Abstract
Low-dose chemotherapy has emerged as a new strategy for control of cancer. However, there is a controversy as to whether low-dose chemotherapy is an effective way to manage many human malignancies. To shed light on this controversy, we performed a meta-analysis of relative merits between low-dose and conventional-dose chemotherapy in different carcinomas. Studies published before February 29, 2016 were reviewed for the meta-analysis and selected according to defined criteria. The effect levels of low-dose chemotherapy regarding overall survival (OS), progression-free survival (PFS) and severe adverse events (SAEs) (Grade≥3) were calculated as risk ratios (ORs) or adjusted hazard ratios (HRs). Six randomized controlled studies (RCTs) have provided data for low-dose chemotherapy versus conventional-dose chemotherapy for 838 cases and 833 cases, respectively. Interestingly, low-dose chemotherapy achieved the same desired potency as conventional-dose chemotherapy, with no differences in pooled ORR (RR=1.00, 95%CI [0.89, 1.13]; (P=0.97), OS (HR=1.07, 95%CI [0.90, 1.26]; P=0.44) and PFS (HR=1.02, 95%CI [0.84, 1.23]; P=0.87) values. Furthermore, pooled data for common SAEs showed that, compared with conventional-dose chemotherapy regimen, low-dose chemotherapy regimen resulted in significant less mucositis (P<0.0001), thrombocytopenia (P<0.00001), anemia (P=0.0001) and febrile neutropenia (P=0.004). At the same time, no statistically significant differences were observed with regard to treatment-related death (P=0.36), diarrhea (P=0.49), leucopenia (P=0.11), neutropenia (P=0.74) and nausea/vomiting (“P”=0.21). Publication bias was assessed by Egger’s test and the funnel plot. In conclusion, the meta-analysis seems to support the idea that low-dose chemotherapy may play an important role in achieving the same desired potency as conventional-dose chemotherapy in managing malignant tumors. Moreover, low-dose regimen seems to possess positive advantages of lower toxicity which is a particular concern for most patients.
Keywords: Low-dose, conventional-dose, chemotherapy, malignant tumors, RCT
Introduction
It is well known that chemotherapy together with surgery and radiotherapy remain the mainstays for dealing with malignant tumors. Generally, conventional chemotherapy is based on the conception of the most optimized efficacy at highest dose. Consistent with this notion, chemotherapy is administered at the maximum tolerated dose density (Norton and Simon, 1986, Frei et al., 1998, Peters et al.,2005). Theoretically, this is reasonable. However, some clinical trials(Kaplan et al., 1997, Moreno-Sol¨®rzano et al.,2007, Coplen, 2010, Inal et al.,2012) challenged this principle for elevating dosage did not yield desired outcomes but led to increasing severe toxicity even therapy-related death. Peters (Peters et al., 2005) reported that thirty-three patients died of causes attributed to high-dose chemotherapy, compared with no therapy-related deaths among individuals treated with low-dose chemotherapy. Virtually, chemotherapy is a two-edged weapon for it indiscriminately kills both malignant lesions and normal tissues. Thus, most patients are intimidated by the severe toxicities of chemotherapy even refuse to undergo the therapy (Fizazi et al., 2002). This urged oncologists to devise an optimal regimen to achieve favorable effect at lowest toxicity. Consequently, some researches concerning low-dose chemotherapy gradually came into sight (Kaplan et al., 1997, Fizazi et al., 2002, Brower, 2015). Nevertheless, whether the low-dose regimen being feasible remains to be established (Ratner et al., 2001). Therefore, we performed this meta-analysis on 6 RCTs incorporating 1671 participants to evaluate the efficacy and toxicity of low-dose versus conventional-dose chemotherapy in managing malignant tumors.
Materials and Methods
Literature search
We searched MEDLINE, EMBASE, Cochrane database, and meeting abstracts of the American Society of Clinical Oncology and European Society of Medical Oncology databases for articles published before September 10, 2016 using both Medical MeSH and free text words, including modified, chemotherapy, low-dose, conventional-dose, standard-dose, reduced, reduction, cancer or malignant tumor. The full details of the search strategy were as follow: modified[All Fields] AND (“drug therapy”[Subheading] OR (“drug”[All Fields] AND “therapy”[All Fields]) OR “drug therapy”[All Fields] OR “chemotherapy”[All Fields] OR “drug therapy”[MeSH Terms] OR (“drug”[All Fields] AND “therapy”[All Fields]) OR “chemotherapy”[All Fields]) AND low[All Fields] AND dose[All Fields] AND standard[All Fields]. The search was conducted with a language restricted to English publication.
Selection criteria
The inclusion criteria were as follows: (1) Randomized controlled trials (RCTs) comparing low-dose with conventional-dose chemotherapy for individuals with malignant tumors; (2) End points were overall survival (OS), progressive free survival (PFS), objective response rate (ORR) and severe adverse events (SAEs) (Grade ≥ 3); (3) All participants had been histologically or cytologically confirmed. Retrospective trials, single arm trials, case reports and systematic reviews were excluded.
Study selection and Data extraction
Two investigators [Yupeng Wu and Shuimei Luo] extracted data respectively employing a predefined data extraction form. Subsequent full-text record screening was fulfilled independently by two investigators [Haitao Yang and Lina Li]. Disagreements on whether an article should be included was resolved using a third reviewer [Xianhe Xie]. When the extracted data were not uniform, consultation was needed to make a final determination [Xianhe Xie]. All of the trials included in the analysis contained the following data: first author’s name, published year, type of study, country of original study, number of patients, median age, interventions and outcomes. But unfortunately, we can’t not obtained the full data from all the trails included, we try to contact study authors to identify additional studies and to request missing data but failed.
Quality assessment
The quality of each trial was separately estimated by two investigators, using the Cochrane Handbook for Systematic Reviews of intervention (Version 5.3.0), based on the following criteria: (1) Random sequence generation; (2) Allocation concealment; (3) Blinding of participants and personnel; (4) Blinding of outcome assessment; (5) Incomplete outcome data; (6) Selective reporting; (7) Other bias. Each trial for bias based on the criteria listed above was marked as ‘low risk’, ‘high risk’ or ‘unclear risk’. The quality of trial was defined as following: A rating: meeting all criteria of low risk; B rating: meeting one or more criteria of unclear risk without criteria of high risk; C rating: appearing one or more criteria of high risk.
Statistical analysis
Statistical analysis was conducted utilizing RevMan5.3. Chi-square and I-square tests were employed to test the heterogeneity of different trials (Higgins et al., 2003); no heterogeneity existed when P > 0.1 and I2 < 50%, a fixed-effects model was applied to pool the trial results. Significant heterogeneity was identified if P <0.1 and I2 > 50%, and a random-effects model was employed. ORR, SAEs (Grade≥3) were determined applying dichotomous variables. OS, PFS were calculated using effect variables. Hazard ratios (HRs) with 95% confidence intervals (CIs) were extracted from the survival curves when HRs were unavailable for PFS and OS (Wu et al., 2016).
Sensitivity analysis
The pooled ORR and 95%CI of sensitivity analysis by removing one study in each turn, the outcomes indicated a robustness main result. When switched fixed-effects model to random-effects model, the ORR and corresponding 95%CI from 1.00 (95%CI [0.89, 1.13]; P=0.97) to 1.02 (95%CI [0.88, 1.18]; P=0.81), that also supported the result was robustness.
Results
Selection of trials
Initially, 272 articles were screened which met our selection criteria after thoroughly searching the relevant databases; subsequently 37 of these studies were excluded due to duplication. After verifying related terms in the titles and abstracts, 202 irrelevant studies were removed and 27 unfit designed studies were eliminated through analyzing the full text. Eventually, 6 RCTs were included.
Study characteristics
Six eligible RCTs (Wood et al., 1994, Kaplan et al., 1997, Fizazi et al., 2002, Mounier et al., 2006, Cortes et al., 2013, Shah et al., 2015) included a total number of 1671 participants with various types of malignant tumors comprising 838 cases underwent low-dose regimen and 833 with conventional-dose chemotherapy. The characteristics of enrolled individuals were presented at Table 1. Chemotherapeutic agents included bleomycin, capecitabine, cisplatin, cyclophosphamide, docetaxel, doxorubicin, fluorouracil, oxaliplatin, tosedostat, vinblastine, vincristine. Five, four and six trials provided ORR, PFS and OS, respectively. The SAEs (Grade ≥ 3) were stated in all trials.
Table 1.
Characteristics of the Included Rcts Comparing Low-Dose Regimen with Conventional-Dose Regimen
| Study ID | Year | Country | Group | No. of patient | Type of Tumor | Median age(range), y | Method | Interventions | , mg/m2 | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORR | OS | PFS | AE | |||||||||||
| DXT | 5-Fu | DDP | ||||||||||||
| Shah | 2015 | American | LD | 54 | Gastric Cancer | 58(22-76) | RCT | 40 | 2400 | 40 | R | R | R | R |
| CD | 31 | 56(30-73) | 75 | 3750 | 75 | |||||||||
| Tosedostat | ||||||||||||||
| Jorge | 2013 | American | LD | 38 | Acute myeloid leukemia | 73(64-86) | RCT | 120 | R | R | NR | R | ||
| CD | 35 | 71(65-86) | 240 to 120 | |||||||||||
| ADM | CTX | VCR | ||||||||||||
| Nicolas | 2006 | France | LD | 82 | Lymphoma | 39(23-67) | RCT | 25 | 400 | 1.4 | R | R | NR | R |
| CD | 95 | 39(23-67) | 50 | 750 | 1.4 | |||||||||
| CTX | ADM | DDP | ||||||||||||
| Fizazi | 2002 | American | LD | 59 | Non-seminomatous germ-cell tumors | 26(14-52) | RCT | 400 | 35 | 100 | R | R | R | R |
| CD | 65 | 28(16-51) | 500 | 45 | 120 | |||||||||
| BLM | ADM | CTX | ||||||||||||
| Lawrence | 1997 | American | LD | 98 | Lymphoma | NR | RCT | 4 U | 25 | 300 | R | R | R | R |
| CD | 94 | NR | 4 U | 45 | 600 | |||||||||
| CTX | ADM | 5-Fu | ||||||||||||
| Wood | 1994 | American | LD | 507 | Breast Cancer | 49 | RCT | 400 | 40 | 400(x2) | NR | R | R | R |
| CD | 513 | 48 | 600 | 60 | 600(x2) | |||||||||
Abbreviations: LD, low-dose; CD, conventional-dose; BLM, bleomycin; Cap, capecitabine; DDP, cisplatin; CTX, cyclophosphamide; DXT, docetaxel; ADM, doxorubicin; 5-Fu, fluorouracil; L-OHP, oxaliplatin; VLB, vinblastine; VCR, vincristine. OS, overall survival; ORR, objective response rate; PFS, progression-free survival; AEs, adverse events.
Methodological quality
In accordance with the recommendations of the Cochrane Handbook for Systematic Reviews, we evaluated the eligible trials using the 7 aspects mentioned above. Four trials (Wood et al., 1994, Kaplan et al., 1997, Fizazi et al., 2002, Mounier et al., 2006) applied the intent-to-treat analysis. Among 6 eligible trials, 4 acquired B quality scores and 2 obtained C quality scores.
Overall response rate
Five out of 6 RCTs (Wood et al., 1994, Kaplan et al., 1997, Fizazi et al., 2002, Mounier et al., 2006, Cortes et al., 2013, Shah et al., 2015) reported ORR. A fixed-effects model was employed to analyze these trials due to no heterogeneity (P=0.22, I2=31%). The results indicated that there was no statistically significant difference in ORR between two arms (RR=1.00, 95%CI [0.89, 1.13]; P=0.97) (Figure 1A). The funnel plot demonstrated that there was no remarkable publication bias (Figure 1B). Egger’s test demonstrated that no significant publication bias was observed (P=0.514) (Figure 1C).
Figure 1.
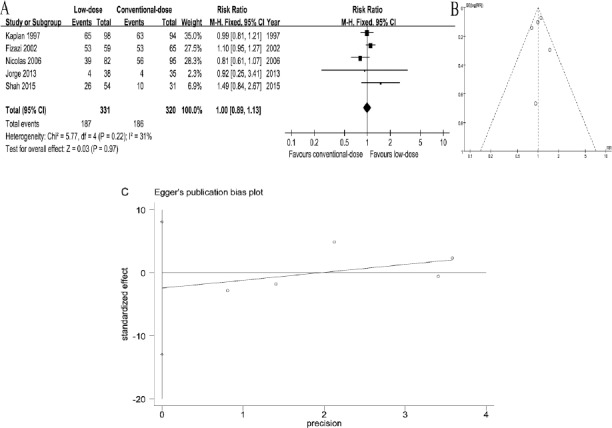
Forest Plots of Associations between Low-Dose Arm and Conventional-Dose Arm and Funnel Plot of RCTs. A, relative risks for overall response rate. B, funnel plot of RCTs. C, Visual assessment of publication bias on egger test
All of trials reported OS (Wood et al., 1994, Kaplan et al., 1997, Fizazi et al., 2002, Mounier et al., 2006, Cortes et al., 2013, Shah et al., 2015). A fixed-effects model was performed owing to no heterogeneity (P=0.1, I2= 47%) (Figure 2A). Four trials (Wood et al., 1994, Kaplan et al., 1997, Fizazi et al., 2002, Shah et al., 2015) referred to PFS was assessed applying a fixed-effects model based on the heterogeneity values (P=0.11, I2= 49%) (Figure 2B). Interestingly, there was no significant differences in OS (HR=1.07, 95%CI [0.90, 1.26]; P=0.44) and PFS (HR=1.02, 95%CI [0.84, 1.23]; P=0.87) between two arms.
Figure 2.
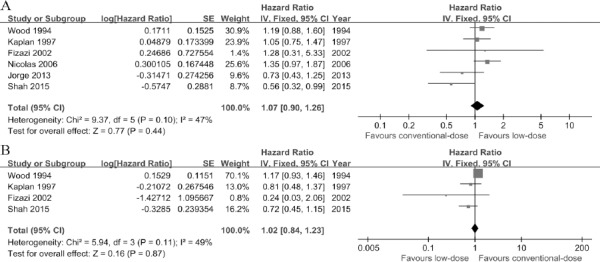
Forest Plots of associations between Low-Dose arm and Conventional-Dose Arm. A, hazard ratios for overall survival. B, hazard ratios for progression-free survival
Severe adverse events
All eligible trials (Wood et al., 1994, Kaplan et al., 1997, Fizazi et al., 2002, Mounier et al., 2006, Cortes et al., 2013, Shah et al., 2015) had estimated the chemotherapy-related adverse events. The most common SAEs were mucositis, thrombocytopenia, anemia, febrile neutropenia, diarrhea, leucopenia, neutropenia, nausea/vomiting and treatment-related death. Low-dose chemotherapy regimen yielded significantly less mucositis (RR=0.31, 95%CI [0.19, 0.53], P<0.001), thrombocytopenia (RR=0.45, 95%CI [0.32, 0.64], P<0.0001), anemia (RR=0.52, 95%CI [0.37, 0.73], P=0.001), febrile neutropenia (RR=0.73, 95%CI [0.58, 0.90], P=0.004) than conventional-dose chemotherapy regimen. Whereas, there were no distinct difference between two arms with regard to diarrhea (RR=1.78, 95%CI[0.35, 8.94], P=0.49), leucopenia (RR=0.50, 95%CI[0.21, 1.17], P=0.11), neutropenia (RR=0.91, 95%CI[0.52, 1.59], P=0.74), nausea/vomiting (RR=0.68, 95%CI[0.37, 1.24], P=0.21) and treatment-related death (RR=0.35, 95%CI[0.04, 3.31], P=0.36) (Figure 3).
Figure 3.
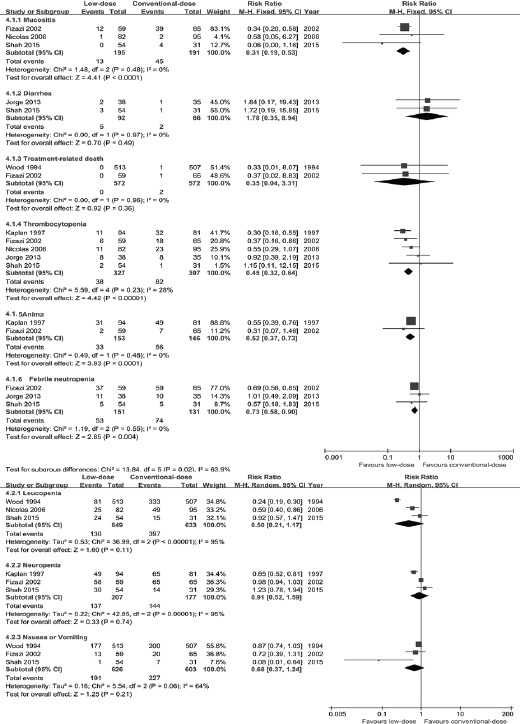
Subgroup Analyses for Severe Adverse Events
Discussion
To the best of our knowledge, this study is the first meta-analysis with a focus on comparing the efficacy and toxicity between low-dose arm and conventional-dose arm. Based on data from 6 RCTs (Wood et al., 1994, Kaplan et al., 1997, Fizazi et al., 2002, Mounier et al., 2006, Cortes et al., 2013, Shah et al., 2015) incorporating 1671 participants with malignant tumors, our study demonstrated that compared with conventional-dose regimen, low-dose regimen achieved a favorable toxicity profile without compromising efficacy, with the pooled ORR (RR=1.00, 95%CI [0.89, 1.13]; P=0.97), OS (HR=1.07, 95%CI [0.90, 1.26]; P=0.44) and PFS (HR=1.02, 95%CI [0.84, 1.23]; P=0.87). Additionally, when compared with conventional-dose arm, we observed that low-dose arm possessed notably less SAEs, including mucositis (P<0.001), thrombocytopenia (P<0.0001), anemia (P=0.0001) and febrile neutropenia (P=0.004) despite the fact that both arms appeared similar rates of diarrhea (P=0.49), leucopenia (P=0.11), neutropenia (P=0.74) and nausea/vomiting (P=0.21).
The potential mechanisms probably are that chemotherapy agents eradicate cancer cells and affect normal cells to some degree. With the escalation of dose, the toxicity grows, which partly counteracts its efficacy (Kaplan et al., 1997, Shah et al., 2015). Recently, the traditional maximum dose density modality has incurred question (Gatenby, 2009, Silva et al., 2012) since it rarely wipes out cancer entirely and ultimately lead to an explosion in tumor growth. Consequently, oncologists assiduously seek an optimal-dose regimen sharing the virtues of both favorable efficacy and minimal toxicity. One of 6 RCTs (Shah et al., 2015) in our study stated that individuals with advanced gastric cancer administered a low-dose regimen of docetaxel, cisplatin, and fluorouracil (mDCF) obtained an improved OS with less toxicities than those underwent its parent DCF. The similar desirable efficacy with less toxicity applied low-dose regimen also occurred in other five RCTs.
With regard to adverse events, previous clinical trial reported that some patients discontinued therapy because of severe toxicity, the number of quit patients in conventional-dose arm was higher than low-dose arm (Fizazi et al., 2002). Noteworthily, the treatment-related death was observed in 3 included RCTs but only 2 provided definite data. Among them, 2 patients died of causes attributed to conventional-dose chemotherapy, compared with no therapy-related deaths among individuals treated with low-dose chemotherapy despite the fact that there was no significant difference between 2 arms.
One limitation must be considered when interpreting our study findings for only 6 RCTs incorporating 1,671 individuals have been included. Thereby, larger scale trials with more participants and more comprehensive cancers are recommended to further confirm the feasibility of low-dose chemotherapy.
Summarily, our study demonstrated that low-dose chemotherapy may play an important role in achieving the same desirable potency as conventional-dose chemotherapy in managing malignant tumors. Moreover, low-dose regimen seems to possess a positive advantage of lower toxicity which would exert a peculiar fascination for most patients. Thus, in routine practice, clinicians should bear the low-dose setting in mind, especially for frail individuals.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Brower V. Modified gastric cancer chemotherapy: more effective, less toxic. Lancet Oncol. 2015;16:e590. doi: 10.1016/S1470-2045(15)00442-8. [DOI] [PubMed] [Google Scholar]
- Coplen DE. The efficacy and safety of reduced-dose docetaxel, cisplatin, and 5-fluorouracil in the first-line treatment of advanced stage gastric adenocarcinoma. Med Oncol. 2010;27:680–4. doi: 10.1007/s12032-009-9268-y. [DOI] [PubMed] [Google Scholar]
- Cortes J, Feldman E, Yee K, et al. Two dosing regimens of tosedostat in elderly patients with relapsed or refractory acute myeloid leukaemia (OPAL): a randomised open-label phase 2 study. Lancet Oncol. 2013;14:354–62. doi: 10.1016/S1470-2045(13)70037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fizazi K, Do KA, Wang X, et al. A 20% dose reduction of the original CISCA/VB regimen allows better tolerance and similar survival rate in disseminated testicular non-seminomatous germ-cell tumors: final results of a phase III randomized trial. Ann Oncol. 2002;13:125–34. doi: 10.1093/annonc/mdf005. [DOI] [PubMed] [Google Scholar]
- Frei ER, Elias A, Wheeler C, Richardson P, Hryniuk W. The relationship between high-dose treatment and combination chemotherapy: the concept of summation dose intensity. Clin Cancer Res. 1998;4:2027–37. [PubMed] [Google Scholar]
- Gatenby RA. A change of strategy in the war on cancer. Nature. 2009;459:508–9. doi: 10.1038/459508a. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inal A, Kaplan MA, Kucukoner M, Isikdogan A. Docetaxel and cisplatin plus fluorouracil compared with modified docetaxel, cisplatin, and 5-Fluorouracil as first-line therapy for advanced gastric cancer: a retrospective analysis of single institution. Neoplasma. 2012;59:233–6. doi: 10.4149/neo_2012_030. [DOI] [PubMed] [Google Scholar]
- Kaplan LD, Straus DJ, Testa MA, et al. Low-dose compared with standard-dose m-BACOD chemotherapy for non-Hodgkin’s lymphoma associated with human immunodeficiency virus infection. National institute of allergy and infectious diseases AIDS clinical trials group. N Engl J Med. 1997;336:1641–8. doi: 10.1056/NEJM199706053362304. [DOI] [PubMed] [Google Scholar]
- Moreno-Solórzano I1, Ibeas-Rollan R, Monzó-Planella M, et al. Two Doses of oxaliplatin with capecitabine (XELOX) in metastatic colorectal cancer. Clin Colorectal Cancer. 2007;6:634–40. doi: 10.3816/CCC.2007.n.031. [DOI] [PubMed] [Google Scholar]
- Mounier N, Spina M, Gabarre J, et al. AIDS-related non-Hodgkin lymphoma: final analysis of 485 patients treated with risk-adapted intensive chemotherapy. Blood. 2006;107:3832–40. doi: 10.1182/blood-2005-09-3600. [DOI] [PubMed] [Google Scholar]
- Norton L, Simon R. The Norton-Simon hypothesis revisited. Cancer Treat Rep. 1986;70:163–9. [PubMed] [Google Scholar]
- Peters WP, Rosner GL, Vredenburgh JJ, et al. Prospective, randomized comparison of high-dose chemotherapy with stem-cell support versus intermediate-dose chemotherapy after surgery and adjuvant chemotherapy in women with high-risk primary breast cancer: a report of CALGB 9082, SWOG 9114, and NCIC MA-13. J Clin Oncol. 2005;23:2191–200. doi: 10.1200/JCO.2005.10.202. [DOI] [PubMed] [Google Scholar]
- Ratner L, Lee J, Tang S, et al. Chemotherapy for human immunodeficiency virus-associated non-Hodgkin’s lymphoma in combination with highly active antiretroviral therapy. J Clin Oncol. 2001;19:2171–8. doi: 10.1200/JCO.2001.19.8.2171. [DOI] [PubMed] [Google Scholar]
- Shah MA, Janjigian YY, Stoller R, et al. Randomized multicenter phase II study of modified docetaxel, cisplatin, and Fluorouracil (DCF) Versus DCF Plus Growth factor support in patients with metastatic gastric adenocarcinoma: A study of the US gastric cancer consortium. J Clin Oncol. 2015;33:3874–9. doi: 10.1200/JCO.2015.60.7465. [DOI] [PubMed] [Google Scholar]
- Silva AS, Kam Y, Khin ZP, et al. Evolutionary approaches to prolong progression-free survival in breast cancer. Cancer Res. 2012;72:6362–70. doi: 10.1158/0008-5472.CAN-12-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood WC, Budman DR, Korzun AH, et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med. 1994;330:1253–9. doi: 10.1056/NEJM199405053301801. [DOI] [PubMed] [Google Scholar]
- Wu YP, Lin TT, Chen SH, et al. Comparison of the efficacy and feasibility of en bloc transurethral resection of bladder tumor versus conventional transurethral resection of bladder tumor: A meta-analysis. Medicine. 2016;95:e5372. doi: 10.1097/MD.0000000000005372. [DOI] [PMC free article] [PubMed] [Google Scholar]


