Abstract
Background:
Possible targeted therapies for metastatic triple negative breast cancer (TNBC) include cytotoxic chemotherapy that causes interstrand breaks (platinum-based drugs). The excision repair cross-complementation 1 (ERCC1) enzyme plays an essential role in the nucleotide excision repair pathway, removing platinum-induced DNA adducts and contributing to cisplatin resistance. Detecting ERCC1 overexpression is important in considering treatment options for metastatic TNBC, including individualized approaches to therapy, and may facilitate improved responses or reduction of unnecessary toxicity. We hypothesized that assigning cisplatin based on pretreatment ERCC1 expression would improve response and survival. This study was conducted to assess the impact of ERCC1 expression on PFS, OS and response rates in metastatic triple negative breast cancer patients treated with platinum-based chemotherapy.
Methods:
From June 2012 to November 2013, 52 metastatic triple negative breast cancer patients were enrolled. ERCC1 protein expression was detected from pretreatment biopsies by Immunohistochemistry. All patients received cisplatin plus paclitaxel. The primary end point was the impact of ERCC1 expression on PFS and OS.
Results:
34 patients (65.4%) showed positive ERCC1 expression while 18 (34.6%) proved negative. Positive ERCC1 expression was associated with short PFS (median, 5 months vs. 7 months; P = 0.043), short OS (median, 9 months vs. 11 months; P = 0.033) and poor response to cisplatin based chemotherapy (P = 0.046).
Conclusions:
This prospective study further validated ERCC1 as a reliable biomarker for customized chemotherapy in metastatic triple negative breast cancer patients. High expression of ERCC1 was thereby fond to be significantly associated with poor outcome in patients treated with platinum based chemotherapy.
Keywords: ERCC1, triple negative, metastatic, breast
Introduction
Breast cancer is a disease with different both histological subtypes and clinical courses. Molecular types are luminal subtype A, luminal subtype B, the human epidermal growth factor receptor 2 (HER2)-overexpressing subtype; and the basal-like subtype (Weigelt et al., 2008).
Expression of ER and/or progesterone receptors (PR) characterizes luminal tumors, and HER2-overexpressing subtype is defined by overexpression and/or amplification of HER2 in tumors negative for ER and PR expression. Triple negative breast cancers (TNBC) is breast tumors that do not express hormone receptors (ER, PR) nor HER2 overexpression and/or amplification (Goldhirsch et al., 2011).
Triple-negative breast cancer (TNBC) represents approximately 10–20 % of breast cancer patients and is an aggressive subtype with poor prognosis (Carey et al., 2010). Chemotherapy is the only systemic treatment available for TNBC; however, many TNBC patients developed relapse within 1–2 years and 30 % of patients survive for five years even with adjuvant chemotherapy (Dent et al., 2007).
The majority of TNBC are of basal-like phenotype, but this group of tumors also includes tumors that lake the expression of basal markers, including the molecular apocrine and claudin-low tumors (Higgins and Baselga, 2011). Targeted treatment for TNBC are not completely available, and the main line of treatment is chemotherapy, including platinum drugs, single or in combination with other chemotherapy drugs (Hudis and Gianni, 2011).
Platinum-based chemotherapy is effective in many malignant diseases. There are three platinum agents more commonly used, namely cisplatin, carboplatin, and oxaliplatin. These platinum drugs form a platinum-DNA adducts, leading to destruction in the helical structure of the DNA (Kelland, 2007). The distortion of the DNA molecule results in cessation of transcription and replication, that will result in cell death.
DNA adducts are detected and repaired by the nucleotide excision repair (NER) pathway, including those caused by platinum agents. The excision repair cross-complementation group 1 (ERCC1) is a nuclease that plays an important role in the NER pathway: ERCC1 forms a heterodimer with xeroderma pigmentosum complementation group F (XPF) protein, and the compound ERCC1-XPF remove the excision of the damaged DNA (Vilmar and Sorensen, 2009). So, the integrity of the NER pathway is a good predictor for resistance to platinum drugs (Kelland, 2007).
Low expression of ERCC1 correlated with in vitro sensitivity to cisplatin in malignant cell lines from cervical cancer (Britten et al., 2000), testicular cancer (Welsh et al., 2004) and malignant effusions collected from patients with non-small cell lung cancer (Wang et al., 2008). Retrospective analysis have shown a relation between increased level of ERCC1 mRNA or protein expression and resistance to the platinum-based chemotherapy in many types of advanced tumors, including gastric and colon cancer (Kwon et al., 2007), non-small cell lung cancer (NSCLC) (Wang et al., 2008), urinary tract cancer (K. H. Kim et al., 2010) and head and neck squamous cell carcinoma (Handra-Luca et al., 2007).
Materials and Methods
Patients’ characteristics
This is a prospective cohort study was conducted from June 2012 to November 2013 in which all 52 enrolled patients were cases of chemotherapy naive metastatic triple negative breast cancer (TNBC). Patients’ clinical history data was acquired from the files of the Medical Oncology Unite, Oncology Center Mansoura University, Mansoura, Egypt. The age of patients ranged from 31 to 69 years. The histology of the formalin-fixed, paraffin embedded sections was confirmed by pathologist, Figure 1. Clinicopathological and molecular data were collected, including age, menopausal state, performance state (PS), loss of weight, site of distant metastasis and the immunohistochemical expression of Ki-67 index. According to the percentage of stained nuclei, two categories were defined for Ki-67 index: <14% (low proliferative) and ≥14% (high proliferative), Figure 2. All patients received 6 cycles of chemotherapy regimen (Cisplatin 75 mg/m2 IV plus Paclitaxel 135 mg/m2 IV on day 1 Cycled every 21 days). Follow-up ranged from a minimum of 3 months to a maximum of 20 months (median 8 months).
Figure 1.
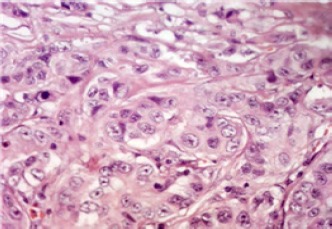
Grade II Invasive Duct Carcinoma (400×)
Figure 2.
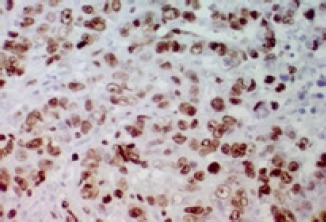
Strong Diffuse Nuclear Expression for Ki-67 in Breast Tumor Cells (>14%) (400×)
Response rate was evaluated by CT scan, PET/CT (whenever possible) and CA 15.3 serum level (when the basal level was elevated before treatment) and patients were categorized according to RECIST (Response Evaluation Criteria in Solid Tumors) (Therasse et al., 2000). Progression free survival (PFS) time was calculated as the duration from the first day of treatment to the date of documented disease progression. Overall survival (OS) time was calculated as the duration from the date of diagnosis to the date of death.
Immunohistochemical study
Immunohistochemical staining was performed on 4 μm FFPE tissue sections using antigen retrieval technique. Slides were deparaffinized in xylene and dehydrated in a graded ethanol series. Endogenous peroxidase was blocked with 0.3% H2O2 in methanol for 15 min. For antigen retrieval, the slides for ERCC1 (clone 8F1, dilution 1:100, Thermo Fisher, Scientific Inc., Fremont, USA) were immersed in 10 mm citric buffer solution (pH 6.0). Slides were heated to 125 ºC by exposure to autoclave irradiation for 15 min and were allowed to cool for 1 h at room temperature, then washed in water and PBS. Non specific binding was blocked by pre-incubation with 2% BSA plus 0.1% NaN3for 30 min. The blocking solution was drained off, and the slides were incubated overnight at 4 ºC with the primary antibodies. Staining with an irrelevant mouse IgG1 or IgG2a was routinely performed as a negative control procedure. After washing three times in PBS, the slides were incubated with a labeled polymer, EnVision + Peroxidase Mouse (DAKO, Glostrup, Denmark), for 30 min. The chromogen used was 2% 3,3`-diaminobenzidine in 50 mM Tris buffer (pH 7.6) containing 0.3% hydrogen. Slides were counterstained with hematoxylin. Breast cancer tissue was used as a positive control. A distinct brown nuclear immunostaining was scored positive. At least, 400 cells from 5 randomly selected fields (X400) were counted. Aberrant expression was defined as staining in excess of normal tissues.
Four semi-quantitative classes were used to describe the percentage of positively stained tumor cells: negative: <10 % positive cells; + positive: minimally positive (10-25% positive cells); ++ positive: moderately positive (25-50% positive cells); and +++ positive: markedly positive (>50% positive cells). Summation of the positive groups was done for proper statistical analysis due to the small sample size, so cases were considered positive if >10% of the tumor cells were positively stained, Figure 3 and negative otherwise Figure 4 because a 10% cutoff level has been used in a previous report (Wachters et al., 2005).
Figure 3.
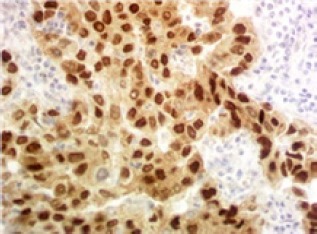
Strong Diffuse Nuclear Expression for Ercc1 in Breast Tumor Cells (400×)
Figure 4.
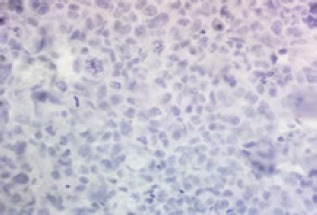
Negative Nuclear Expression For ERCC1 in Breast Tumor Cells (400×)
Statistical Analysis
Descriptive statistics comparing ERCC1 expression with the clinicopathological characteristics were analyzed by the chi-square test. Survival curves were calculated by the Kaplan–Meier method, and the differences were assessed by the log-rank test. Statistical analysis software (Dr SPSSII, Windows, version 20) was used to perform the analyses, and a significant level of ≤0.05 was considered statistically significant.
Results
ERCC1 expression and clinicopathologic features of patients
From June 2012 to November 2013, 52 newly diagnosed chemotherapy naive metastatic triple negative breast cancer patients were enrolled. With age from 31 to 69 years; mean ± SD (51 ± 11). Fourteen patients (26.9%) were premenopausal while 38 patients (73.1%) were postmenopausal, 26 patients (50%) presented with weight loss and 25 patients (48.1%) had performance status 2. ERCC1 expression was positive in 34 patients (65.4%) while 18 patients (34.6%) showed negative ERCC1 expression. Twenty four patients (46.2%) were expressing low Ki 67 while 28 patients (53.8%) expressing high Ki 67. Both visceral and bone metastasis at diagnosis were presented in 23 patients (44.2%) while visceral metastasis only and bone metastasis only were presented in 17 patients (32.7%) and 12 patients (23.1%), respectively.
Impact of ERCC1 expression on response rate, progression free survival (PFS) and overall survival (OS)
After 6 cycles of chemotherapy regimen (Cisplatin 75 mg/m2 IV plus Paclitaxel 135 mg/m2 IV on day 1 Cycled every 21 days), complete response (CR) observed in three cases (5.8%) confirmed by PET/CT while 18 patients (34.6%) showed partial response (PR). However stable disease (SD) and progression were observed in 14 patients (26.9%) and 17 patients (32.7%).
This study did not found statistically significant relation between ERCC1 expression and clinicopathological criteria of the patients as age (P 0.24), menopausal state (P 0.15), performance state at start of treatment (P 0.33), site of metastasis at diagnosis (P 0.6) and Ki 67 expression (P 0.32) while weight loss at diagnosis was statistically significant with high ERCC1 expression (P 0.02) as shown in Table (1).
Table 1.
ERCC1 Expression and Patients Characteristics
| Characteristics | ERCC1 protein -ve expression (18 ptn) No. (%) | (52 patients) +ve expression (34 ptn)No. (%) | P |
|---|---|---|---|
| Age (years) | 0.32 | ||
| <51 | 10 (55.6%) | 14 (41.2%) | |
| ≥51 | 8 (44.4%) | 20 (58.8%) | |
| Menopausal state | 0.15 | ||
| Premenopausal | 7 (38.9%) | 7 (20.6%) | |
| postmenopausal | 11 (61.1%) | 27 (79.4%) | |
| Weight loss | |||
| No loss | 13 (72.2%) | 13 (38.2%) | 0.02 |
| Weight loss | 5 (27.8%) | 21 (61.8%) | |
| Performance state | |||
| PS 0+1 | 11 (61.1%) | 16 (47.1%) | 0.33 |
| PS 2 | 7 (38.9%) | 18 (52.9%) | |
| Ki67 expression | 0.32 | ||
| Ki 67 ≤ 20% | 10 (55.6%) | 14 (41.2%) | |
| Ki 67 >20% | 8 (44.4%) | 20 (58.8%) | |
| Site of metastasis | 0.6 | ||
| Visceral and bone | 8 (44.4%) | 15 (44.1%) | |
| Bone only | 3 (16.7%) | 9 (26.5%) | |
| Visceral only | 7 (38.9%) | 10 (29.4%) | |
| Response | 0.046 | ||
| Responders (CR and PR) | 11 (50%) | 11 (50%) | |
| Non responders (SD and Progressed) | 7 (23.3%) | 23 (76.7%) |
CR, complete response; PR, partial response; SD, stable disease
Patients with CR and PR were considered responders while patients with SD and progressed disease were considered non responders and the present study found that patients with high ERCC1 expression showed poor response (SD and progressed disease) to cisplatin plus paclitaxel while patients with low ERCC1 expression showed an improved response (CR and PR) with a statistically significant relation (P 0.046) as shown in Table (1).
The median follow up duration was eight month (range: 3 – 20 month) and at the end of the study there were 36 patients (69.2%) died, six patients (11.5%) lost follow up and ten patients (19.3%) continued alive till end of the study. High ERCC1 expression was associated with short PFS (median, 5 months vs. 7 months; P = 0.043), show Table (2) and Figure 5. Also, high ERCC1 expression was associated with short OS (median, 9 months vs. 11 months; P = 0.033) as shown in table (2) and Figure 6.
Table 2.
Univariate Analysis of PFS and OS with ERCC1 Expression
| Prognostic factors | Median (months) | 95% Confidence interval (CI) | Log Rank | P value | |
|---|---|---|---|---|---|
| PFS | Negative (18) | 7 | 4.93 – 9.06 | 4.08 | 0.043 |
| Positive (34) | 5 | 4.19 – 5.80 | |||
| OS | Negative (18) | 11 | 8.50 – 13.49 | 4.54 | 0.033 |
| Positive (34) | 9 | 6.74 – 11.25 |
Figure 5.
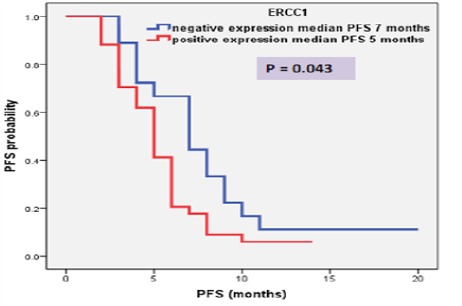
PFS of Patients according to RECC1 Expression
Figure 6.
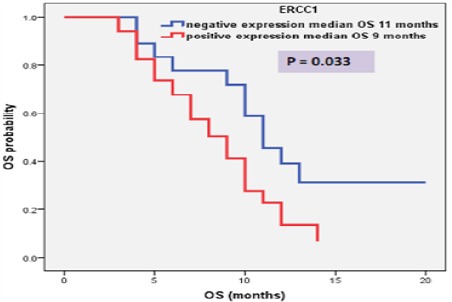
OS of Patients according To RECC1 Expression.
Discussion
The present study showed higher ERCC1 expression in TNBC (high expression versus low expression was 65.4% vs. 34.6%, respectively) and this was difference from Kim et al., (2011) who found that TNBC tend to be ERCC1 negative and this was due to larger number of studied cases in that study which was comparing ERCC1 expression in all other molecular subtypes of breast cancer as luminal A, Luminal B and HER-2 amplifier. Based on their results, Kim et al., (2011) document that the possibility of using platinum-based chemotherapy as a treatment option for TNBC can be expected which was supported by a phase II clinical trial for neoadjuvant cisplatin chemotherapy in 22 TNBC patients, showing almost 64% response rate and 22% pathologically complete response (Silver et al., 2010).
There are little studies as regard the expression of ERCC1 in breast cancer. Some series showed that the expression of ERCC1 is lower in TNBC. Sidoni et al., (2008) analyzed ERCC1 expression in 81 TNBC and found that one third of the studied cases (32.0%) was positive for ERCC1 that may be predictive of a poor response to platinum-based drugs. Therefore, they suggested that the immunohistochemical screening for ERCC1 in TNBC could be a method for better identification of patients who may respond to platinum-containing chemotherapies. In contrast to our results Gerhard et al., (2013) found that TNBC were more frequently negative for ERCC1 (61.5% of the cases) however this study examine ERCC1 expression in primary tumor and not in metastatic breast cancer as our study. Another different study by Shao et al., (2010) found that positive ERCC1 expression was presented in 50% of both locally advanced and metastatic breast cancer patients.
The current study did not demonstrate any significant relation between ERCC1 expression and clinicopathological criteria of the patients as age, menopausal state, performance state at start of treatment, site of metastasis at diagnosis and Ki 67 expression except weight loss at diagnosis which was statistically significant with high ERCC1 expression. Using a quantitative immunofluorescence technique, Metro et al., (2010) analyzed the expression of ERCC1 protein in 55 patients with metastatic breast cancer. In agreement with our study, the authors did not find any statistically significant associations between ERCC1 and the following variables: age (≥ vs. < 50 years), tumor stage at diagnosis (localized vs. advanced), histology (ductal vs. other), presence of visceral disease (yes vs. no), and pretreatment for metastatic disease (yes vs. no). Also, no difference in expression was observed within metastatic sites classified as visceral vs. nonvisceral metastase (Metro et al., 2010).
In general, our results and those from Shao et al., (2010) and Deng et al., (2014) suggest that ERCC1 expression is not associated with many clinicopathological features in patients with breast cancer as age, performance status, tumor size and hormonal status.
On the other hand, ERCC1 expression in breast cancer was analyzed in 504 women with early stage breast cancer treated with breast-conserving surgery and postoperative breast irradiation, Goyal et al., (2010) found that increased ERCC1 expression was associated with criteria of a better prognosis, including age >50 years-old, lower T stage, negative nod, positivity of ER, and non-triple negative status but, this study was conducted on early breast cancer and included all molecular subtypes of breast cancer patients.
Gerhard et al., (2013) analyzed the immunohistochemical expression of ERCC1 in a series of primary breast cancers and showed an association between ERCC1-positive expression and tumor size smaller than 2.0 cm (P = 0.007), but no correlation was found with other clinicopathological features however, this study examine ERCC1 expression in primary tumor and not in metastatic breast cancer as our study.
ERCC1 positivity has been shown to predict poor treatment response of platinum-based chemotherapy in ovarian, gastric, cervical, colon and non-small cell lung cancer (Britten et al., 2000), (Dabholkar et al., 1992), (Metzger et al., 1998), (Olaussen et al., 2006), (Shirota et al., 2001). The present study found an improved response to platinum based chemotherapy in patients with low ERCC1 expression and a poor response in patients with high ERCC1 expression and this explain the role of ERCC1 in platinum resistance.
Also, in a study involving 107 breast cancer patients treated with neoadjuvant chemotherapy composed of paclitaxel plus carboplatin showed that ERCC1-negative tumors were related to a higher pathological complete remission (pCR) than tumors positive for ERCC1 (Chen et al., 2012).
However, Metro et al., (2010) did not find a statistically significant associations between RRM1, ERCC1 or BRCA1, and response to treatment (P 0.49 for RRM1, P 0.13 for ERCC1, P 0.41 for BRCA1) which can be explained by this study was conducted on primary and metastatic breast cancer patients and also included TNBC and other molecular subtypes. Also, Shao et al., (2010) found that ERCC1 expression was not significantly associated with ORR and this is not consistence with our result which can be explained by Shao et al., (2010) was conducted on 54 locally advanced or metastatic breast cancer patients including TNBC and other molecular subtypes and an explanation is the intrinsic differences in tumor biology among different cancers.
Chemotherapy composed by platinum drugs results in the formation of platinum-DNA adducts, leading destruction of the DNA molecule. These DNA adducts are repaired by and important enzyme (ERCC1) which is related to the NER pathway, (Kelland, 2007), (Martin et al., 2008), (Vilmar and Sorensen, 2009). So, the activity of DNA repair systems, specially the NER pathway, is an important indicator of platinum drugs resistance (Reed, 2005).
Many tumors are treated with platinum-based doublets, and the ERCC1 has been considered as a possible useful predictive and/or prognostic marker to platinum chemotherapy agents. According to Olaussen et al., (2006) the expression status of ERCC1 is a predictive factor for the sensitivity to platinum-based chemotherapy in NSCLC patients. The authors analyzed two groups of patients with completely resected NSCLC: the adjuvant chemotherapy group received cisplatin-based adents and the control group was observed only. Patients with low ERCC1 tumors in the adjuvant chemotherapy group had a statistically significant better OS and DFS when compared to the control group; in contrast, there was no survival difference between the two groups in patients with high ERCC1 tumors (Olaussen et al., 2006). In another study, the negativity for ERCC1 expression in patients with locally advanced NSCLC treated with neoadjuvant platinum-based concurrent chemoradiotherapy was associated with an improved survival compared to patients whose tumors were ERCC1-positive (Hwang et al., 2008).
The current study found a longer PFS and OS for patients with low ERCC1 expression than patients with high ERCC1 expression and this explain the importance of biological assessment of metastatic disease before treatment with platinum based chemotherapy. Our result was in agreement with few studies regarding patients with breast cancer that were treated with platinum-based regimens. Shao et al., (2010) analyzed the expression of ERCC1 in patients with advanced breast cancer treated with platinum-based chemotherapies on 54 patients with locally advanced or metastatic breast cancer treated with paclitaxel and cisplatin and found that, in multivariate analysis, increased ERCC1 expression was associated with poor progression-free survival (PFS).
In contrast, Gerhard et al., (2013) and Goyal et al., (2010) did not find an association between ERCC1 expression and survival for patients with breast cancer. Also Kim et al., (2011) did not find any association between ERCC1 expression and survival of the patients after platinum based chemotherapy which was different from our result and can be explained by in Kim et al., (2010) the expression of ERCC1 was examined in early breast cancer patient not in metastatic setting. Likewise, Metro et al., (2010) did not found significant associations between ERCC1 expression and PFS or OS but this study was conducted on both primary and metastatic breast cancer patients and also included TNBC and other molecular subtypes.
Shao et al., (2010) found that ERCC1 expressions were not significantly associated with PFS or OS and this difference from our results can be explained by Shao et al., (2010) was conducted on 54 locally advanced or metastatic breast cancer patients including TNBC and other molecular subtypes and an explanation is the intrinsic differences in tumor biology among different cancers.
In a series by Deng et al., (2014), the median DFS was not significantly different between the low and high ERCC1 mRNA expression groups in the entire cohort (54.99 vs. 45.59 months, P 0.433). No significant difference in OS was observed between the low and high ERCC1 mRNA expression groups (58.02 vs. 48.52 months, P 0.760) however this study was conducted on 363 patients with breast cancer, 344 had invasive cancer (94.8%) and 19 non-invasive cancer (5.2%); 30 (8.3%) received plati¬num-based adjuvant chemotherapy, 35 (9.6%) received surgery alone, and 298 (82.1%) received non-platinum-based adjuvant chemotherapy and this design different from our inclusion criteria that included metatstatic triple negative breast cancer patients. This prospective study was conducted on a small number of patients and in single institution however, it further indicate ERCC1 as an important marker for customized chemotherapy in metastatic TNBC patients and showed that high ERCC1 expression was significantly associated with poor response, PFS and OS in patients treated with platinum based chemotherapy. This may indicate further prospective studies with large numbers of patients to confirm the role of ERCC1 as a prognostic factor, since there are limited prognostic markers in chemotherapy for TNBC.
References
- Britten RA, Liu D, Tessier A, Hutchison MJ, Murray D. ERCC1 expression as a molecular marker of cisplatin resistance in human cervical tumor cells. Int J Cancer. 2000;89:453–57. [PubMed] [Google Scholar]
- Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010;7:683–92. doi: 10.1038/nrclinonc.2010.154. [DOI] [PubMed] [Google Scholar]
- Chen X, Wu J, Lu H, Huang O, Shen K. Measuring beta-tubulin III, Bcl-2, and ERCC1 improves pathological complete remission predictive accuracy in breast cancer. Cancer Sci. 2012;103:262–68. doi: 10.1111/j.1349-7006.2011.02135.x. [DOI] [PubMed] [Google Scholar]
- Dabholkar M, Bostickbruton F, Weber C, et al. ERCC1 and ERCC2 expression in malignant-tissues from ovarian-cancer patients. J Natl Cancer Inst. 1992;84:1512–17. doi: 10.1093/jnci/84.19.1512. [DOI] [PubMed] [Google Scholar]
- Deng QH, Yang HH, Lin YP, et al. Prognostic value of ERCC1 mRNA expression in non-small cell lung cancer, breast cancer, and gastric cancer in patients from Southern China. Int J Clin Exp Pathol. 2014;7:8312–18. [PMC free article] [PubMed] [Google Scholar]
- Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- Gerhard R, Carvalho A, Carneiro V, et al. Clinicopathological significance of ERCC1 expression in breast cancer. Pathol Res Pract. 2013;209:331–36. doi: 10.1016/j.prp.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22:1736–47. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S, Parikh RR, Green C, et al. Clinicopathologic significance of excision repair cross-complementation 1 expression in patients treated with breast-conserving surgery and radiation therapy. Int J Radiat Oncol Biol Phys. 2010;76:679–84. doi: 10.1016/j.ijrobp.2009.02.050. [DOI] [PubMed] [Google Scholar]
- Handra-Luca A, Hernandez J, Mountzios G, et al. Excision repair cross complementation group1 immunohistochemical expression predicts objective response and cancer-specific survival in patients treated by cisplatin-based induction chemotherapy for locally advanced head and neck squamous cell carcinoma. Clin Cancer Res. 2007;13:3855–59. doi: 10.1158/1078-0432.CCR-07-0252. [DOI] [PubMed] [Google Scholar]
- Higgins MJ, Baselga J. Targeted therapies for breast cancer. Eur J Clin Invest. 2011;121:3797–3803. doi: 10.1172/JCI57152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudis CA, Gianni L. Triple-negative breast cancer: An unmet medical need. Oncologist. 2011;16:1–11. doi: 10.1634/theoncologist.2011-S1-01. [DOI] [PubMed] [Google Scholar]
- Hwang IG, Ahn MJ, Park BB, et al. ERCC1 expression as a prognostic marker in N2(+) nonsmall-cell lung cancer patients treated with platinum-based neoadjuvant concurrent chemoradiotherapy. Cancer. 2008;113:1379–86. doi: 10.1002/cncr.23693. [DOI] [PubMed] [Google Scholar]
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–84. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- Kim D, Jung W, Koo JS. The Expression of ERCC1, RRM1, and BRCA1 in breast cancer according to the immunohistochemical phenotypes. J Korean Med Sci. 2011;26:352–9. doi: 10.3346/jkms.2011.26.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Do IG, Kim HS, et al. Excision repair cross-complementation group 1 (ERCC1) expression in advanced urothelial carcinoma patients receiving cisplatin-based chemotherapy. Apmis. 2010;118:941–8. doi: 10.1111/j.1600-0463.2010.02648.x. [DOI] [PubMed] [Google Scholar]
- Kwon HC, Roh MS, Oh SY, et al. Prognostic value of expression of ERCC1, thymidylate synthase, and glutathione S-transferase P1 for 5-fluorouracil/oxaliplatin chemotherapy in advanced gastric cancer. Ann Oncol. 2007;18:504–9. doi: 10.1093/annonc/mdl430. [DOI] [PubMed] [Google Scholar]
- Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: The role of DNA repair pathways. Clinical Cancer Res. 2008;14:1291–95. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- Metro G, Zheng Z, Fab A, et al. In situ protein expression of RRM1, ERCC1, and BRCA1 in metastatic breast cancer patients treated with gemcitabine-based chemotherapy. Cancer Invest. 2010;28:172–80. doi: 10.3109/07357900903095722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger R, Leichman CG, Danenberg KD, et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol. 1998;16:309–16. doi: 10.1200/JCO.1998.16.1.309. [DOI] [PubMed] [Google Scholar]
- Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–91. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- Reed E. ERCC1 and clinical resistance to platinum-based therapy. Clinical Cancer Res. 2005;11:6100–2. doi: 10.1158/1078-0432.CCR-05-1083. [DOI] [PubMed] [Google Scholar]
- Shao YY, Kuo KT, Hu FC, et al. Predictive and prognostic values of Tau and ERCC1 in advanced breast cancer patients treated with paclitaxel and cisplatin. Jpn J Clin Oncol. 2010;40:286–93. doi: 10.1093/jjco/hyp184. [DOI] [PubMed] [Google Scholar]
- Shirota Y, Stoehlmacher J, Brabender J, et al. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol. 2001;19:4298–4304. doi: 10.1200/JCO.2001.19.23.4298. [DOI] [PubMed] [Google Scholar]
- Sidoni A, Cartaginese F, Colozza M, Gori S, Crino L. ERCC1 expression in triple negative breast carcinoma: the paradox revisited. Breast Cancer Res Treat. 2008;111:569–70. doi: 10.1007/s10549-007-9804-4. [DOI] [PubMed] [Google Scholar]
- Silver DP, Richardson AL, Eklund AC, et al. Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28:1145–53. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- Vilmar A, Sorensen JB. Excision repair cross-complementation group 1 (ERCC1) in platinum-based treatment of non-small cell lung cancer with special emphasis on carboplatin: A review of current literature. Lung Cancer. 2009;64:131–39. doi: 10.1016/j.lungcan.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Wachters FM, Wong LS, Timens W, Kampinga HH, Groen H J. ERCC1, hRad51, and BRCA1 protein expression in relation to tumour response and survival of stage III/IV NSCLC patients treated with chemotherapy. Lung Cancer. 2005;50:211–19. doi: 10.1016/j.lungcan.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Wang L, Wei J, Qian X, et al. ERCC1 and BRCA1 mRNA expression levels in metastatic malignant effusions is associated with chemosensitivity to cisplatin and/or docetaxel. BMC Cancer. 2008;8:97. doi: 10.1186/1471-2407-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt B, Horlings HM, Kreike B, et al. Refinement of breast cancer classification by molecular characterization of histological special types. J Pathol. 2008;216:141–50. doi: 10.1002/path.2407. [DOI] [PubMed] [Google Scholar]
- Welsh C, Day R, McGurk C, et al. Reduced levels of XPA, ERCC1 and XPF DNA repair proteins in testis tumor cell lines. Int J Cancer. 2004;110:352–61. doi: 10.1002/ijc.20134. [DOI] [PubMed] [Google Scholar]


