymptomatic participants, aged 50-75 years, were tested with one-time quantitative FIT (OC-SENSOR, Eiken Chemical Co.,Ltd., Tokyo, Japan) and all participants underwent colonoscopy. We assessed test performance in detecting advanced neoplasia (advanced adenoma and CRC) and measured the burden of colonoscopy with different cut-offs [25 (FIT25), 50 (FIT50), 100 (FIT100), 150 (FIT150), and 200 (FIT200)ng/ml].
Results:
Among 1,479 participants, advanced neoplasia and CRC were found in 137 (9.3%) and 14 (0.9%), respectively. From FIT25 to FIT200, the positivity rate decreased from 18% to 4.9%. For advanced neoplasia, an increased cut-off decreased sensitivity from 42.3% to 16.8% but increased specificity from 84.2% to 96.3%. The increased cut-off increased the positive predictive value (PPV) from 21.5% to 31.5%. However, all cut-off points provided a high negative predictive value (NPV) (>90%). For CRC, the miss rate for FIT25 to FIT 150 was the same (n=3, 21%), whereas that with FIT200 increased to 35% (n=5).
Conclusions:
In a country with limited-colonoscopy resources, using FIT150 may be preferred because it offers both high PPV and NPV for advanced neoplasia detection. It could also decrease colonoscopy workload, while maintaining a CRC miss rate similar to those with lower cut-offs.
Keywords: Fecal occult blood test, colorectal cancer screening, colonoscopy, colorectal cancer
Introduction
The incidence of colorectal cancer (CRC) has been increasing rapidly in many Asian countries (Sung et al., 2005). In Thailand, between 1998 through 2012, the age-standardize incidence rate (ASR) for CRC increased from 8.8 to 14.4 per 100,000 for males and from 7.6 to 11.2 per 100,000 for females. Unfortunately, to date, a national policy for CRC screening has not been implemented in Thailand. Although screening colonoscopy is the most effective CRC screening method (Dominic et al., 2009; Sung et al., 2015), it requires expensive equipment and endoscopists. Therefore, implementing primary screening colonoscopy as a mass CRC screening program in a country with limited colonoscopy resources and finances is impossible.
According to the Asia-Pacific consensus recommendations for CRC screening, fecal immunochemical test (FIT) is recommended as the first choice for the mass CRC screening in limited-resource countries (Sung et al., 2015). However, FIT is a two-step screening method, and individuals with positive FIT have to be referred for colonoscopy. The different cut-off hemoglobin levels (range 10 to 75 ng/ml) provide different positive rates of FIT (range 4.5% to 46.4%) to be referred for colonoscopy (Hundt et al., 2009; van Rossum et al., 2009; Quintero et al., 2012; Aniwan et al., 2015).
With quantitative FIT, the cut-off can be adjusted according to the colonoscopy resource and the target population size (Halloran et al., 2012). Hypothetically, a lower cut-off will increase sensitivity and consequently, it will decrease specificity, increasing the number of colonoscopies, including unnecessary ones (van Rossum et al., 2009; Hamza et al., 2013). FIT performance has been evaluated in many large population-based studies; however, as only participants with positive FIT results underwent diagnostic colonoscopy, the CRC miss rate cannot be evaluated (Grazzini et al., 2009; van Rossum et al., 2009; Hamza et al., 2013; Raginel et al., 2013). In addition, the results from many previous studies on FIT performance at different cut-offs could not be generalizable because different commercial products utilizing a variety of analytical methods were used (Hundt et al., 2009; Brenner et al., 2010; Brenner and Tao, 2013).
Therefore the aim of our study was to determine the optimum cut-off of FIT to strike a balance among the positive predictive value (PPV) for advanced neoplasia detection, the negative predictive value (NPV) for advanced neoplasia exclusion, with the lowest CRC miss rate and the lowest colonoscopy burden.
Materials and Methods
Study population
We conducted a cross-sectional study between December 2014 and June 2016. Asymptomatic participants, aged 50-75 years, who participated in the health promotion program in the six university hospitals across Thailand (Chulalongkorn University Hospital, Siriraj Hospital, Rajavithi Hospital, ChiangMai University Hospital, Prince of Songkla University Hospital, Khon Kaen University Hospital), were invited. According to the National Statistical Office of Thailand, Thailand is divided into four geographical regions; Central, North, Northeast, and South regions. Half of participants were invited from 3 hospitals in the central region and 50% were enrolled from the other 3 hospitals representing all regions of Thailand (20% North, 15% Northeast and 15% South region) (Figure 1). The exclusion criteria were prior colon examination (endoscopy/ radiologic imaging), previous colonic resection, previous history of CRC, inflammatory bowel disease, and family history of hereditary CRC such as familial adenomatous polyposis and hereditary nonpolyposis colorectal cancer (at least 1 first degree relative with CRC before 60 years or at least 2 first degree relatives with CRC)(Castells et al., 2009; Stoffel et al., 2015). We also excluded participants who had a positive FOBT in the past year. All participants provided written informed consent. The study was approved by the Institutional Review Board of each hospital (Thai Clinical Trial Registry, TCTR20140228001).
Figure 1.
Study Population from the Different Geographical Regions of Thailand
Fecal immunochemical test (FIT)
One-day stool sample was collected within 3 days prior to colonoscopy and before bowel preparation. The quantitative fecal immunochemical test (OC-SENSOR, Eiken Chemical Co., Ltd., Tokyo, Japan) was used. Participants received an explanation for stool collection. No restrictions in diet and medications were required. The stool collection, storage, and analysis were performed according to the manufacturer’s instructions. The participants submitted the stool-filled bottle on the colonoscopy day. The date of stool sampling was recorded. The stool-filled bottle was analyzed using an automated analyzer machine (OC-SENSOR DIANA machine). The samples were analyzed within 7 days from the collection date. The medical laboratory scientists who performed FIT analysis were blinded to the colonoscopy results. We assessed the test performance in detecting advanced neoplasia at different cut-off levels [25 (FIT25), 50 (FIT50), 100 (FIT100), 150 (FIT150), and 200 (FIT200) ng/ml].
Colonoscopy
All participants underwent colonoscopy, regardless of the FIT results. The instruction for bowel preparation and bowel preparation regimen were prescribed, as described previously (Aniwan et al., 2016). The colonoscopy was performed under conscious sedation with intravenous midazolam and meperidine/fentanyl. The quality of bowel preparation was rated by Aronchick scale (Saltzman et al., 2015). All identified polyps were removed, labeled separately and sent to the pathologist at each hospital. Polyp size was measured by using the open jaws of biopsy forceps, which were 7-mm size (Aniwan et al., 2015). The cecal intubation rate, withdrawal time, and characteristic of polyps were recorded. Polyps were categorized as adenoma (tubular, tubulovillous, villous, or serrated adenoma) and non-adenoma. Advanced adenoma was defined as adenoma with high-grade dysplasia, or villous adenoma (≥25% villous), or adenoma ≥10 mm (Aniwan et al., 2015). CRC was defined when malignant cells were present in the intramucosal layer (Levin et al., 2008). Advanced neoplasia included advanced adenoma and CRC. The location of polyp was classified as the proximal colon and the distal colon. The region proximal to the splenic flexure and the splenic flexure were defined as the proximal colon. The region distal to the splenic flexure was defined as the distal colon (Aniwan et al., 2015). The endoscopists and the pathologists were blinded to the FIT results.
Statistical analysis
The colonoscopic findings were used as a diagnostic reference standard to determine the performance of FIT on advanced neoplasia. At the different cut-offs, the positive rate, sensitivity, specificity, PPV, NPV and number needing colonoscopy with 95% CIs were calculated. The diagnostic tests were analyzed using MedCalc Statistical Software version 16.4.3 (MedCalc Software bvba, Ostend, Belgium). We used sensitivity and specificity to detect advanced neoplasia as the primary outcome. The sensitivity and specificity of advanced neoplasia detection that have been reported previously have ranged from 24% to 59%(Brenner et al., 2010; Haug et al., 2010; Aniwan et al., 2015) and from 58% to 96%(Brenner et al., 2010; Aniwan et al., 2015), respectively. We assumed that sensitivity and specificity for detecting advanced neoplasia were 25% and 90%, respectively. Based on the prevalence of advanced neoplasia at 11% (Aniwan et al., 2015), the calculated sample size responded to type I error (α=0.05) and estimated 10% drop-out rate was 1470. Statistical analysis was performed with SPSS (version 18.0; PSS Inc, Chicago III).
Results
Demographic data
A total of 1,580 asymptomatic participants who participated in a health promotion program were invited, 1,539 participants underwent CRC screening. Hence our participation rate of CRC screening from invited asymptomatic participants was 97%. Among 1,539 screened participant, 60 participants were excluded due to age >75 years (n=39), missed stool collection (n=12) and poor bowel preparation (n=9). A total of 1,479 participants were eligible. All participants underwent complete colonoscopy. Adenoma, advanced neoplasia, and CRC were present in 547 (37%), 137 (9.3%) and 14 (0.9%) participants, respectively. The characteristics of participants are shown in Table 1.
Table 1.
Characteristics of the Study Population
| Number of participants | |
|---|---|
| (N=1,479) (%) | |
| Age (mean ± SD, years) | 60.4±7.2 |
| 50-59 | 732 (49.5%) |
| 60-69 | 559 (37.8%) |
| 70-75 | 188 (12.7%) |
| Sex | |
| Male | 566 (38.3%) |
| Female | 913 (61.7%) |
| BMI (mean ± SD, kg/m2) | 23.9±3.9 |
| Smoking | 141 (9.5%) |
| First-degree family history of colorectal cancer | 254 (17.2%) |
| Daily aspirin and/or NSAID user | 99 (6.7%) |
| Prevalence of colorectal neoplasia at colonoscopy | |
| Adenoma | 547 (37%) |
| Advanced neoplasia | 137 (9.3%) |
| Colorectal cancer | 14 (0.9%) |
| Participants with advanced neoplasia stratified by location (N) | 137 |
| Proximal | 44 (32%) |
| Distal | 85 (62%) |
| Both | 8 (6%) |
| Participants with advanced neoplasia stratified by size (N) | 137 |
| < 10 mm. | 7 (5%) |
| 10 - 14 mm. | 91 (67%) |
| 15 - 19 mm. | 25 (18%) |
| ≥ 20 mm. | 14 (10%) |
| Participants with advanced neoplasia stratified by morphology (N) | 137 |
| Pedunculated | 67 (49%) |
| Sessile | 64 (47%) |
| Flat | 6 (4%) |
FIT performance on advanced neoplasia
Positivity rate of FIT
From FIT25 to FIT200, the positivity rate of FIT decreased from 18% to 4.9%. At all cut-offs, participants with positive FIT results had a significantly higher detection rate for advanced neoplasia than participants with negative FIT results [(21.5% vs. 6.5%), (25% vs. 7.1%), (28.7% vs. 7.7%), (27.3% vs. 8.1%), and (31.5% vs. 8.1%); P<0.001, respectively]. Among the different cut-offs, participants with positive FIT200 had the highest advanced neoplasia detection rate (Odds ratio 5.21, 95%CI 3.07-8.85) compared with participants with negative FIT200.
Accuracy, sensitivity, and specificity
From FIT 25 to FIT 200, the accuracy rates of FIT performance in detecting advanced neoplasia increased from 80.3% to 88.9%. Although increasing cut-off decreased sensitivity for advanced neoplasia from 42.3% to 16.8%, it increased the specificity for advanced neoplasia from 84.2% to 96.3%. The sensitivity/and specificity of FIT25, FIT50, FIT100, FIT150 and FIT200 for advanced neoplasia were 42.3%/84.2%, 32.1%/90.2%, 22.6%/94.3%, 17.5%/95.2% and 16.8%/96.3%, respectively (Table 2).
Table 2.
Performance of Quantitative FIT
| Quantitative FIT | |||||
|---|---|---|---|---|---|
| 25 ng/ml | 50 ng/ml | 100 ng/ml | 150 ng/ml | 200 ng/ml | |
| Positive rate (%) | 18.3 | 11.9 | 7.3 | 5.9 | 4.9 |
| Advanced neoplasia | |||||
| Accuracy (95% CI) | 80.3 (78.0-82.0) | 84.8 (83.0-86.6) | 87.6 (85.9-89.3) | 88 (86.3-89.7) | 88.9 (87.2-90.4) |
| Sensitivity (95% CI) | 42.3 (34.0-51.1) | 32.1 (24.2-40.6) | 22.6 (15.9-30.6) | 17.5 (11.6-24.9) | 16.8 (11.5-23.9) |
| Specificity (95% CI) | 84.2 (82.1-86.1) | 90.2 (88.4-91.7) | 94.3 (92.9-95.5) | 95.2 (94.0-96.3) | 96.3 (95.1-97.1) |
| Positive predictive value (95% CI) | 21.5 (16.7-26.9) | 25 (18.8-32.1) | 28.7 (20.4-38.2) | 27.3 (18.3-37.8) | 31.5 (22.0-42.9) |
| Negative predictive value (95% CI) | 93.5 (91.9-94.8) | 92.9 (91.3-94.2) | 92.3 (90.7-93.6) | 91.9 (90.3-93.3) | 91.9 (90.4-93.2) |
| False positive rate (%) | 15.8 | 9.8 | 5.7 | 4.8 | 3.7 |
| False negative rate (%) | 57.7 | 67.9 | 77.4 | 82.5 | 83.2 |
| Number needed to colonoscope (N) | 4.7 | 4 | 3.5 | 3.7 | 3.1 |
| Advanced neoplasia stratified by location | |||||
| Proximal advanced neoplasia | |||||
| Sensitivity (95% CI) | 43.2 (28.4-59.0) | 34.1 (20.5-49.9) | 22.7 (11.5-37.8) | 20.5 (9.8-35.3) | 20.5 (9.8-35.3) |
| Specificity (95% CI) | 82.5 (80.4-84.4) | 88.8 (87.0-90.4) | 93.7 (91.7-94.4) | 94.5 (93.2-95.6) | 95.5 (94.3-96.6) |
| Positive predictive value (95% CI) | 7 (4.3-10.8) | 8.5 (4.9-13.7) | 9.3 (4.5-16.4) | 10.2 (4.8-18.5) | 12.3 (5.8-22.1) |
| Negative predictive value (95% CI) | 97.9 (97.0-98.7) | 97.8 (96.8-98.5) | 97.5 (96.6-98.3) | 97.5 (96.5-98.2) | 97.5 (96.6-98.3) |
| Distal advanced neoplasia | |||||
| Sensitivity (95% CI) | 40.0 (29.5-51.2) | 28.2 (19.0-39.0) | 21.2 (13.1-31.4) | 16.5 (9.3-26.1) | 15.3 (8.4-24.7) |
| Specificity (95% CI) | 83.1 (81.0-85.0) | 89.1 (87.3-90.7) | 93.5 (92.1-94.8) | 94.7 (93.4-95.8) | 95.7 (94.5-96.7) |
| Positive predictive value (95% CI) | 12.6 (8.8-17.2) | 13.6 (8.9-19.6) | 16.7 (10.2-25.1) | 15.9 (9.0-25.3) | 17.8 (9.8-28.5) |
| Negative predictive value (95% CI) | 95.8 (94.5-96.8) | 95.3 (94.0-96.4) | 95.1 (93.8-96.2) | 94.9 (93.6-96.0) | 94.9 (93.6-96.0) |
| Colorectal cancer | |||||
| Sensitivity (95% CI) | 78.6 (49.2-95.3) | 78.6 (51.9-95.7) | 78.6 (49.2-95.3) | 78.6 (49.2-95.3) | 64.3 (38.4-88.2) |
| Specificity (95% CI) | 82.3 (80.3-84.2) | 88.7 (87.0-90.3) | 93.4 (92.0-94.6) | 94.7 (93.5-95.8) | 95.6 (94.5-96.7) |
| Positive predictive value (95% CI) | 4.1 (2.3-7.6) | 6.3 (3.2-10.9) | 10.2 (5.2-17.5) | 12.5 (6.4-21.3) | 12.3 (5.8-22.1) |
| Negative predictive value (95% CI) | 99.8 (99.3-99.9) | 99.8 (99.3-99.9) | 99.8 (99.4-99.9) | 99.8 (99.8-99.9) | 99.6 (99.2-99.9) |
| False positive rate (%) | 95.9 | 93.8 | 89.8 | 87.5 | 87.7 |
| False negative rate (%) | 25 | 23 | 22 | 22 | 35 |
| Number needed to colonoscope (N) | 24.3 | 15.9 | 9.8 | 8 | 8.1 |
The overall performance of quantitative FIT for detecting advanced neoplasia that was calculated by the area under the ROC curves (AUC) was 0.69 (95%CI; 0.64-0.73). Using FIT25, FIT50, FIT100, FIT150 and FIT200 as thresholds did not result in significantly different AUC (Figure 3).
Figure 2.
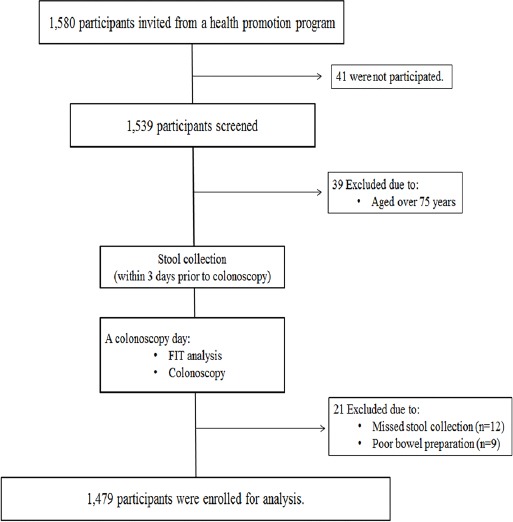
Study Enrollment
Figure 3.
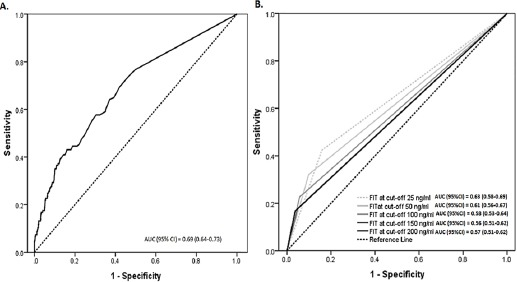
ROC Curve of Overall FIT (A) and at the Different Cut-Offs of FIT (B) Performance on Advanced Neoplasia
Positive predictive value and negative predictive value
From FIT25 to FIT200, the PPV for advanced neoplasia decreased 10% (from 21.5% to 31.5%). In contrast, from FIT25 to FIT200, the NPV were similar and were higher than 90% (91.9%-93.5%). At FIT25, FIT50, FIT100, FIT150 and FIT200, the numbers of participant with positive FIT that needed colonoscopy (NNC) to detect advanced neoplasia were 4.7, 4.0, 3.5, 3.7 and 3.1, respectively.
False positive rate and false negative rate
From FIT25 to FIT200, the false positive rate for advanced neoplasia decreased significantly (15.8% vs. 9.8% vs. 5.7% vs. 4.8% vs. 3.7%; p=0.02). The false positive rate for advanced neoplasia of FIT25 was 4-fold higher than that of FIT200 (15.8% vs. 3.7%; p=0.007). Whereas, there was no significant difference in the false negative rate among all cut-offs (57.7% vs. 67.9% vs. 77.4% vs. 82.5% vs. 83.2%; p=0.18). The false negative rate for advanced neoplasia of FIT25 was 1.4-fold lower than that of FIT200 (57.7% vs. 83.2%; p=0.04).
Diagnostic performance of FIT on proximal advanced neoplasia
At all cut-offs, the PPV for proximal advanced neoplasia was slightly lower (5%) than for distal advanced neoplasia. However, this difference was not statistically significant (p>0.05). The NPV for proximal advanced neoplasia was ≥97.5%. (Table 2).
Colorectal cancer missed by FIT
Of the 14 participants with CRC, 3 participants with CRC had FIT that was negative according to all cut-offs. Of the 3 missed CRC cases, two had 12-mm size in the sigmoid colon and rectum. The other had 15-mm size in the transverse colon. All 3 missed CRCs were Tis stage of TNM staging (Edge and Compton, 2010). Using cut-offs ≤ FIT150, FIT were positive in all the remaining 11 participants with CRC. Using FIT200, 2 additional participants with CRC were missed. One had 20-mm size in the descending colon. The other had 15-mm size in the sigmoid colon (Table 3).
Table 3.
Colorectal Cancer Characteristics and the Hemoglobin Level of FIT in the Stool Sampling
| No | Location | Size (mm.) | T stage | TNM stage | Hb level of FIT in the stool sampling (ng/ml) |
|---|---|---|---|---|---|
| 1 | Sigmoid | 12 | Tis | 0 | 6 |
| 2 | Rectum | 12 | Tis | 0 | 12 |
| 3 | Transverse | 15 | Tis | 0 | 24 |
| 4 | Descending | 20 | T3 | IIA | 171 |
| 5 | Sigmoid | 15 | Tis | 0 | 176 |
| 6 | Rectum | 15 | Tis | 0 | 208 |
| 7 | Sigmoid | 15 | T3 | IIIA | 232 |
| 8 | Cecum | 15 | T3 | IIA | 345 |
| 9 | Ascending | 25 | T4 | IIIC | 408 |
| 10 | Rectum | 15 | T3 | IIA | 475 |
| 11 | Rectum | 20 | T3 | IIA | 651 |
| 12 | Sigmoid | 30 | T3 | IIA | 4298 |
| 13 | Ascending | 20 | T3 | IIA | 7726 |
| 14 | Ascending | 30 | T3 | IIA | 14606 |
The diagnosis performance of FIT for CRC is shown in Table 2. The AUC of the ROC curves for CRC was 0.92 (95% CI 0.86-0.98) (Figure 4).
Figure 4.
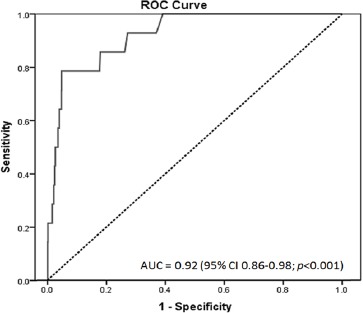
ROC Curve of Overall FIT Performance on CRC
Discussion
The objective of selecting cut-off of FIT for CRC screening is to strike a balance between the advanced neoplasia detection rate, CRC miss rate and the burden of colonoscopy. Accordingly, the selection is based on sensitivity and specificity, the false positive rate and the number requiring colonoscopy to detect advanced neoplasia and CRC (Halloran et al., 2012). Hypothetically, lowering the cut-off could increase sensitivity for detecting advanced neoplasia and CRC. Unfortunately, this may increase the false positive rate. We confirmed this hypothesis by showing that the sensitivity of FIT25 increased 2.5-fold compared to FIT200 (42.3% vs. 16.8%), while the false positive rate increased 4.3-fold compared to FIT200 (15.8% vs. 3.7%). Furthermore, the rate of participants requiring colonoscopy increased 3.7-fold compared to FIT200 (18.3% vs. 4.9%). Consequently, using the lower cut-off FIT would increase not only the burden of unnecessary colonoscopy but also its expense.
Currently, many commercial FITs are widely available. The cut-off limits are usually pre-set from the manufacturers, and could result in heterogeneity in the diagnostic performances (Hundt et al., 2009). Based on the recent systemic review by the US Preventive Services Task Force, the Food and Drug Administration (FDA)-approved OC FIT-CHEK (for instance OC–sensor Diana, OC-micro) provided the most evidence-based support for the good performance of one-day stool sample FIT (Lin et al., 2016). In our study, we demonstrated the diagnostic performances of the one-day quantitative FIT (OC-sensor Diana) in the cut-offs from FIT25 to FIT200, had both relatively high sensitivity (range 64.3%-78.6%) and specificity (range 82.3%-95.6%) for CRC and fair sensitivity (range 16.8%-42.3%) with high specificity (range 84.2%-96.3%) for advanced neoplasia. Our results are in line with the results from previous studies using one-day stool quantitative FIT at varying cut-offs (50-100 ng/ml) in which the overall sensitivity and specificity for CRC ranged from 60%-100% and 87%-96%, respectively, and the overall sensitivity and specificity for advanced neoplasia ranged from 18%-44% and 85.8%-97%, respectively (Lin et al., 2016). In our study, we extended the range of cut-off, including both cut-offs below and above the manufacturers’ threshold (100 ng/ml), to evaluate the false positive rate and CRC miss rate that may occur during the use of these extreme cut-offs. We found that decreasing the cut-off from FIT150 to FIT25 did not increase the CRC detection, whereas increasing the cut-off from FIT150 to FIT200 resulted in two more CRC being missed.
Until recently, the recommended cut-off of FIT had not been determined. Several studies have suggested cut-offs based on the results from a variety of target populations and different FIT kits (Hundt et al., 2009; Brenner et al., 2010; Brenner and Tao, 2013; Hamza et al., 2013; Raginel et al., 2013). Some studies were conducted in patients who were symptomatic (Terhaar sive Droste et al., 2011), while others were carried out in participants who were FIT positive (Grazzini et al., 2009; van Rossum et al., 2009; Hamza et al., 2013; Raginel et al., 2013; Alvarez-Urturi et al., 2016). Since the FIT negative participants did not undergo colonoscopy, hence the missed CRC data were not available. Some studies evaluated only the cut-off recommended from the manufacturers’, the cut-offs above or below the thresholds were not evaluated (Haug et al., 2010; Park et al., 2010; de Wijkerslooth et al., 2012). The strength of our study is that we only recruited participants from an asymptomatic population and we only used one brand of quantitative FIT. This FIT avoids interpreter error because it is read by an automated machine that can analyze a wide range of hemoglobin cut-offs. Importantly, we performed colonoscopy in all participants, regardless to the FIT results. Therefore, we were able to collect data on missed CRC and advanced neoplasia. We found that there were 1,209 (81.3%) participants with negative FIT (<25 ng/ml) underwent colonoscopy. In this group, there were 3 cases of missed CRC and 79 cases of missed advanced neoplasia. The other strong point of our study is that our colonoscopies were performed according to the quality colonoscopy indicators (Rex et al., 2015).
Our results support that there is no “one-size fits all” for FIT. Because quantitative FIT can provide the exact hemoglobin level in the stool, and the cut-off of FIT affects its performances and the number of positive cases that need to be scoped. The selecting optimal cut-off is crucial. In our opinion, an important factor should be considered that is achieving a low CRC miss rate. We showed that both FIT150 and FIT200 yielded similar PPV (12.5% vs. 12.3%), NPV (99.8% vs. 99.6%) and NNC to find CRC (8.0 vs. 8.1). Both FIT25 and FIT50 yielded similar PPV (4.1% vs. 6.3%) and NPV (99.8% vs. 99.8%), but FIT25 required a greater NNC to find CRC than FIT50 (24.3 vs. 15.9). When the CRC miss rate was taken into account, the cut-offs from FIT25 to FIT150 had the same CRC miss rates (n=3, 21%), whereas at FIT200, the CRC miss rate increased to 35% (n=5).
Regarding to the colonoscopy rate, FIT150 and FIT200 yielded the same positivity rate of FIT for colonoscopy (5.9% vs. 4.9%). In Thailand, there are 12 million participants aged 50-75 who are eligible for CRC screening but there are only 1,054 endoscopists. This proportion can result in a workload that overwhelms the very limited number of endoscopists. With FIT150, the volume and subsequent cost of colonoscopy only slightly increased, by 1.2-fold, compared to FIT200, while the CRC miss rate from FIT150 was lower (21% vs. 35%). Hence, we purpose that the optimal cut-off for CRC screening in our population should be 150 ng/ml.
In other countries with a more capacity to perform colonoscopies, the lowest cut-off can be used (van Rossum et al., 2009). The Netherlands group studied the quantitative FIT at different cut-offs in CRC screening (van Rossum et al., 2009). In 6,157 participants, the 428 participants with positive FIT at ≥50 ng/ml underwent colonoscopy. Of note, they did not perform colonoscopy in the participants with negative FIT. At FIT50, CRC was found in 28 participants. When the cut-off was increased from FIT50 to FIT75, the CRC miss rate compare to FIT50 was 3.6% (n=1). When the cut-off was increased from FIT50 to FIT100, the CRC miss rate compared to FIT50 was 14% (n=4). As such, the Dutch Ministry of Health advocated starting the national CRC screening program with the cut-off 75 ng/ml. We speculate that the number of truly missed CRC in this study could be underreported because they did not perform colonoscopy in the individuals with FIT<50 ng/ml. As our study, we carried out colonoscopy even in individuals with FIT <50 ng/ml, and we found that the rate of missed CRC in the FIT-negative group (<FIT50) was 24%.
There are some limitations in our study. This is a hospital-based study, selection bias might be present. Since participants were enrolled from the health promotion program, they may have increased health awareness than general population. However, our demographic data were comparable with that reported in a population-based study (Grazzini et al., 2009; de Wijkerslooth et al., 2012). Because colonoscopy is not a perfect test (Rex et al., 2015). The advanced neoplasia miss rate may come from the suboptimal performance of the endoscopists. A recent back-to-back colonoscopy study that reflected the performance of the endoscopist demonstrated that the advanced neoplasia miss rate was 17.5% and 10% of missed adenomas had size ≥10 mm (Aniwan et al., 2016).
In conclusion, using a high cut-off FIT not only provides a high PPV for advanced neoplasia detection but also maintains a high NPV for advanced neoplasia exclusion. The main advantage of this strategy is a decreased colonoscopy workload, while maintaining CRC miss rate that is similar to the lower cut-offs. In Thailand, setting FIT150 may be optimal. However, this recommendation may not be generalizable. The optimal cut-off may vary from country to country, we recommend that similar studies should be carried out in each country to define and tailor the optimal cut-off.
Acknowledgements
The quantitative fecal immunochemical test (OC-SENSOR) and FIT kits were supported by Eiken Chemical Co., Ltd., Tokyo, Japan.
Funding Statement
This research was supported by the National Research Council of Thailand and Health Systems Research Institute Fund (NRCT-HSRI-2013-04) and Grant for International Research Integration: Chula Research Scholar Rachadaphiseksomphot Endowment Fund (2300052001).
References
- Alvarez-Urturi C, Andreu M, Hernandez C, et al. Impact of age- and gender-specific cut-off values for the fecal immunochemical test for hemoglobin in colorectal cancer screening. Dig Liver Dis. 2016;48:542–51. doi: 10.1016/j.dld.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Aniwan S, Orkoonsawat P, Viriyautsahakul V, et al. The secondary quality indicator to improve prediction of adenoma miss rate apart from adenoma detection rate. Am J Gastroenterol. 2016;111:723–9. doi: 10.1038/ajg.2015.440. [DOI] [PubMed] [Google Scholar]
- Aniwan S, Rerknimitr R, Kongkam P, et al. A combination of clinical risk stratification and fecal immunochemical test results to prioritize colonoscopy screening in asymptomatic participants. Gastrointest Endosc. 2015;81:719–27. doi: 10.1016/j.gie.2014.11.035. [DOI] [PubMed] [Google Scholar]
- Brenner H, Haug U, Hundt S. Inter-test agreement and quantitative cross-validation of immunochromatographical fecal occult blood tests. Int J Cancer. 2010;127:1643–9. doi: 10.1002/ijc.25154. [DOI] [PubMed] [Google Scholar]
- Brenner H, Tao S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer. 2013;49:3049–54. doi: 10.1016/j.ejca.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Castells A, Castellvi-Bel S, Balaguer F. Concepts in familial colorectal cancer: where do we stand and what is the future? Gastroenterology. 2009;137:404–9. doi: 10.1053/j.gastro.2009.06.015. [DOI] [PubMed] [Google Scholar]
- de Wijkerslooth TR, Stoop EM, Bossuyt PM, et al. Immunochemical fecal occult blood testing is equally sensitive for proximal and distal advanced neoplasia. Am J Gastroenterol. 2012;107:1570–8. doi: 10.1038/ajg.2012.249. [DOI] [PubMed] [Google Scholar]
- Dominic OG, McGarrity T, Dignan M, et al. American college of gastroenterology guidelines for colorectal cancer screening 2008. Am J Gastroenterol. 2009;104:2626–7. doi: 10.1038/ajg.2009.419. [DOI] [PubMed] [Google Scholar]
- Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- Grazzini G, Visioli CB, Zorzi M, et al. Immunochemical faecal occult blood test: number of samples and positivity cutoff. What is the best strategy for colorectal cancer screening? Br J Cancer. 2009;100:259–65. doi: 10.1038/sj.bjc.6604864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran SP, Launoy G, Zappa M. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition-Faecal occult blood testing. Endoscopy. 2012;44:65–87. doi: 10.1055/s-0032-1309791. [DOI] [PubMed] [Google Scholar]
- Hamza S, Dancourt V, Lejeune C, et al. Diagnostic yield of a one sample immunochemical test at different cut-off values in an organised screening programme for colorectal cancer. Eur J Cancer. 2013;49:2727–33. doi: 10.1016/j.ejca.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Haug U, Hundt S, Brenner H. Quantitative immunochemical fecal occult blood testing for colorectal adenoma detection: evaluation in the target population of screening and comparison with qualitative tests. Am J Gastroenterol. 2010;105:682–90. doi: 10.1038/ajg.2009.668. [DOI] [PubMed] [Google Scholar]
- Hundt S, Haug U, Brenner H. Comparative evaluation of immunochemical fecal occult blood tests for colorectal adenoma detection. Ann Intern Med. 2009;150:162–9. doi: 10.7326/0003-4819-150-3-200902030-00005. [DOI] [PubMed] [Google Scholar]
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American cancer society, the US multi-society task force on colorectal cancer, and the American college of radiology. Gastroenterology. 2008;134:1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: Updated evidence report and systematic review for the US preventive services task force. JAMA. 2016;315:2576–94. doi: 10.1001/jama.2016.3332. [DOI] [PubMed] [Google Scholar]
- Park DI, Ryu S, Kim YH, et al. Comparison of guaiac-based and quantitative immunochemical fecal occult blood testing in a population at average risk undergoing colorectal cancer screening. Am J Gastroenterol. 2010;105:2017–25. doi: 10.1038/ajg.2010.179. [DOI] [PubMed] [Google Scholar]
- Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366:697–706. doi: 10.1056/NEJMoa1108895. [DOI] [PubMed] [Google Scholar]
- Raginel T, Puvinel J, Ferrand O, et al. A population-based comparison of immunochemical fecal occult blood tests for colorectal cancer screening. Gastroenterology. 2013;144:918–25. doi: 10.1053/j.gastro.2013.01.042. [DOI] [PubMed] [Google Scholar]
- Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110:72–90. doi: 10.1038/ajg.2014.385. [DOI] [PubMed] [Google Scholar]
- Saltzman JR, Cash BD, Pasha SF, et al. Bowel preparation before colonoscopy. Gastrointest Endosc. 2015;81:781–94. doi: 10.1016/j.gie.2014.09.048. [DOI] [PubMed] [Google Scholar]
- Stoffel EM, Mangu PB, Gruber SB, et al. Hereditary colorectal cancer syndromes: American society of clinical oncology clinical practice guideline endorsement of the familial risk-colorectal cancer: European society for medical oncology clinical practice guidelines. J Clin Oncol. 2015;33:209–17. doi: 10.1200/JCO.2014.58.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JJ, Lau JY, Goh KL, et al. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol. 2005;6:871–6. doi: 10.1016/S1470-2045(05)70422-8. [DOI] [PubMed] [Google Scholar]
- Sung JJ, Ng SC, Chan FK, et al. An updated Asia Pacific Consensus Recommendations on colorectal cancer screening. Gut. 2015;64:121–32. doi: 10.1136/gutjnl-2013-306503. [DOI] [PubMed] [Google Scholar]
- Terhaar sive Droste JS, Oort FA, van der Hulst RW, et al. Higher fecal immunochemical test cutoff levels: lower positivity rates but still acceptable detection rates for early-stage colorectal cancers. Cancer Epidemiol Biomarkers Prev. 2011;20:272–80. doi: 10.1158/1055-9965.EPI-10-0848. [DOI] [PubMed] [Google Scholar]
- The gastroenterological association of Thailand. Member of the gastroenterological association of Thailand (GAT) [accessed on 11 August 2016]. Available at: http://www.gastrothai.net/eng/about8_member.php//
- Institute of population and social research Mahidol university. population of Thailand BE 2559. [accessed on 11 August 2016]. Available at: http://www.thailandometers.mahidol.ac.th/#population//
- National cancer institute. Minitry of public helath. Cancer in Thailand Vol IV 1998-2000 and Vol VIII 2010-2012. [accessed on 11 August 2016]. Available at: http://www.nci.go.th/th/index1.html//
- van Rossum LG, van Rijn AF, Laheij RJ, et al. Cutoff value determines the performance of a semi-quantitative immunochemical faecal occult blood test in a colorectal cancer screening programme. Br J Cancer. 2009;101:1274–81. doi: 10.1038/sj.bjc.6605326. [DOI] [PMC free article] [PubMed] [Google Scholar]


