Abstract
Background:
Raising breast cancer awareness is a key strategy to reduce associated mortality. While a paucity of adequately validated instruments for breast cancer awareness is applicable across cultures, even outside the health care setting such instruments have been developed.
Objective:
This study investigated the validity and psychometric properties of a breast cancer awareness scale in Indonesia (BCAS-I).
Methods:
This cross-sectional study was carried out among Indonesian women conveniently selected within three provinces (Yogyakarta, South of Sumatera and East Nusa Tenggara) located in rural-urban areas using stratified random sampling. First, we translated all questionnaires from English to the local language and then back-translated. The third step was to perform psychometric testing of the adapted instrument by establishing internal consistency (Cronbach’s alpha score 0.79) and construct validity by confirmatory factor analysis (CFA).
Results:
In the 856 participants who responded (responded rate = 98.28%), the age ranged from 18 to 80 years old (mean = 30, SD = 11). The BCAS-I was shown to have good internal consistency, and CFA demonstrated the model fit data adequately (χ2 = 922.267, df = 515, p <0.001, comparative fit index = 0.965, Tucker-Lewis Index = 0.96, goodness-of-fit index = 0.97, adjusted goodness-of-fit index = 0.97, root-mean-square error of approximation = 0.03 95% confidence interval: 0.027, 0.034). The final version of BCAS-I consists of 33 items across 5 domains that cover most key aspects of breast cancer awareness for this population.
Conclusion:
The BCAS-I demonstrated good psychometric properties and was found to be valid to provide a measurement of breast cancer awareness in Asian women in general and Indonesian women in particular.
Keywords: Breast cancer, breast cancer awareness, instrument validation, Indonesian women
Introduction
The World Health Organization (WHO) identifies breast cancer as the leading cancer in females in both developed and developing countries (1.7 million) (Jemal et al., 2010; Jemal et al., 2011; Ferlay et al., 2015). Breast cancer accounts for over a half of new cancer cases occurring in women in developing countries, representing a quarter of all new cancer cases (Ferlay et al., 2015). The increasing incidence of breast cancer in economically developing countries is likely to be due to population aging and lifestyle changes, including lower physical activity, higher levels of obesity, and alcohol consumption (Jemal et al., 2011). Perhaps more importantly, breast cancer mortality rates are on the rise in many Asian countries (Salim et al., 2010). In Indonesia, a lower-middle-income Asian country, breast cancer is the most frequently diagnosed cancer in women (Ng et al., 2011).
One of the problems in developing countries such as Indonesia is that diagnosis of breast cancer occurs later in the disease progression, resulting in poorer prognosis and higher breast cancer mortality. While several campaigns to reduce risk factors of breast cancer have been trialled in developing countries, difficulties deploying the campaigns has led to low efficacy of such programs in developing countries (Hossain et al., 2014). This problem is compounded by the fact that in most lower- and middle-income countries, such as Indonesia, no routine mammographic screening programs are in place due to limited resources available in these health care settings (Coughlin and Ekwueme, 2009; El Saghir et al., 2011). Given the infrastructural and other resource limitations in these countries, breast cancer awareness is particularly important, as it is the individual more than health professionals who will prompt timely clinical breast examination or mammography.
Poverty and social-cultural habits represent additional factors that have been shown to be associated with delayed diagnosis of breast cancer (Toure et al., 2013), and poor health-seeking behaviour in both diagnosis and treatment of breast cancer has been demonstrated as shortening survival time in patients (Bish et al., 2005; Liu et al., 2014; Youlden et al., 2014). In such a setting, early detection based on awareness of early signs and symptoms of breast cancer are particularly important in decreasing mortality rates of breast cancer. Furthermore, increasing awareness represents a feasible and cost-effective method in countries with resource-limited health care, especially those that do not have ongoing organized population-based screening (Youlden et al., 2014).
In countries such as Indonesia, self-examination rather than clinical-breast examination is likely to be the trigger leading to earlier breast cancer diagnosis, and breast cancer awareness is vital in encouraging health-seeking behaviour in those at risk of breast cancer. Most research into breast cancer awareness has been conducted in Western countries (Stubbings et al., 2009; Linsell et al., 2010; Özalp et al., 2014; Pud, 2015), and breast cancer awareness instruments used for these studies have been developed especially for this context and are unlikely to be valid in developing countries. Indeed, at present, there is a paucity of adequately validated instruments for breast cancer awareness applicable across cultures or even outside the health care setting in which such instruments were developed.
Several breast-cancer awareness instruments have been developed that the authors report as valid. For example, Breast-CAM (Linsell et al., 2010) was developed for women in the UK. However, instruments like those are highly contextualized to particular health care settings. Indeed, BCAM actually mentions the British mammographic screening program among its items, reducing its usefulness outside the UK setting. In addition, the inadequacy of Western- developed breast cancer-awareness tools for application to Asian populations is likely to lead to several problems. Beliefs and misconceptions are likely to be driven, at least in part, by cultural context (Mohammed et al., 2009; Ahmadian and Samah, 2012; Simon et al., 2012; Gonzalez et al., 2015). An instrument developed and validated on Western women may not be assumed to be valid in Asian populations.
Recently, the B-CAS (Breast Cancer Awareness Scale) tool was developed and validated for measuring breast cancer awareness in Thai women (Rakkapao et al., 2016). Unlike many previously developed instruments for measuring breast cancer awareness, the B-CAS underwent an appropriate and thorough validation. This study investigated the validity and psychometric properties of the breast cancer awareness scale in Indonesian (BCAS-I) women.
Material and Methods
A two-phase study was designed to evaluate the psychometric properties of BCAS-I. The first phase involved translation of the existing English language version into Indonesian, thereby establishing translational validity. The second phase was evaluating the psychometric properties of the BCAS-I.
Phase I: Instrument translation and face validity
The original version of the B-CAS is a self-administered scale containing 35 items distributed across 5 domains: knowledge of risk factors, knowledge of signs and symptoms, attitude to breast cancer prevention, barrier of breast screening and health behaviour related to breast cancer awareness (Rakkapao et al., 2016). The B-CAS items were translated from English into Indonesian using the forward and backward translation technique advocated by WHO (2015) and Epstein et al., (2015).
Four Indonesian-English bilingual translators were identified. Of these, two were used to forward translate the original English version of the B-CAS instrument into Indonesian, while the remaining two translators were used to back translate the instrument from Indonesian to English. The original and back-translated versions of B-CAS were then compared by two native English speakers. All translators were not previously familiar with the content and have no clinical background. Any apparent discrepancies between the two translated versions were modified, and the back-translation cycle was repeated until the investigator was satisfied with the semantic equivalence. Finally, the BCAS-I instrument was field tested in a pilot group of 20 Indonesian women to evaluate the translation quality and other practical aspects of test administration. Participants were asked to read and listen to each item to ensure their understanding of each item. The purpose of this pilot study was to establish face validity and find true any superficial problems with the instrument.
Phase II: psychometric validation
Participants
Indonesian women aged 18 to 80 years living in three provinces participated in this study. A stratified random sample of Indonesian women collected from three Indonesian provinces (South of Sumatera, Yogyakarta and East Nusa Tenggara) by two location combinations (rural, urban) were collected. We felt these province-location combinations adequately represented the spectrum of cultures, religions, and socioeconomics in Indonesian women. They represented the provinces with the highest, middle and lowest incidence rates of breast cancer in Indonesia based on the result of the Indonesian research of Oemiati et al., (2011). Then, the questionnaire was administered in March to May 2016 to women with no history of breast cancer, who were neither pregnant nor breast feeding and were literate in the Indonesian language.
We based our sample size on the recommendations of Comrey and Lee (1992) for factor analysis where n = 50-very poor, n = 100-poor, n = 200-fair, n = 300-good, n = 500-very good, and n = 1,000 or more-excellent. Finally, data from 856 Indonesian women were obtained from 871 questionnaires (response rate = 98.3%). Informed consent was obtained from all participants included in this study. The study protocol was approved by the Khon Kaen University Ethics Committee for Human Research (HE582369) and a letter research permit from The Indonesian Ministry of Home Affairs (No.440.02/1085/Polpum).
Measurement
The BCAS-I is an instrument that measures the breast cancer awareness scale in Indonesian women. This instrument’s 35 items are distributed across 5 domains: knowledge of risk factors (RF: 9 items), knowledge of signs and symptoms (SS: 8 items), attitude to breast cancer prevention (AT: 6 items), barrier of breast screening (BAR: 4 items) and health behaviour related to breast cancer awareness (BEH: 8 items). The two knowledge domains were measured as yes/don’t know/no. The domains of both attitude to breast cancer prevention and barriers of breast screening were measured using a 5-point Likert-type scale from 1 (strongly disagree) to 5 (strongly agree). The health behaviour related to breast cancer awareness domain was rated using a 5-point frequency scale. The questionnaire also included 14 questions relating to sociodemographics, including age, province, region, educational level, marital status, monthly income, religion, current occupation, health insurance, family breast cancer history, family (any) cancer history, tobacco use, alcohol use, and breastfeeding history.
Statistical analysis
Demographic data of the participants were summarized using descriptive statistics with means and standard deviation for continuous variables and counts and percentages, for categorical data. Confirmatory factor analysis (CFA) was employed to affirm the BCAS-I measurement model. We deemed a successful model to have the goodness-of-fit index (GFI) >0.9, the adjusted goodness-of-fit index (AGFI) >0.8, the Comparative Fit Index (CFI) >0.9, the Root-Mean-Square Error of Approximation (RMSEA) ≤0.06, and the Tuker-Lewis Index (TLI) >0.9 (Hu and Bentler, 1998; Schreiber et al., 2006; Jackson et al., 2009), and the χ2 statistic although a poor measure of measurement model fit was also provided for reasons of convention. The Kaiser-Meyer-Olkin (KMO) test was generated along the CFA to provide evidence of construct validity; it measures the sampling adequacy, which should be greater than 0.5 for a satisfactory analysis to proceed. Further, the value of Bartlett’s Test of Sphericity was provided (Hair et al., 2010). The proposed measurement model is provided in Figure 1.
Figure 1.
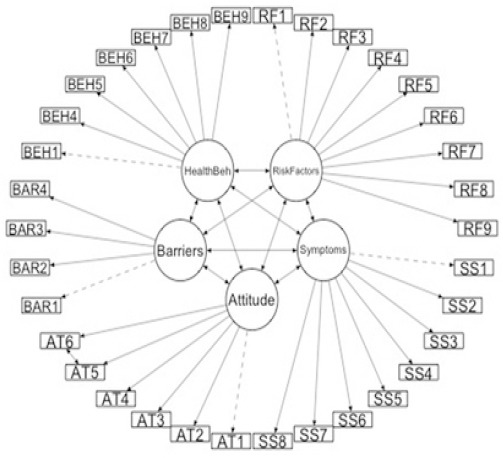
Measurement Model for CFA of BCAS-I
Cronbach’s alpha coefficient was used to examine the internal consistency of the BCAS-I tool. Reliability coefficients over 0.70 were considered acceptable (Sijtsma, 2009). Finally, the association of the BCAS-I subscales with participant characteristics was undertaken using proportional odds ordinal logistic regression. The data were coded in Epidata software version 3.1, and all analysis was performed using the R statistics package (R CRAN team version 2.3.0, 2015), the R libraries Lavaan (Rosseel, 2012), and semPlot (Epskamp, 2015). A significance level of 0.05 will be used throughout all analyses.
Results
Sample characteristics
Of the total 856 Indonesian women who completed the questionnaire, the average age was 30 (standard deviation = 11) and ranged from 18 to 80 years old. Most participants resided in rural areas (62%), and 60% did not have health insurance. Participant characteristics are shown in Table 1.
Table 1.
Participant Characteristics In The Confirmatory Factor Analysis In Indonesian Women
| Characteristics | Number | % |
|---|---|---|
| Age | ||
| Early adulthood (18-34y) | 611 | 71.3 |
| Adulthood (35-59y) | 223 | 26.1 |
| Elderly (>60y) | 22 | 2.6 |
| Province | ||
| Yogyakarta | 194 | 22.7 |
| East Nusa Tenggara | 255 | 29.8 |
| South of Sumatera | 407 | 47.5 |
| Locale | ||
| Rural | 530 | 62 |
| Urban | 326 | 38 |
| Education level | ||
| Primary school | 66 | 7.7 |
| Junior high school | 57 | 6.7 |
| Senior high school | 326 | 38.1 |
| Bachelor degree | 380 | 44.3 |
| Postgraduate degree | 27 | 3.2 |
| Marital status | ||
| Single | 426 | 49.8 |
| Married | 399 | 46.6 |
| Widowed/separate/discovered | 31 | 3.6 |
| Monthly income | ||
| < 2,000,000 IDR* (<152 USD**) | 647 | 75.6 |
| 2,000,000 to 6,000,000 IDR (152 to 457 USD) | 198 | 23.1 |
| ≥ 6,000,000 IDR (≥457 USD) | 11 | 1.3 |
| Religion | ||
| Muslim | 584 | 68.2 |
| Christians | 262 | 30.6 |
| Others | 10 | 1.2 |
| Occupation | ||
| No employment | 287 | 33.5 |
| Farmer | 47 | 5.5 |
| Trader | 70 | 8.2 |
| Labourer | 96 | 11.2 |
| Government/official/enterprise/business | 181 | 21.1 |
| Student | 148 | 17.3 |
| Others | 27 | 3.2 |
| Health insurance | ||
| Yes | 337 | 39.4 |
| No | 519 | 60.6 |
| Smoking history | ||
| Yes | 18 | 2.1 |
| No | 838 | 97.9 |
| Alcohol history | ||
| Yes | 24 | 2.8 |
| No | 832 | 97.2 |
, Indonesian Rupiah Rate;
, United States Dollar
Construct validity
Unweighted Least Squares confirmatory factor analysis was employed to assess the BCAS-I measurement model fit, and the resulting standardized loadings are provided in Table 2. Confirmatory factor analysis revealed the factors structure of the BCAS-I model fit the data adequately (χ2 = 922.267, df = 515, p <0.001, CFI = 0.965, TLI = 0.96, GFI = 0.97, AGFI = 0.97, RMSEA = 0.03 95% CI: 0.027, 0.034). All items significantly loaded on their respective factors except the constraint items of each subscale, for which no significant test was performed (Table 2). The KMO was 0.87, and Bartlett’s sphericity test was significant (χ2 = 11414.92, df = 561, p <0.001) indicating reasonable adequacy of the data for factor analysis.
Table 2.
Standardized Loading Factors for Confirmatory Factor Analysis of the BCAS-I
| Domains items | Risk factors | Signs and symptoms | Attitude | Barriers | Health behaviour |
|---|---|---|---|---|---|
| Knowledge of risk factors (9 items) | |||||
| aRF1 Family history of breast cancer | 0.569+ | ||||
| RF2 Use of birth control pills | 0.583 | ||||
| RF3 Having undergone hormone replacement therapy | 0.718 | ||||
| RF4 Beginning your menses before the age of 12 | 0.455 | ||||
| RF5 Menopause after the age of 55 | 0.572 | ||||
| RF6 Infertility | 0.593 | ||||
| RF7 Giving birth after the age of 30 | 0.533 | ||||
| RF8 Eating fatty foods | 0.587 | ||||
| RF9 Obesity | 0.575 | ||||
| Knowledge signs and symptoms (8 items) | |||||
| bSS1 Bleeding or liquid discharge from the nipple | 0.777+ | ||||
| SS2 Swelling in the breast or armpit area. | 0.858 | ||||
| SS3 Changes in the shape, size or colour around the breast or nipple | 0.801 | ||||
| SS4 Pain in the breast or armpit area | 0.839 | ||||
| SS5 Sensation of the nipple being pulled from the inside | 0.743 | ||||
| SS6 A lump or thickness of skin beneath the armpit | 0.808 | ||||
| SS7 Puckering or dimpling/scaling around the breast | 0.772 | ||||
| SS8 A lump or thickness of skin in the breast area | 0.862 | ||||
| Attitude to breast cancer prevention (6 items) | |||||
| cAT1 Breast cancer can be avoided by decreasing the risk factors of breast cancer | 0.570+ | ||||
| AT2 Breast cancer can be cured if it is detected in its first stage | 0.652 | ||||
| AT3 Breast cancer can be detected in its first stage by having routine check-ups with a doctor/health specialist | 0.717 | ||||
| AT4 Routine mammogram tests can detect breast cancer in its first stage. | 0.602 | ||||
| AT5 Exercise can reduce the risk of breast cancer | 0.497 | ||||
| AT6 Reducing fatty foods can decrease the risk of breast cancer | 0.507 | ||||
| Barrier of breast screening (4 items) | |||||
| dBAR1 I don’t feel comfortable going to the doctor for a breast screening | 0.716+ | ||||
| BAR2 Breast check-ups involve too much time waiting in line | 0.792 | ||||
| BAR3 I am busy and don’t have time to go the doctor for a breast check-up | 0.745 | ||||
| BAR4 I don’t know how to check a breast self-examination | 0.476 | ||||
| Health behaviour related to breast cancer awareness (7 items) | |||||
| eBEH1 How often do you eat fried food? | 0.167+ | ||||
| BEH4 How often do you eat fresh vegetables? | 0.064 | ||||
| BEH5 How often do you exercise? | 0.206 | ||||
| BEH6 How often do you hear about breast check-up programs organized by health professionals in your area? | 0.518 | ||||
| BEH7 How often did you perform a breast self-examination in the past year? | 0.712 | ||||
| BEH8 How often do you get breast check-ups at health clinics? | 0.379 | ||||
| BEH9 How often do you get mammogram tests? | 0.169 |
, Risk factors;
, Signs and symptoms;
, Attitude;
, Barriers;
, Behaviours;
, item constrained
The interfactor correlation matrix revealed significant association between several subscales of the BCAS-I instrument. The knowledge of risk factors subscale was positively associated with three other subscales, such as knowledge of signs and symptoms subscale, attitude subscale, and behaviour subscale. The attitude subscale was negatively associated with behaviour subscale. Although some of the other interfactor correlations were statistically significant, the small magnitude of these correlations suggests the analysis was overpowered. Details of the correlation analysis of the BCAS-I subscales are presented in Table 3.
Table 3.
Inter Factors Correlation Of The BCAS-I Subscales Score
| Domains | Sign and symptoms | Attitude | Barriers | Behaviour |
|---|---|---|---|---|
| Risk factors | 0.532*** | 0.140*** | 0.023 | 0.060* |
| Signs and symptoms | 0.142*** | -0.034* | 0.132*** | |
| Attitude | -0.123*** | -0.281*** | ||
| Barriers | 0.017 |
, p<0.001;
**, p<0.01;
, p<0.05
Reliability
The reliability of the BCAS-I was evaluated for internal consistency (Cronbach’s alpha). The alpha value of 0.79 indicates sufficiently high reliability to provide confidence interpreting the score overall. The correlation item total score range was 0.76 to 0.92.
Breast cancer awareness and demographic characteristics
In this study, we also investigated the association of the BCAS-I subscales against participant characteristics. Table 4 shows the odds of better knowledge of risk factors is 31% lower in urban women than in rural women (AdjOR = 0.69, 95% CI 0.09 - 0.78, p <0.05). The odds of a higher level of both attitude- and behaviour-related breast cancer awareness also found in urban women compared those odds in rural women (AdjOR attitude = 1.39, 95% CI: 1.02, 1.89, p <0.05), (AdjOR behaviour = 1.40, 95% CI: 1.02, 1.91, p <0.05).
Table 4.
The Adjusted Odds Ratio Of Logistic Regression Analysis For The Five Breast Cancer Awareness Domains Regarding The BCAS-I
| Effect | Risk factors | Signs and symptoms | Attitude | Barriers | Health behaviour |
|---|---|---|---|---|---|
| Age | 1.07 (0.90, 1.28) | 1.14 (0.97, 1.36) | 1.06ns (0.88, 1.26) | 1.15 (0.96, 1.38) | 0.95 (0.80, 1.14) |
| Province (ref: East Nusa Tenggara) | χ 2LRT = 2.82, df = 2, p = 0.24 | χ 2LRT = 4.26, df = 2, p = 0.12 | χ 2LRT =0.64, df = 2, p = 0.73 | χ 2LRT = 13.70, df = 2, p = 0.001*** | χ 2LRT = 1.35, df = 2, p = 0.51 |
| South of Sumatera | 1.38 (0.59, 3.20) | 0.70 (0.30, 1.62) | 0.81 (0.35, 1.84) | 5.03*** (2.09, 12.09) | 1.09 (0.48, 2.48) |
| Yogyakarta | 1.01 (0.43, 2.41) | 0.50 (0.21, 1.19) | 0.73 (0.31, 1.71) | 3.84** (1.57, 9.43) | 1.36 (0.58, 3.16) |
| Locale (ref:urban) | 0.69* (0.09, 0.78) | 0.90 (0.66, 1.22) | 1.39 * (1.02, 1.89) | 1.06 (0.77, 1.44) | 1.40* (1.02, 1.91) |
| Education (ref: primary school) | χ 2LRT = 3.12, df = 4, p = 0.54 | χ 2LRT = 1.81, df = 4, p = 0.77 | χ 2LRT =28.37, df = 4, p = 0.001*** | χ 2LRT = 8.08, df = 4, p = 0.09 | χ 2LRT = 33.73, df = 4, p = 0.001*** |
| Junior high school | 0.80 (0.42, 1.54) | 0.77 (0.40, 1.49) | 0.81 (0.40, 1.64) | 0.80 (0.41, 1.54) | 0.73 (0.35, 1.50) |
| Senior high school | 0.93 (0.54, 1.60) | 0.70 (0.40, 1.24) | 0.38** (0.21, 0.69) | 1.04 (0.59, 1.84) | 0.34*** (0.18, 0.63) |
| Bachelor degree | 0.84 (0.48, 1.48) | 0.70 (0.39, 1.25) | 0.30*** (0.17, 0.56) | 0.68 (0.38, 1.23) | 0.21*** (0.11, 0.40) |
| Postgraduate degree | 0.48 (0.19, 1.20) | 0.61 (0.25, 1.50) | 0.11*** (0.04, 0.29) | 0.54 (0.20, 1.42) | 0.16*** (0.06, 0.42) |
| Marital status (ref: married) | χ 2LRT = 0.11, df = 2, p = 0.95 | χ 2LRT = 0.03, df = 2, p = 0.99 | χ 2LRT = 3.24, df = 2, p = 0.20 | χ 2LRT = 3.97, df = 2, p = 0.05* | χ 2LRT =1.64, df = 2, p = 0.44 |
| Single | 1.03 (0.71, 1.49) | 0.99 (0.69, 1.43) | 1.31 (0.90, 1.90) | 1.56* (1.07, 2.28) | 0.88 (0.60, 1.28) |
| Widowed/separate/ discovered | 0.90 (0.45, 1.82) | 0.95 (0.48, 1.88) | 0.65 (0.30, 1.39) | 1.31 (0.65, 2.64) | 1.51 (0.71, 3.18) |
| Monthly income (ref: <2,000,000 IDR (<152 USD)) | χ 2LRT = 0.82, df = 2, p = 0.66 | χ 2LRT = 1.24, df = 2, p = 0.54 | χ 2LRT = 0.26, df = 2, p = 0.88 | χ 2LRT =0.42, df = 2, p = 0.81 | χ 2LRT = 4.84, df = 2, p = 0.09 |
| 2,000,000 to 6,000,000 IDR (152 to 457 USD) | 0.89 (0.62, 1.27) | 0.92 (0.65, 1.31) | 0.91 (0.64, 1.31) | 0.89 (0.62, 1.27) | 0.69* (0.47, 0.99) |
| ≥ 6,000,000 IDR (≥ 457 USD) | 0.65 (0.19, 2.25) | 0.54 (0.18, 1.66) | 0.92 (0.29, 2.96) | 0.97 (0.29, 3.32) | 0.51 (0.16, 1.58) |
| Religion (ref: Christians) | χ 2LRT = 0.56, df = 2, p = 0.76 | χ 2LRT = 2.43, df = 2, p = 0.30 | χ 2LRT = 0.95, df = 2, p = 0.62 | χ 2LRT = 3.05, df = 2, p = 0.22 | χ 2LRT = 1.27, df = 2, p = 0.53 |
| Muslim | 0.88 (0.39, 1.99) | 1.90 (0.85, 4.27) | 1.46 (0.66, 3.25) | 0.50 (0.21, 1.16) | 0.63 (0.29, 1.40) |
| Others | 0.60 (0.16, 2.31) | 1.53 (0.39, 5.98) | 1.50 (0.42, 5.42) | 1.05 (0.24, 4.53) | 0.83 (0.24, 2.87) |
| Occupation (ref: farmer) | χ 2LRT = 3.07, df = 6, p = 0.80 | χ 2LRT = 4.35, df = 6, p = 0.63 | χ 2LRT =8.63, df = 2, p = 0.20 | χ 2LRT =8.85, df = 6, p = 0.18 | χ 2LRT =10.89, df = 6, p = 0.09 |
| No employment (include housewives) | 1.37 (0.72, 2.58) | 1.07 (0.56, 2.05) | 1.08 (0.55, 2.09) | 1.16 (0.61, 2.23) | 1.09 (0.57, 2.09) |
| Trader | 1.52 (0.74, 3.11) | 1.17 (0.58, 2.40) | 1.99 (0.93, 4.29) | 1.43 (0.69, 2.97) | 2.35 * (1.11, 4.95) |
| Labourer | 1.17 (0.58, 2.35) | 0.98 (0.49, 1.98) | 0.95 (0.46, 1.96) | 1.25 (0.61, 2.54) | 1.42 (0.70, 2.88) |
| Government/official/ enterprise/business | 1.04 (0.52, 2.05) | 0.72 (0.36, 1.42) | 1.19 (0.59, 2.41) | 1.39 (0.69, 2.78) | 1.33 (0.67, 2.65) |
| Student | 1.30 (0.63, 2.67) | 0.95 (0.46, 1.98) | 0.92 (0.44, 1.94) | 0.70 (0.34, 1.45) | 0.84 (0.41, 1.74) |
| Others | 1.26 (0.47, 3.39) | 1.02 (0.40, 2.62) | 0.67 (0.25, 1.78) | 1.20 (0.46, 3.14) | 1.35 (0.51, 3.58) |
, p<0.001;
, p<0.01;
, p<0.05
In addition, relatives to those with primary school education of less (the referent group) and those with at least senior education showed a poorer level of both attitudes to breast cancer awareness and breast cancer awareness behaviour. Specifically, the odds of those who had completed high school (senior) were 62% and 66% less than women with only a primary school level of education, respectively (AdjOR = 0.38, 95% CI: 0.21, 0.69, p <0.01), (AdjOR = 0.34, 95% CI : 0.18, 0.63, p <0.001). This difference became more pronounced amongst those with higher levels of education. Compared to women with a primary school education, women with a Bachelors degree and a postgraduate qualification, on average, had 70% and 89%, respectively, less odds of better breast cancer awareness attitude (AdjOR = 0.30, 95% CI: 0.17,0.56, p <0.001; (AdjOR = 0.11, 95% CI: 0.04, 0.29, p <0.001). Moreover, in behaviour-related breast cancer awareness, those odds had 79% and 84% poorer in their subscale, respectively (AdjOR = 0.21, 95% CI: 0.11, 0.40, p <0.00; AdjOR = 0.16, 95% CI: 0.06, 0.42, p <0.001).
Moreover, women in both south of Sumatera and Yogyakarta had odds of higher perceived barriers of breast screening compared with those in East Nusa Tenggara women (AdjOR South of Sumatera = 5.03, 95% CI: 2.09, 12.09, p <0.001), (AdjOR Yogyakarta = 5.03, 95% CI: 1.57, 9.43, p <0.001) as well as single women compared with those to single women (AdjOR = 1.56, 95% CI: 1.07, 2.28, p <0.05). All the coefficients for each subscale (knowledge of risk factors, knowledge of signs and symptoms, attitude to breast cancer prevention, barrier of breast screening and health behaviour related to breast cancer awareness) of the BCAS-I are shown in Table 4.
Discussion
Establishing comprehensive measures of breast cancer awareness plays an effective role towards developing and employing an early-detection program for breast cancer in low- and middle-income countries such as Indonesia. Measuring breast cancer awareness is particularly important to increase awareness of breast cancer screening in the Indonesian population. Many breast cancer cases in low -and middle-income countries such as Indonesia are diagnosed in advanced stages because of a low level of awareness of signs and symptoms and screening methods among a general population of women (Tazhibi and Feizi, 2014). Lack of awareness and late detection of breast cancer in developing countries are the key points contributing to higher breast cancer mortality. Thus, early detection through breast self-examination is a simple and inexpensive approach and provides an appropriate avenue of detection in a limited-resource setting (Sullivan et al., 2015). Our results demonstrate that the BCAS-I, which is a wide variety ethnic and cultural context, is a strongly valid and acceptable measure of breast cancer awareness and achieves a high response rate in a population-based sample of Asian women, particularly Indonesian women. To the best of our knowledge, this is the first large population-based study to investigate the BCAS-I, particularly in Indonesia.
The BCAS-I showed good psychometric properties and is valid for use in the Indonesian population. According to our findings, only 33 items were included in 5 domains that covered most key aspects of breast cancer awareness for this population. Two items of health behaviour breast cancer awareness (How often do you eat food or dessert containing coconut milk? and How often do you eat chicken, beef, or duck complete with fat and skin?) are removed from the model fit to the BCAS-I, which they did not load significantly on their respective structure in the BCAS-I; nonetheless, the strong internal consistency reliability was reached in our instrument. However, all domains of the BCAS-I were consistent with the previous study (Rakkapao et al., 2016), the number of factors was identical to the original version, and there were some difference of items in this subscale.
Furthermore, the BCAS-I presented good internal consistency (overall 0.7, and its subscales ranged from 0.76 to 0.92), which is in line with the original version applied to Thai women (α = 0.86, the range of factor score = 0.71-0.83) (Rakkapao et al., 2016). This is a necessary requisite for assessing existing levels of awareness to plan comprehensive health programs of breast cancer such as early detection and treatment of breast cancer in low- and middle-income countries such as Indonesia. A previous study on breast cancer awareness was trialed and developed (e.g., the BCAM) (Linsell et al., 2010) specially for the UK health system and actually refers to specific programs (e.g., mammography screening) offered in this health setting. Breast-BCAM was unlikely to be appropriate outside the UK. Our findings of this study are likely to be useful for measuring breast cancer awareness in the Indonesian population.
Moreover, after adjusting for other covariates using multivariable binary mixed effect modelling, our results showed that breast cancer awareness was poor in the Indonesian women participating in the validation process. This was due to lack of knowledge of risk factors for breast cancer, in which only about 31% of women were urban women with slightly higher levels of awareness. This finding is in line with previous studies about poor levels of awareness in developing countries (Okobia et al., 2006; Noreen et al., 2015) as well as in developed countries (Linsell et al., 2010). Those findings may reflect women with a university and high school education had a higher chance of being in class with higher levels of awareness than women with less education. Having adequate knowledge of breast cancer drives women to engage in prevention and screening programs.
Some limitations should also be acknowledged. First, our study included only three provinces in Indonesia. We did attempt to choose provinces with diverse culture, religion, and socio-economics in Indonesian women. However, this limited number of provinces may not represent the general population of women with breast cancer in Indonesia. Second, the measurement was conducted at a single time-point, so we could only conclude concurrent associations of breast cancer awareness (as measured by BCAS-I) and breast-self examination. Finally, the single measured means establish temporal stability of the BCAS-I.
However, our study also had some strength. This study is a large population-based sample with higher of response rate that representatives spectrum of cultures in Indonesian women. Moreover, this study was validated for the general population. While our validation was on adult women that was in line with a previous study (Rakkapao et al., 2016), those represented are ethnically and socioculturally diverse. Finally, we concluded that the BCAS-I demonstrated good psychometric properties and was valid in measuring breast cancer awareness in Asian women in general and Indonesian women in particular. Currently, the BCAS-I has conducted a large population-based study, which simply and comprehensively developed interventions of breast cancer awareness. In the future, we plan to gauge the gap of breast cancer awareness interventions.
Statement conflict of interest
All authors declare that they have no conflict of interest.
Acknowledgements
The authors would like to thank all participants for their warm cooperation. Solikhah was granted to International Doctoral Program in Public Health at the Faculty of Public Health, Khon Kaen University.
References
- Ahmadian M, Samah AA. A literature review of factors influencing breast cancer screening in Asian countries. Life Sci J. 2012;9:585–94. [Google Scholar]
- Bish A, Ramirez A, Burgess C, Hunter M. Understanding why women delay in seeking help for breast cancer symptoms. J Psychosom Res. 2005;58:321–6. doi: 10.1016/j.jpsychores.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Comrey AL, Lee H. A first course in factor analysis. Lawrence Erlbaum Associates Publishers; 1992. pp. 299–322. [Google Scholar]
- Coughlin SS, Ekwueme DU. Breast cancer as a global health concern. Cancer Epidemiol. 2009;33:315–8. doi: 10.1016/j.canep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- El Saghir NS, Adebamowo CA, Anderson BO, et al. Breast cancer management in low resource countries (LRCs): Consensus statement from the Breast Health Global Initiative. The Breast. 2011;20:3–11. doi: 10.1016/j.breast.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Epskamp S. semPlot: unified visualizations of structural equation. Struct Equ Model Multidiscip J. 2015;22:1–10. [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Gonzalez P, Lim J-W, Wang-Letzkus M, et al. Breast cancer cause beliefs: Chinese, Korean, and Mexican American breast cancer survivors. West J Nurs Res. 2015;37:1081–99. doi: 10.1177/0193945914541518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair JF, Black WC, Babin BJ, Anderson RE. Seventh. New Jersey: Pearson Prentice Hall; 2010. Multivariate Data Analysis; pp. 604–724. [Google Scholar]
- Hossain MS, Ferdous S, Karim-Kos HE. Breast cancer in South Asia: A Bangladeshi perspective. Cancer Epidemiol. 2014;38:465–70. doi: 10.1016/j.canep.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Fit Indicies in covariance structure modelling: sensitivity to underparameterized model Misspecication. Psychol Methods. 1998;3:424–53. [Google Scholar]
- Jackson DL, Gillaspy JAJ, Stephenson RP. Reporting practices in confirmatory factor analysis: An overview and some recommendations. Psychol Methods. 2009;14:6–23. doi: 10.1037/a0014694. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- Linsell L, Forbes LJL, Burgess C, et al. Validation of a measurement tool to assess awareness of breast cancer. Eur J Cancer. 2010;46:1374–81. doi: 10.1016/j.ejca.2010.02.034. [DOI] [PubMed] [Google Scholar]
- Liu L-Y, Wang F, Yu L-X, et al. Breast cancer awareness among women in Eastern China: a cross-sectional study. BMC Public Health. 2014;14:1–8. doi: 10.1186/1471-2458-14-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A-A, Huda A-A, Mansour A-M. Coping with a diagnosis of breast cancer-literature review and implications for developing countries. Breast J. 2009;15:615–22. doi: 10.1111/j.1524-4741.2009.00812.x. [DOI] [PubMed] [Google Scholar]
- Noreen M, Murad S, Furqan M, Sultan A, Bloodsworth P. Knowledge and awareness about breast cancer and its early symptoms among medical and non-medical students of Southern Punjab, Pakistan. Asian Pac J Cancer Prev. 2015;16:979–84. doi: 10.7314/apjcp.2015.16.3.979. [DOI] [PubMed] [Google Scholar]
- Okobia MN, Bunker CH, Okonofua FE, Osime U. Knowledge, attitude and practice of Nigerian women towards breast cancer: A cross-sectional study. World J Surg Oncol. 2006;4:1–9. doi: 10.1186/1477-7819-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özalp E, Karslıoğlu EH, Aydemir Ö, et al. Validating the sexual adjustment and body Image scale (Sabis) with breast cancer patients. Sex Disabil. 2014;33:253–67. [Google Scholar]
- Pud D. The psychometric properties of the Hebrew version of the memorial symptom assessment scale (MSAS-Heb) in patients with breast cancer. J Pain Symptom Manage. 2015;49:790–5. doi: 10.1016/j.jpainsymman.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Rakkapao N, Promthet S, Moore MA, Hurst CP. Development of a breast cancer awareness scale for Thai Women: Moving towards a validated measure. Asian Pac J Cancer Prev. 2016;17:851–6. doi: 10.7314/apjcp.2016.17.2.851. [DOI] [PubMed] [Google Scholar]
- Rosseel Y. lavaan: An R package for structural equation modeling. J Stat Softw. 2012;48:1–36. [Google Scholar]
- Schreiber JB, Nora A, Stage FK, Barlow EA, King J. Reporting structural equation modeling and confirmatory factor analysis results: A review. J Educ Res. 2006;99:323–38. [Google Scholar]
- Sijtsma K. On the use, the Misuse, and the very limited usefulness of Cronbach’s Alpha. Psychometrika. 2009;74:107–20. doi: 10.1007/s11336-008-9101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AE, Forbes LJL, Boniface D, et al. An international measure of awareness and beliefs about cancer: Development and testing of the ABC. BMJ Open. 2012;2:1–8. doi: 10.1136/bmjopen-2012-001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbings S, Robb K, Waller J, et al. Development of a measurement tool to assess public awareness of cancer. Br J Cancer. 2009;101:13–7. doi: 10.1038/sj.bjc.6605385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan T, Sullivan R, Ginsburg OM. Cancer: disease control priorities, screening for cancer: Considerations for low- and middle-income countries. Washington (DC): The international bank for reconstruction and development/The World Bank; 2015. pp. 211–22. [PubMed] [Google Scholar]
- Tazhibi M, Feizi A. Awareness levels about breast cancer risk factors, early warning signs, and screening and therapeutic approaches among Iranian adult women: A large population based study using latent class analysis. Bio Med Res Int 2014. 2014:e306352. doi: 10.1155/2014/306352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton H, Pillarisetti RR. “Breast awareness” and “breast self-examination” are not the same. What do these terms mean? Why are they confused? What can we do? Eur J Cancer Oxf Engl. 2008;44:2118–21. doi: 10.1016/j.ejca.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Toure M, Nguessan E, Bambara AT, et al. Factors linked to late diagnosis in breast cancer in Sub-Saharan Africa: Case of Côte d’Ivoire. Gynecol Obstet Fertil. 2013;41:696–700. doi: 10.1016/j.gyobfe.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Youlden DR, Cramb SM, Yip CH, Baade PD. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol Med. 2014;11:101–15. doi: 10.7497/j.issn.2095-3941.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


