Abstract
Background:
Urothelial carcinoma (UC) is the malignancy most frequently encountered in the urinary bladder. The primary aim of this study was to make a reappraisal of histopathologic features, recurrence and progression.
Materials and Methods:
The records of cases consecutively diagnosed with UC in the state hospital pathology laboratory were collected. Cases were classified according to age, gender, histologic grade, pathologic staging [primary Tumor (pT)],tumor configuration, primary or recurrent status, and progression.
Results:
A total of 35 (29 male and 6 female) cases were examined. The mean age was 68.9 years with a male-to-female incidence ratio of 4.8:1. Low-grade UCs accounted for 20 (57.1%) and high-grade for 15 (42.9%). A papillary pattern was observed in 80% of the UCs, classified into the following pathological stages: 11 (31.4%) pTa, 22 (62.9%) pT1, and 2 (5.9%) pT2 cases. Eleven patients progressed to a higher stage (pT1 to pT2), and three cases from low to higher grade. We analyzed results for 26 (74.3%) cases aged 65 years or older.
Conclusions:
UCs have a great tendency for recurrence but potentially may be amenable to effective local or systemic treatments.
Keywords: Bladder, carcinoma, grade, stage, progression, recurrence, urothelial
Introduction
Worldwide, urinary bladder cancer is the seventh most common malignancy (Lopez-Beltran et al., 2004). Approximately 74,690 new patients and 15,580 deaths are estimated to have occurred in 2014 in the USA (Siegel et al., 2014). These cancers usually occur in the elderly population with a male predominance (male to female, ratio; 4.9:1). The mean age at the time of diagnosis is 65.1 years (Shen et al., 2016). An epidemiological analysis have reported potential association of occupational factors for urinary bladder carcinomas are smoking, exposure to aromatic amines, genetic sensitivity, environmental pollution, socioeconomic status, urinary tract disease, schistosoma haematobium infection, ionizing radiation, cyclophosphamide, and other drugs (Negri et al., 2007; Burger et al., 2012). Urothelial carcinoma with invasion of the stroma is subclassified into two major categories: Noninvasive papillary UC, and invasive UC (Cheng et al., 2012). Seventy five percent of patients of urothelial carcinoma (UC) have at presentation a limited mucosa and lamina propria (Stage pTa or pT1), while 25% at the time of diagnosed patients present with invasive carcinoma(muscularis propria, detrusor muscle) (Cao et al., 2010). Urothelial carcinoma (synonym;transitional cell) is the most frequent type of urinary bladder malignancies. The significant prognostic factor for urothelial tumors is the histologic grade. The recurrence rate for stage pTa or pT1 15–70%, and 10-15% of patients eventually of progression with advanced disease (stage pT2) (Pan et al., 2010).
The aim of the present study was to clarify the recurrence, progressionhistopathologic features and case characteristics of UC diagnosed in urinary bladder.
Materials and Methods
Study design
In this study, we re-examined 4 μm micrometer sections of formalin-fixed and paraffin-embedded transurethral resection of bladder tumor (TURBT) specimens of cases with urothelial carcinoma, and stained them with hematoxylin and eosin, except for others malignancies of the urinary bladder. All patients were categorized according to age, gender, histologic grade, tumor configuration, microscopic tumor extension (primary tumor, pT), recurrent tumors and patient age; ≤40 years, 41 to 50 years, 51 to 60 years, 61 to 70 years, 71 to 80 years, and >81 years. Histologic grade was classified as low-grade or high-grade; pathologic staging was categorized as pTa, noninvasive papillary carcinoma; pT1, tumor invades subepithelial connective tissue (lamina propria); pT2, tumor invades muscularis propria (detrusor muscle) according to the classification of the College of American Pathologists (CAP, Revised: October 1, 2013).
Results
Patient demographic features
A total of 35 carcinomas of the urinary bladder with histologic urothelial carcinoma (UC) in patientsanalyzed.
Age
The mean age of the cases was 68.9 ± 11.9 years (range 37 to 86 years). The median age detected in man for UC was 68.3 years, and in female was 71.6 years (see Table 1). Distribution of cases among age groups were: ≤40 years, 2.9%; 41 to 50 years, 2,9%; 51 to 60 years, 17.1%; 61 to 70 years, 28.6%; 71 to 80 years, 31.4%; and >81 years, 17.1%. All cases of age group were as≤60 years of age, 20%; ≥61 years of age, 80%. No statistically significant difference was found between age or age groups and histologic grade or pathologic staging (P=0.873, 0.254, 0.753, 0.444, respectively).
Table 1.
Clinicopathologic Features and Tumor Recurrences
| n | ||
|---|---|---|
| Gender | ||
| Male | 29 | 82.9% |
| Female | 6 | 17.1% |
| Age (year) | ||
| Mean | 35 | 68.9 |
| Male mean age | 68.3 | |
| Female mean age | 71.6 | |
| Older patients (≥65 years) | 26 | 74.3% |
| Histologic grade | ||
| LGUC | 20 | 57.1% |
| HGUC | 15 | 42.9% |
| Pathologic staging (pT) | ||
| pTa | 11 | 31.4% |
| pT1 | 22 | 62.9% |
| pT2 | 2 | 5.9% |
| Tumor configuration | ||
| Papillary | 28 | 80.0% |
| Solid/nodule | 2 | 5.7% |
| Mixed | 5 | 14.3% |
| Recurrence of year | ||
| ≤1 year | 23 | 65.7% |
| >1 year | 12 | 34.3% |
| Pathologic Stage progression | 11 | 31.4% |
| Histologic Grade progression | 3 | 8.6% |
| Adequacy of detrusor muscle | 28 | 80.0% |
Gender
There were 29 male (89.9%) and 6 female (17.1%) patients in this study. No statistically significant difference was found betweenthe histologic grade and pathologic staging in gender (P = 0.164, 0.48, respectively).
Histologic grade
Low-grade UCs (LGUCs) were observed in 20 (57.1%) cases while high-grade UCs were detected in 15 (42.9%) of the patients (see Figure 1). LGUCs were accounted in 20 of 35 patients, including 10 (50%) with pTa; HGUCs was encountered in all cases, including 12 (80%), and 2 (13.3%) with pT1, pT2, respectively. There was no correlation or significant statistical association between histologic grade and pathologic staging (P = 0.002).
Figure 1.
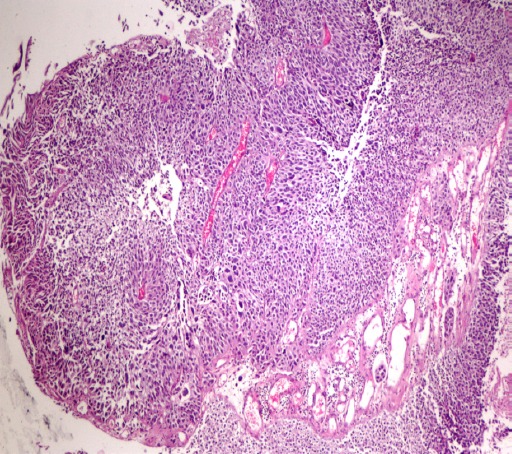
Urothelial Carcinoma. High-Grade Urothelial Carcinoma, Papillary, Pathologic Staging, pT1; Tumor Invades Subepithelial Connective Tissue-Lamina Propria
Tumor configuration
The predominantly of tumors showed a papillary configuration (80%), and less often a mixed (papillary and solid), solid/nodule. Tumors with a predominantly papillary configuration were found in 39.3% of all patients in pTa, in 53.6.1% of all patients pT1, in 7.1% of all patients with pT2, in 67.9% of all patients with low-grade malignancy, and in 32.1% of all patients high-grade malignancy. No significant difference was observed between tumor configuration and histologic grade or pathologic staging (P= 0.020, 0.197, respectively).
Microscopic tumor extension or pathologic staging (primary Tumor, pT)
pTa was observed in 11 (31.4%), pT1 and pT2 in 22 (62.9%), 2 (5.9%) patients, respectively. Fifteencases (42.9%) with UC was classified as noninvasive carcinoma, 20 cases as (57.1%) invasive carcinoma (lamina propria invasion, muscularis propria-detrusor muscle invasion) and 33 patients (94.3%) as nonmuscle invasive bladder cancer. Of those, noninvasive carcinoma occurred in 33 of 35 patients, including 10 (66.7%) LGUC, and 5 (33.3%) with HGUC. Invasive carcinoma was encountered in 20 of 35 patients, including 10 (50%) with LGUC, and 10 (50%) with HGUC. There was no correlation or significant statistical association between invasive status and histologic grade of tumor (P= 0.339). Muscle invasive urothelial carcinomaall cases reported were in males.
Recurrence of year
Twenty seven (77.1%) cases had one recurrence, six (17.1%) cases had two recurrences, and two (5.7%) patient had three recurrences. The mean count of episodes was 1.28. Recurrence of UC within ≤1 year following the first transurethral resection was seen in 23 (65.7%) patients, >1 years 12 (34.3%) patients, respectively. The mean process time to first recurrence follow-up was 11.11 months (1 to 36 months). Patients with UC recurrence had a rate of 82.8% at follow-up of 24 months. All cases had no evidence of extravesical extension, no metastasis and no evidence of death. The no association recurrence for the years and also pathologic staging were statistically significant (P = 0.229). There was no correlation between recurrence for years and histologic grade (P = 0.002).
Stage or grade progression
Eleven patients (31.4%, all total recurrent cases) were diagnosed as having tumour progression in pT1, pT2 carcinomas as a secondary pathological diagnosis after the first transurethral resection. Three patients showed progression from a low to high grade tumor. In four (11.4%)cases with tumour recurrence, the tumor progressed to muscle invasion.
Older patients (≥65 years)
We analyzed the results in 26 patients (74.3%) 65 years of age and older. Low-grade and high-grade UC was found approximately 42.9% and 31.4% in older patients, respectively. Seven patients (20%) were pTa and 17 patients (48.6%) were pT1 and 2 patients (5.7%) were pT2. No statistically significant relationship was found between older patients and histologic grade of tumor, pathologic staging (P = 0.914, 0.251, respectively).
Adequacy of material to identify muscularis propria (detrusor muscle)
Muscularis propria (detrusor muscle) was identified in 28 (80%) transurethral resection specimens in total and the muscularis propria was not identified in 7 (20%) in all cases. The muscularis propria was absent in 1 case high-grade Ta and 1 case T1 specimens.
Discussion
Urothelial carcinoma is the most common carcinoma of the urinary bladder, accounting for more than 90% of all primary carcinomas (Lopez-Beltran et al., 2004). Horstmann et al., (2008) and Kim et al., (2016) reported the incidence of urinary bladder cancer male-to-female ratio to be 2:1 and 5:1. Thus, the ratio of male to women often differs between studies. Gupta et al., (2009) reported a male to female ratio of 8.6:1. In our study, gender differences were seen in men more than women, with their onset being closer to those mentioned in the Gupta et al., (2009) In our study we found, the overwhelming majority of in men, with a male:female ratio of 9.4:1. As far as we know bladder cancer occurs almost exclusively in the geriatric population. Urinary bladder cancer tends to occur in the elderly (Hoke et al., 1999). Kim et al., (2016) reported the mean age in cases to be 64.5. In our experience, urothelial carcinoma presentation had a mean age 68.9 (men, women mean age: 68.3 years vs 71.6 years) likely to that published in the literature. The latest data, Gupta and and coworkers reported in 44.7% UC to be a low-grade and 55.3% to be a high-grade tumor and, Cheng et al., (2000) have found 105 transurethral resection of bladder tumor (TURBT) biopsies of cases with low-grade, and high-grade carcinoma to be 12.3%, and 87.7%. In our consultation practices, low-grade UC was seen in more than half of the cases, and thus our rates were different from the literature findings. Pan et al., (2010) showed low to high grade urothelial carcinoma that encountered for 46.6% and 39.4% in transurethral resection biopsies. In our study the percentageshowed is in consistency with the literature. LGUC was seen in 20 (57.1%) patients while 15 (42.9%) patients were HGUCs. At the same time, LGUCs are correlates with longer disease-free interval (Schapers et al., 1994). On the other hand, Jimenez et al., (2000) demonstrated that the pathologic T stage was relationship between with the progression of invasive muscle tumors. The authors evaluated that the histologic grade is not a prognostic sign for UC to invade the muscularis propria.
Schned et al., (2008) found LGUC with a papillary configuration for 60% of tumors and HGPUCs account for 22.6% while non-papillary UC accounts for 10.1%. In our study, the papillary tumor configuration was seen at a rate similar to from the literature. Mostofi et al., (1973) discuss that the growth pattern of the tumour beneficial role a important prognostic indicator. Papillary carcinoma during have generally carries a good prognosis, as despite to aninfiltrative or mixed growth pattern. In other tissues, the cells usually display an infiltrative pattern also has been associated with an adverse prognosis (Lopez-Beltran et al., 2004). In some studies, pathologic staging in TURBT specimens with UC has been described, in 14.3% (15 cases) with pTa carcinoma, in 52.4% (55 cases) with pT1 carcinoma and 33.3% (35 cases) with pT2 carcinoma (Cheng et al., 2000). In our study we found, the overwhelming majority of in pT1, and thus our rates were accordance with the literature. Recent studies have shown by Chamie and colleagues, they ratio UC recurrence rates of 39.1% and a rate of progression of 33%. A recent study has demonstrated the presence of the ten-year recurrence, progression and mortality was 74.3%, 33.3%, and 12.3%, in order of frequency (Chamie et al., 2013). Superficial bladder cancer (non muscle invasive; pTa, pT1) has a described recurrence rates of 50.8% (Akagashi et al., 2006). Herr et al., (2007) showed that LGPUCs show a mean number of recurrence episodes of 6.6 and found a 67% recurrence rate. The authors demonstrated a 17% progression rate grade or stage acquired in 215 cases treated. Several studies have demonstrated of LGPUC and HGUC, recurrence rates range from 34-72% and43-74%, for LGPUC progression rates range from %4-10.5. Inother studies reported that LGPUC had a higher rate (53.8%) of recurrence. In the study, the mean duration at diagnosis of recurrence and progression was 13.9 months (2 - 72 months) and 25.1 months (Miyamoto H et al., 2010). In different study, the recurrence and progression rate was 37%, 0% and 54%, 15% for low and high grade superficial UC (Millán-Rodríguez et al., 2000). Alsheikh et al., (2001) detected 48.2% of the LGPUC in series recurred (4 months to 7 years) and 2 of the patients had a grade progression and 2 of the patients had invasion of the muscularis propria. However, other investigations have demonstrated that nonmuscle invasive UC is correlated with a increased risk of 1 year and 5 year recurrence rate of 15–61% and 31–78% (Van der Heijden et al., 2009). In our patients, carcinoma progression was observed in 11 cases (31.4%), divided into muscle invasion (4 cases). In our experience, the recurrence, progression and mean episod of time to first recurrence was seen at a rate in accordance with the literature. Some of these factors are opinion to contribute to the development of this recurrence, including incomplete TUR, implantation of tumour cells, de novo/new tumor (Van der Heijden et al., 2009; Bryan et al., 2010). In another opinion, recurrence carcinomas are a multiple or large tumours (Anastasiadis et al., 2012). Recent studies have identified residual tumors correlation with early recurrence. In this study, 33.3% of patients had entirely re-TUR had residual tumours (Chen et al., 2016). We believe this is a cause for the early recurrence of UC both for mainly residual tumour/incomplete therapy and de novo tumour. More than half of the cases in our experience had a recurrence of less than 1 year (65.7%). A more recent studyof %45.6 patients, cases’ median age from <65 years, and %54.4 cases with median aged more 65 years in the bladder cancer (Kim et al., 2016), in other study of 404 cases with bladder carcinoma, the mean age was 74 years and 13% being more than 80 years old (Figueroa et al., 1998). On the other hand, these appearances correlate with an increased risk of mortality and patients aged >70 (and older) and older had a death rate of 2.8% (Chamie et al., 2013; Figueroa et al., 1998). In our study, 20% of the cases did not demonstrate a muscularis propria in their TUR specimen and their rate was different compared to published studies. Maruniak et al and Kim et alfound the muscularis propria was not identified in 51% and 61.5% of patients (Kim HS et al., 2016; Maruniak NA et al., 2002).
In conclusion, the overall mortality rate is low at the present time. The grade of recurrent disease was most frequently low grade. Initial recurrence of UC recurrence was found in 65.7%) cases, with a median time to recurrence of 11.1 months. Progression to muscle invasionwas noted four (11.4%)cases. The patient’s pathology stage or grade such as prognostic factors depends on incomplete or complete TUR. This made five-year survival, especially in the first year, easy to plan for recurrence or progression. Presumably as a consequence of early patient diagnosis was enough, this is said to be associated with a increased survival.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Akagashi K, Tanda H, Kato S, et al. Recurrence pattern for superficial bladder cancer. Int J Urol. 2006;13:686–91. doi: 10.1111/j.1442-2042.2006.01386.x. [DOI] [PubMed] [Google Scholar]
- Alsheikh A, Mohamedali Z, Jones E, et al. Comparison of the WHO/ISUP classification and cytokeratin 20 expression in predicting the behavior of low-grade papillary urothelial tumors, World/health organization/internattional society of urologic pathology. Mod Pathol. 2001;14:267–72. doi: 10.1038/modpathol.3880300. [DOI] [PubMed] [Google Scholar]
- Anastasiadis A, de Reijke TM. Best practice in the treatment of nonmuscle invasive bladder cancer. Ther Adv Urol. 2012;4:13–32. doi: 10.1177/1756287211431976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan RT, Collins SI, Daykin MC, et al. Mechanisms of recurrence of Ta/T1 bladder cancer. Ann R Coll Surg Engl. 2010;92:519–24. doi: 10.1308/003588410X12664192076935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M, Catto JW, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–41. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- Cao D, Vollmer RT, Luly J, et al. Comparison of 2004 and 1973 World Health Organization grading systems and their relationship to pathologic staging for predicting long-term prognosis in patients with urothelial carcinoma. Urology. 2010;76:593–9. doi: 10.1016/j.urology.2010.01.032. [DOI] [PubMed] [Google Scholar]
- Chamie K, Litwin MS, Bassett JC, et al. Urologic diseases in America project. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer. 2013;119:3219–27. doi: 10.1002/cncr.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Li T, Ning XH, et al. Clinical significance of residual tumors at repeat transurethral resection in patients with T1 bladder cancer. Zhonghua Yi Xue Za Zhi. 2016;96:1124–7. doi: 10.3760/cma.j.issn.0376-2491.2016.14.013. [DOI] [PubMed] [Google Scholar]
- Cheng L, Lopez-Beltran A, Bostwick DG. Bladder cancer: general features. first ed. New Jersey: A John Wiley & Sons, Inc; 2012. pp. 138–52. [Google Scholar]
- Cheng L, Neumann RM, Weaver AL, et al. Grading and staging of bladder carcinoma in transurethral resection specimens. Correlation with 105 matched cystectomy specimens. Am J Clin Pathol. 2000;113:275–9. doi: 10.1309/94B6-8VFB-MN9J-1NF5. [DOI] [PubMed] [Google Scholar]
- Figueroa AJ, Stein JP, Dickinson M, et al. Radical cystectomy for elderly patients with bladder carcinoma: an updated experience with 404 patients. Cancer. 1998;83:141–7. doi: 10.1002/(sici)1097-0142(19980701)83:1<141::aid-cncr19>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Gupta P, Jain M, Kapoor R, et al. Impact of age and gender on the clinicopathological characteristics of bladder cancer. Indian J Urol. 2009;25:207–10. doi: 10.4103/0970-1591.52916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr HW, Donat SM, Reuter VE. Management of low grade papillary bladder tumors. J Urol. 2007;178:1201–5. doi: 10.1016/j.juro.2007.05.148. [DOI] [PubMed] [Google Scholar]
- Hoke GP, Stone BA, Klein L, Williams KN. The influence of gender on incidence and outcome of patients with bladder cancer in Harlem. J Natl Med Assoc. 1999;91:144–8. [PMC free article] [PubMed] [Google Scholar]
- Horstmann M, Witthuhn R, Falk M, Stenzl A. Gender-specific differences in bladder cancer: a retrospective analysis. Gend Med. 2008;5:385–94. doi: 10.1016/j.genm.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Jimenez RE, Gheiler E, Oskanian P, et al. Grading the invasive component of urothelial carcinoma of the bladder and its relationship with progression-free survival. Am J Surg Pathol. 2000;247:980–7. doi: 10.1097/00000478-200007000-00009. [DOI] [PubMed] [Google Scholar]
- Kim HS, Ku JH, Kim SJ, et al. Prognostic factors for recurrence and progression in Korean non-muscle-invasive bladder cancer patients: A retrospective, multi-institutional study. Yonsei Med J. 2016;57:855–64. doi: 10.3349/ymj.2016.57.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Beltran A, Sauter G, Gasser T, et al. Tumours of the urinary system. In: Eble JN, sauter G, Epstein JI, Sesterhenn IA, editors. World Health Organization classification of tumours pathology and genetics: Tumours of the urinary system and male genital organs. Lyon: IARC Press; 2004. pp. 88–157. [Google Scholar]
- Lopez-Beltran A, Bassi P, Pavone-Macaluso M, Montironi R. Handling and pathology reporting of specimens with carcinoma of the urinary bladder, ureter, and renal pelvis. Eur Urol. 2004;45:257–66. doi: 10.1016/j.eururo.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Maruniak NA, Takezawa K, Murphy WM. Accurate pathological staging of urothelial neoplasms requires better cystoscopic sampling. J Urol. 2002;167:2404–7. [PubMed] [Google Scholar]
- Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J, et al. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol. 2000;164:680–4. doi: 10.1016/s0022-5347(05)67280-1. [DOI] [PubMed] [Google Scholar]
- Miyamoto H, Brimo F, Schultz L, et al. Low-grade papillary urothelial carcinoma of the urinary bladder: a clinicopathologic analysis of a post-World Health Organization/International Society of Urological Pathology classification cohort from a single academic center. Arch Pathol Lab Med. 2010;134:1160–3. doi: 10.5858/2009-0403-OA.1. [DOI] [PubMed] [Google Scholar]
- Mostofi FK, Sobin LH, Torloni H. International histological classification of tumors, No: 10. Geneva, Switzerlend: WHO; 1973. Histological typing of urinary bladder tumours; pp. 9–33. [Google Scholar]
- Negri E, Vecchia CL. Bassi PF, Pagano F. Invasive Bladder Cancer. London: Springer; 2007. Epidemiology and prevention of bladder cancer; pp. 1–14. [Google Scholar]
- Pan CC, Chang YH, Chen KK, et al. Prognostic significance of the 2004 WHO/ISUP classification for prediction of recurrence, progression, and cancer-specific mortality of non-muscle-invasive urothelial tumors of the urinary bladder: a clinicopathologic study of 1,515 cases. Am J Clin Pathol. 2010;133:788–95. doi: 10.1309/AJCP12MRVVHTCKEJ. [DOI] [PubMed] [Google Scholar]
- Schapers RF, Pauwels RP, Wijnen JT, et al. A simplified grading method of transitional cell carcinoma of the urinary bladder: reproducibility, clinical significance and comparison with other prognostic parameters. Br J Urol. 1994;73:625–31. doi: 10.1111/j.1464-410x.1994.tb07546.x. [DOI] [PubMed] [Google Scholar]
- Schned AR, Andrew AS, Marsit CJ, et al. Histological classification and stage of newly diagnosed bladder cancer in a population-based study from the Northeastern United States. Scand J Urol Nephrol. 2008;42:237–42. doi: 10.1080/00365590801948166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, Jemal A. Cancer Statistics, 2014. Ca Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Shen Z, Xie L, Chen T, et al. Risk factors predictive of recurrence and progression for patients who suffered initial recurrence after transurethral resection of stage pT1 bladder tumor in Chinese population: A retrospective study. Medicine. 2016;95:e2625. doi: 10.1097/MD.0000000000002625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Heijden AG, Witjes JA. Recurrence, progression, and follow-up in non–muscle-invasive bladder cancer. Eur Urol. 2009;8:556–62. [Google Scholar]


