Abstract
Objective:
Although there are a few studies investigating the relation between X-Ray Repair Cross Complementing 3 (XRCC3) gene rs861539 polymorphism and osteosarcoma (OSA), the results are inconsistent. Therefore, we performed this systematic review and meta-analysis to clarify the associations between XRCC3 rs861539 polymorphism and OSA risk.
Methods:
We have retrieved published literature from PubMed, Google scholar, and ISI Web of Knowledge up to 25 January 2017. Odds ratios were pooled using either fixed-effects or random effects models. Overall and subgroup analyses were performed. Statistical analysis was performed running comprehensive meta-analysis (CMA) 2.0 software.
Results:
A total of four studies with 515 cases and 1,109 controls were identified in order to investigate the association between XRCC3 rs861539 polymorphism and OSA risk. The results showed that XRCC3 rs861539 polymorphism was associated with OSA in allelic (T vs. C: OR= 1.563, 95% CI: 1.244-1.963, p= <0.001), homozygote (TT vs. CC: OR= 2.574, 95% CI: 1.573-4.212, p= <0.001), dominant (TT+TC vs. CC: OR= 1.255, 95% CI: 1.011-1.558, p= 0.039), and recessive (TT vs. TC+ CC: OR= 2.224, 95% CI: 1.393-3.552, p= 0.001), but not with heterozygote (TC vs. CC: OR= 1.361, 95% CI: 0.982-1.885, p= 0.064). The XRCC3 rs861539 polymorphism conferred susceptibility to OSA in Asians, but not in Caucasians. Additionally, we observed no evidence of publication bias.
Conclusion:
To the best of our knowledge, this is the first meta-analysis investigating the association between XRCC3 rs861539 polymorphism and OSA risk. Our results revealed a significant association between the XRCC3 rs861539 polymorphism and risk of OSA, especially in Asian populations. Future more comprehensive and well-designed case control studies with larger sample size are needed to warrant these findings.
Keywords: Osteosarcoma, XRCC3, rs861539 polymorphism, meta-analysis
Introduction
Osteosarcoma (OSA) is the most common primary bone malignancy in children and adolescents (Lauvrak et al., 2013). OSA is one of the three most common genuine primary bone malignancies (osteosarcoma, chondrosarcoma, and Ewing’s sarcoma), of which these malignancies account more than 75% of malignant bone tumors (Davies et al., 2009). OSA is the most frequent malignant bone tumor, which approximately comprising 47% of all bone neoplasms among adolescents and young adults aged 15 to 29 years old (Bleyer et al., 2006). Approximately, 750-900 new cases are diagnosed each year in the USA, which 400 cases arise in pediatrics and adolescents younger than 20 years of age (Mirabello et al., 2009). According to current/available studies, the peak incidence of OSA occurs in the second decade of life which can be due to rapid bone growth and turnover associated with adolescence (Messerschmitt et al., 2009). According to the statics, the incidence of OSA in males was higher than in females; however, it occurred at an earlier age in females than in males (Bleyer et al., 2006). OSA is characterized by the production of immature bone or osteoid by the malignant cells; however, the diagnosis of OSA is also made based on these characters (Alpantaki et al., 2013; Sarkar 2014). OSA variants classified based on morphology as telangiectatic OSA, low-grade intraosseous OSA, and small cell OSA (Yarmish et al., 2010). In addition to humans, OSA is reported in the many other mammals, in particular, domestic dogs and canine (Mueller et al. 2007). It is held that the majority of pediatrics OSA is sporadic, while inheritance accounts for a minority of cases (Calvert et al., 2012). In older adults, nearly one-third of cases arise in the setting of Paget disease of bone or as a second or later cancer (Geller et al., 2010). Exposure to ionizing radiation is the most well documented environmental risk factor for OSA implicated in 3% of OSA cases (Kalra et al., 2007).
The etiology and role of the OSA in bone microenvironment is not well known (Kundu 2014). Since OSA has complex karyotypes and is typically aneuploid, it is characterized by a high level of genomic instability (Martin et al., 2012). To date, several specific genes have been identified in the pathogenesis of OSA. Particularly, tumor suppressor gene mutations of p53, RB, and MDM2 are frequently implicated in OSA development (Kawaguchi et al., 2002; Martin et al., 2012). However, genome wide association studies identified different gene alterations in human including TP53, IGF-1/IGF-1R, HGF/MET, ERBB-2/HER-2, PTEN, RB, CDKN2A, SIS/PDGF, matrix metalloproteinases (MMPs), Ezrin (EZR), COX-2, 14 angiogenic factors (VEGF and angiostatin), and telomerase reverse transcriptase gene (TERT) (Mueller et al. 2007).
The XRCC3 polymorphism is associated with the risks of numerous types of cancer, such as lung, ovarian or gastric cancer; however, there is limited information regarding the analyzed gene polymorphisms in osteosarcoma (Talar-Wojnarowska). Although a few studies have investigated the relation between XRCC3 DNA repair gene variants and OSA (Guo et al., 2015; Goričar et al., 2015; Jin et al., 2015; Yang et al., 2015), the results are conflicting rather than conclusive. One thing must be noted. A single study might not be powered sufficiently to detect a small effect of the polymorphisms on condition, particularly in relatively small sample sizes. In addition, various types of study populations and study designs might also have contributed to the disparate results. It is clear that meta-analysis can be used to pool data from individual studies to obtain sufficient statistical power to detect the potential effect of small to moderate sizes associated with the polymorphism. To clarify the effect of the XRCC3 rs861539 gene polymorphism on the OSA risk, we performed a systematic review and meta-analysis on all eligible case–control studies.
Materials and Methods
Search strategy
We searched the electronic databases of PubMed, Google Scholar, ISI Web of Knowledge, and Medline to identify all eligible published case-control and cohort studies had evaluated the associations between XRCC3 rs861539 polymorphism and OSA up to 25 January 2017. The key words used for searching were as follows: ‘‘X-ray repair cross-complementing group 3’’, ‘‘XRCC3’’, ‘‘XRCC3 C241T’’, or ‘‘XRCC3 Thr241Met’’, ‘‘XRCC3 rs861539’’ and ‘‘bone tumors’’, ‘‘bone malignancy’’, ‘‘osteosarcomas’’, and ‘‘polymorphism’’, ‘‘polymorphisms’’, ‘‘variant’’, or ‘‘mutation’’. The language was restricted to English. In addition, we hand searched the references of all identified publications for additional studies.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) independent case-control or cohort design studies evaluated the association between XRCC3 rs861539 polymorphism and OSA; (2) studies provided sufficient published data for estimating an odds ratio (OR) with a 95% confidence interval (95% CI). Major reasons for exclusion were as follows: (1) case report or reviews, (2) cell line studies, (3) having irrelevant data. When there was more than one eligible article with overlapping data conducted by the same author, we included the recent or the comprehensive one.
Data Extraction
In the current meta-analysis, two authors (MM and HN) independently searched and identified the eligible articles based on the inclusion criteria. The authors independently extracted the following data: first author’s name, year of publication, ethnicity or country, numbers and genotypes of cases and controls, and Hardy-Weinberg equilibrium (HWE) of controls.
Statistical methods
All analyses were performed with the comprehensive meta-analysis (CMA) V2.0 software (Biostat, USA). Two-sided P.values < 0.05 were considered statistically significant. The statistical significance of the pooled OR was determined using the Z-test and P.value less than 0.05 was considered statistically significant. The pooled ORs with 95 % CIs were calculated in five genetic models: allelic (T vs. C), heterozygote (TC vs. CC), homozygote (TT vs. CC), dominant (TT+TC vs. CC), and recessive (TT vs. TC+CC). Due to lack of heterozygote and minor allele homozygote genotypes frequency, subgroup analyses by ethnicity was available only in dominant genetic model throughout Jin et al., (2015) and Goričar et al., (2015) studies. Both the Cochran’s Q statistic test for heterogeneity and the I2 statistic test to quantify the proportion of total variation were used to measure heterogeneity between studies. An I2 value of 25%, 50%, and 75 % represents low, moderate, and high heterogeneity, respectively (Higgins et al., 2003). Moreover, a random effects model using the DerSimonian was utilized to calculate the OR and 95% CI for comparisons with moderate to high heterogeneity (P-value > 0.1 and I2 > 25%) (DerSimonian et al., 1986). Otherwise, a fixed-effects model using the Mantel–Haenszel method was used (Mantel et al., 1959). To assess the reliability of the outcomes in the current meta-analysis, a sensitivity analysis was performed by sequential omission of individual studies for various genetic models in the overall population and also for subgroup analysis by ethnicity. Then, publication bias was estimated graphically by Begg’s funnel plot test and statistically Egger’s linear regression test (Egger et al., 1997). Additionally, we applied graphically Begg’s funnel plot test and statistically Egger’s linear regression test to estimate the publication bias, and P<0.05 was considered statistically significant (Egger et al., 1997).
Results
Study characteristics
Out of the 8 identified potential relevant studies, only four case–control studies met all inclusion criteria. Finally, four studies comprising 515 cases with OSA and 1,109 controls were included into the current meta-analysis (Guo et al., 2015; Goričar et al., 2015; Jin et al., 2015; Yang et al., 2015). All the eligible studies were written in English and all included studies were conducted during 2015. Among those studies, 3 studies were performed in China (Guo et al., 2015; Jin et al., 2015; Yang et al., 2015) and one study was conducted in Slovenia (Goričar et al., 2015). All genotype frequencies in the control group fitted well in the Hardy-Weinberg equilibrium (P > 0.05). The main characteristics of studies included in the current meta-analysis are presented in Table 1.
Table 1.
Main Characteristics of All Studies Included in the Meta-Analysis.
| First author | Country (Ethnicity) | Case/Control | Cases | Controls | HWE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | Allele | Genotype | Allele | ||||||||||
| CC | CT | TT | C | T | CC | CT | TT | C | T | ||||
| Jin et al. 2015 | China (Asian) | 148/296 | 77 | 71 | 173 | 125 | |||||||
| Guo et al. 2015 | China (Asian) | 136/136 | 54 | 55 | 27 | 163 | 109 | 70 | 52 | 14 | 192 | 80 | 0.355 |
| Yang et al. 2015 | China (Asian) | 152/304 | 66 | 63 | 22 | 195 | 107 | 166 | 117 | 21 | 449 | 159 | 0.95 |
| Goričar et al. 2015 | Slovenia (Caucasian) | 79/373 | 39 | 40 | 153 | 220 | |||||||
Quantitative synthesis
Table 2 depicts the main results of the meta-analysis regarding XRCC3 rs861539 polymorphism and OSA risk. When all the eligible studies were pooled into the meta-analysis of polymorphism, an obvious association between XRCC3 rs861539 polymorphism and increased risk of OSA in allelic (T vs. C: OR= 1.563, 95% CI: 1.244-1.963, p= <0.001), homozygote (TT vs. CC: OR= 2.574, 95% CI: 1.573-4.212, p= <0.001), dominant (TT+TC vs. CC: OR= 1.255, 95% CI: 1.011-1.558, p= 0.039; Figure 1A) and recessive (TT vs. TC+CC: OR= 2.224, 95% CI: 1.393-3.552, p= 0.001) was observed, but not heterozygote (TC vs. CC: OR= 1.361, 95% CI: 0.982-1.885, p= 0.064). In the stratified analysis by ethnicity, only dominant genetic model was available for Caucasian. The present meta-analysis showed that the XRCC3 rs861539 polymorphism was not associated with OSA risk in Caucasian (TT+TC vs. CC: OR= 0.713, 95% CI: 0.438-1.161, p= 0.174). However, there was a significant association between XRCC3 rs861539 polymorphism and risk of OSA in Asians in the dominant genetic model (TT+TC vs. CC: OR= 1.442, 95% CI: 1.133-1.835, p= 0.003; Figure 1B).
Table 2.
Results of Meta-Analysis for XRCC3 rs861539 Polymorphism and OSA Risk
| Genetic model | No. study | Type of model | Heterogeneity | Odds ratio | Publication Bias | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | PH | OR | 95% CI | POR | PBeggs | PEggers | ||||
| Pooled | ||||||||||
| T vs. C | 2 | Fixed | 0 | 0.848 | 1.563 | 1.244-1.963 | <0.001 | NA | NA | |
| TC vs. CC | 2 | Fixed | 0 | 0.971 | 1.361 | 0.982-1.885 | 0.064 | NA | NA | |
| TT vs. CC | 2 | Fixed | 0 | 0.917 | 2.574 | 1.573-4.212 | <0.001 | NA | NA | |
| TT+TC vs. CC | 4 | Fixed | 58.12 | 0.067 | 1.255 | 1.011-1.558 | 0.039 | 0.308 | 0.529 | |
| TT vs. TC+CC | 2 | Fixed | 0 | 0.909 | 2.224 | 1.393-3.552 | 0.001 | NA | NA | |
| Caucasian | ||||||||||
| TT+TC vs. CC | 1 | Fixed | 0 | 1 | 0.713 | 0.438-1.161 | 0.174 | NA | NA | |
| Asian | ||||||||||
| TT+TC vs. CC | 3 | Fixed | 0 | 0.697 | 1.442 | 1.133-1.835 | 0.003 | 1 | 0.612 | |
Figure 1.
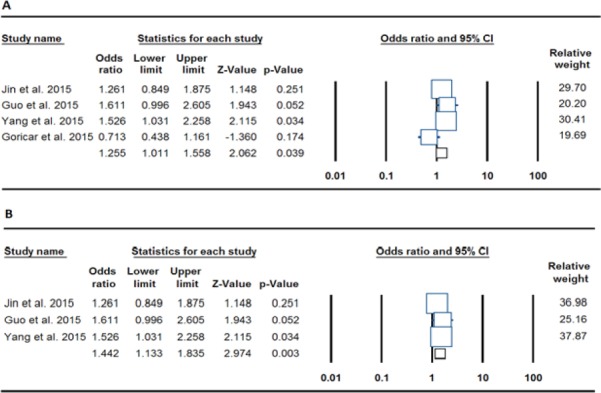
Forest Plot for the Association of the XRCC3 rs861539 Polymorphism and OSA Risk. A: Pooled (dominant: TT+TC vs. CC), B: Asians (dominant: TT+TC vs. CC)
Sensitivity Analysis
We conducted the sensitivity analysis to evaluate the stability of the current meta-analysis results through removing each study sequentially. However, no obvious changes were found in the results confirming the stability of our results e under the five genetic contrasts for XRCC3 rs861539 polymorphism.
Heterogeneity
There was a moderate but not significant heterogeneity among these studies for dominant model; however, the heterogeneity obviously was disappeared after stratified analysis by ethnicity. Therefore, it can be concluded that ethnicity contribute to substantial heterogeneity among the meta-analysis.
Publication Bias
Egger’s test and Begg’s funnel plot were used to evaluate publication bias quantitatively and qualitatively, respectively. For pooling and stratifying by ethnicity, the examination of publication bias was conducted only for dominant genetic model because only two studies were included. However, the Begg’s and Egger’s tests did not show any obvious publication bias under dominant genetic model in terms of pooling (PBeggs = 0.308, PEggers=0.529; Figure 2A) and for Asians ((PBeggs = 1.000, PEggers=0.612; Figure 2B).
Figure 2.
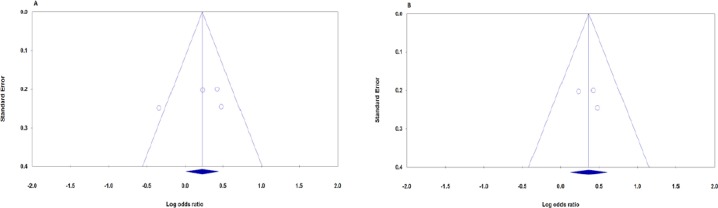
Begg’s Funnel Plots of the XRCC3 rs861539 Polymorphism and OSA Risk for Publication Bias Test. Each Point Represents a Separate Study for the Indicated Association. A: Pooled (dominant: TT+TC vs. CC), B: Asians (dominant: TT+TC vs. CC)
Discussion
Three excision repair (ER) pathways were involved in single stranded DNA (ssDNA) damage responses including Nucleotide excision repair (NER), base excision repair (BER), and DNA mismatch repair (MMR) (Shaheen et al., 2011; Iyama et al., 2013). In addition, organisms evolved two main DNA double-strand breaks (DSBs) repair mechanisms to preserve genome integrity including nonhomologous end-joining (NHEJ) and homologous recombination (HR) (Mao et al., 2008; Lieber 2010).
To date, several polymorphisms in NER genes (e.g., XPD, XPF, ERCC1, XRCC1, XRCC3, XPA, XPB, XPC and hOGG1) have been identified (Improta et al., 2008), of which the known genetic polymorphisms of the XRCC3 have been studied most commonly (Yeh et al., 2005; Forat-Yazdi et al., 2015). XRCC3 gene was originally identified due to its ability to complement the DNA repair defect in a Chinese hamster cell line (Tebbs et al., 1995). It is localized on chromosome 14q32.3 by fluorescence in situ hybridization (FISH) and Southern blot hybridization by genomic DNA from panels of two independent hybrid clones (Tebbs et al., 1995). It consists of 7 exons, which lied in the region taking 13.5 kbs and its product is a small protein of 346 amino acids (Huang et al., 2015). The XRCC3 gene plays a critical role in maintaining genomic integrity through repairing ionizing radiation induced DSBs through homologous recombination (HR) pathway (Chistiakov et al., 2008; Borrego-Soto et al., 2015).
The SNPs of XRCC3 gene have been indicated in the susceptibility to different malignancies, such as breast cancer, lung cancer, and head and neck cancer (Namazi et al., 2015; Ali et al., 2016). To date, several polymorphisms have been identified in XRCC3 gene as Thr241Met (C18067T, rs861539), 5-UTR (A4541G rs1799794), and IVERSUS 5–14 (A17893G, rs1799796of which XRCC3 Thr241Met (C18067T, rs861539) in exon 7 is one of the most extensively investigated SNPs in the literatures (Chen et al., 2014). The XRCC3 Thr241Met gene polymorphism is characterized by impaired function of repair which may influence the function of the enzyme by removing a phosphorylation site (Talar-Wojnarowska). The XRCC3 Met/Met genotype has been associated with higher DNA adduct levels in lymphocytes of healthy subjects (Matullo et al., 2001) and individuals with 241Met or 241Thr allele repaired the DSBs to the same extent (Araujo et al., 2002). In addition, XRCC3 Thr241Met is associated with an increased number of micronuclei in lymphocytes of humans exposed to ionizing radiation (Zhao et al., 2013).
A few studies reported the association between DNA repair genes variants and risk of osteosarcoma. However, several studies investigated the influence of genetic variability of DNA repair genes in OSA treatment outcome (Jin et al., 2015). For example, Wang et al., and Jin et al., have reported that some variants of NER and HRR pathways genes such as ERCC1 rs11615, ERCC2 rs1799793, and NBN rs1805794 modulate the risk of developing OSA or may be useful genetic prognostic markers for OSA in a Chinese population (Jin et al., 2015; Wang et al., 2015). In 2015, Yang et al., in a case control study on 152 OSA cases and 304 healthy controls found that the XRCC3 rs861539 polymorphism was significantly associated with increased risk of OSA in a Chinese population. However, a few months later, Goričar et al., (2015) did not observe any association in a Slovenian population. In the current meta-analysis, we found an association between XRCC3 rs861539 polymorphism and OSA was found under four allelic, homozygote, dominant, and recessive genetic models, but not under heterozygote model. Additionally, the XRCC3 rs861539 polymorphism conferred susceptibility to OSA in Asians, but not in Caucasians. However, due to lack of sufficient data especially in the Caucasian populations, the results are curious.
Heterogeneity between-study is to be expected in the meta-analyses. In the current study, there was a moderate heterogeneity in the dominant genetic model, the only model applied in all included studies, which could distorted the results of meta-analysis. In the subgroup analysis by ethnicity, the heterogeneity disappeared among both Asians and Caucasians. Therefore, it can be concluded the differences in the subjects’ genetic backgrounds can result in heterogeneity.
Meta-analysis has advantages compared to individual studies; however, some potential limitations in the current meta-analysis should be considered. First limitation concerns the number of included studies and their sample sizes which were moderately small restricting the ability to detect the possible risk for XRCC3 rs861539 polymorphism with acceptable power. Second out offour included studies, three studies were conducted on Asians and only one on Caucasians; thereforem the results must be interpreted carefully. Further studies concerning populations in Caucasians and other ethnicity such as west Asians, North American and African are needed to distinguish the ethnic variation related biases. Third, because we included only published papers written in English, publication bias may have occurred; even though, statistical tests revealed nothing. Finally, gene–gene and gene–environment interactions were not addressed in the current meta-analysis. The pathogenesis of OSA has a genetic and environmental basis because in some cases OSA was associated with high doses of ionizing radiation from therapeutic or occupation-related exposures. However, most studies did not provide the data stratified by these risk factors. In addition, in this meta-analysis, we pooled the overall outcomes based on individual unadjusted ORs without adjustment for other risk factors such as age, sex, environmental exposures, OSA subtypes etc.
To our best knowledge, this is the first meta-analysis examined the association between XRCC3 rs861539 polymorphism and OSA risk. This meta-analysis suggests the association between the XRCC3 rs861539 polymorphism and OSA in Asians. However, more convincing evidence is required to draw comprehensive conclusion. Therefore, well-designed studies in large sample and in different ethnicity are recommended to confirm these findings.
References
- Ali A, AbdulKareem H, Al Anazi M, et al. Polymorphisms in DNA repair gene XRCC3 and susceptibility to breast cancer in Saudi females. Biomed Res Int. 2016;2016:1–9. doi: 10.1155/2016/8721052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpantaki K, Katonis P, Datsis G, et al. Spinal osteosarcoma. Clinical medicine insights. Oncology. 2013:199. doi: 10.4137/CMO.S10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleyer A, O’Leary M, Barr R, et al. National Cancer Institute, NIH Pub No. 06-5767. Bethesda, MD 2006: 2006. Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival 1975-2000. [Google Scholar]
- Borrego-Soto G, Ortiz-López R, Rojas-Martínez A. Ionizing radiation-induced DNA injury and damage detection in patients with breast cancer. Genet Mol Biol. 2015;38:420–32. doi: 10.1590/S1415-475738420150019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert G, Randall R, Jones K, et al. At-risk populations for osteosarcoma: The syndromes and beyond. Sarcoma. 2012;2012:1–9. doi: 10.1155/2012/152382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang Z, Yan Y, et al. XRCC3 C18067T polymorphism contributes a decreased risk to both basal cell carcinoma and squamous cell carcinoma: Evidence from a meta-analysis. PLoS One. 2014;9:e84195. doi: 10.1371/journal.pone.0084195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov DA, Voronova NV, Chistiakov PA. Genetic variations in DNA repair genes, radiosensitivity to cancer and susceptibility to acute tissue reactions in radiotherapy-treated cancer patients. Acta Oncol. 2008;47:809–24. doi: 10.1080/02841860801885969. [DOI] [PubMed] [Google Scholar]
- Davies A, Sundaram M, James S. Imaging of bone tumors and tumor-like lesions. 1st ed. Berlin: Springer-Verlag; 2009. [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, et al. Bias in metaanalysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forat-Yazdi M, Gholi-Nataj M, Neamatzadeh H, et al. Association of XRCC1 Arg399Gln polymorphism with colorectal cancer risk: A HuGE meta-analysis of 35 studies. Asian Pac J Cancer Prev. 2015;16:3285–91. doi: 10.7314/apjcp.2015.16.8.3285. [DOI] [PubMed] [Google Scholar]
- Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8:705–18. [PubMed] [Google Scholar]
- Goričar K, Kovač V, Jazbec J, et al. Genetic variability of DNA repair mechanisms and glutathione-S-transferase genes influences treatment outcome in osteosarcoma. Cancer Epidemiol. 2015;39:182–8. doi: 10.1016/j.canep.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Guo J, Lv HC, Shi RH, et al. Association between XRCC3 Thr241Met polymorphism and risk of osteosarcoma in a Chinese population. Genet Mol Res. 2015;14:16484–90. doi: 10.4238/2015.December.9.20. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JY, Yang JF, Qu Q, et al. DNA repair gene XRCC3 variants are associated with susceptibility to glioma in a Chinese population. Genet Mol Res. 2015;14:10569–75. doi: 10.4238/2015.September.8.18. [DOI] [PubMed] [Google Scholar]
- Improta G, Sgambato A, Bianchino G, et al. Polymorphisms of the DNA repair genes XRCC1 and XRCC3 and risk of lung and colorectal cancer: a case-control study in a Southern Italian population. Anticancer Res. 2008;28:2941–6. [PubMed] [Google Scholar]
- Lauvrak S, Munthe E, Kresse S, et al. Functional characterisation of osteosarcoma cell lines and identification of mRNAs and miRNAs associated with aggressive cancer phenotypes. Br J Cancer. 2013;109:2228–36. doi: 10.1038/bjc.2013.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G, Wang M, Chen W, et al. Single nucleotide polymorphisms of nucleotide excision repair and homologous recombination repair pathways and their role in the risk of osteosarcoma. Pak J Med Sci. 2015;31:269–73. doi: 10.12669/pjms.312.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra S, Grimer RJ, Spooner D, et al. Radiationinduced sarcomas of bone: factors that affect outcome. J Bone Joint Surg Br. 2007;89:808–13. doi: 10.1302/0301-620X.89B6.18729. [DOI] [PubMed] [Google Scholar]
- Kawaguchi K, Oda Y, Sakamoto A, et al. Molecular analysis of p53, MDM2, and H-ras genes in osteosarcoma and malignant fibrous histiocytoma of bone in patients older than 40 years. Mod Pathol. 2002;15:878–88. doi: 10.1097/01.MP.0000024264.48690.EA. [DOI] [PubMed] [Google Scholar]
- Kong C, Hansen MF. Biomarkers in osteosarcoma. Expert Opin Med Diagn. 2009;3:13–23. doi: 10.1517/17530050802608496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu ZS. Classification, imaging, biopsy and staging of osteosarcoma. Indian J Orthop. 2014;48:238–46. doi: 10.4103/0019-5413.132491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyama T, Wilson DM. DNA repair mechanisms in dividing and non-dividing cells. DNA Repair (Amst) 2013;12:620–36. doi: 10.1016/j.dnarep.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- Mao Z, Bozzella M, Seluanov A, et al. DNA repair by nonhomologous end joining and homologous recombination during cell cycle in human cells. Cell Cycle. 2008;7:2902–6. doi: 10.4161/cc.7.18.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Squire J, Zielenska M. The genetics of osteosarcoma. Sarcoma. 2012;2012:1–11. doi: 10.1155/2012/627254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerschmitt PJ, Garcia RM, Abdul-Karim FW, et al. Osteosarcoma. J Am Acad Orthop Surg. 2009;17:515–27. doi: 10.5435/00124635-200908000-00005. [DOI] [PubMed] [Google Scholar]
- Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004. Cancer. 2009;115:1531–43. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller F, Fuchs B, Kaser-Hotz B. Comparative biology of human and canine osteosarcoma. Anticancer Res. 2007;27:155–64. [PubMed] [Google Scholar]
- Namazi A, Abedinzadeh M, Nourbaksh P, et al. Association between the XRCC3 Thr241Met polymorphism and risk of colorectal cancer: a meta-analysis of 5,193 cases and 6,645 controls. Asian Pac J Cancer Prev. 2015;16:2263–68. doi: 10.7314/apjcp.2015.16.6.2263. [DOI] [PubMed] [Google Scholar]
- Sarkar R. Pathological and clinical features of primary osseous tumours of the jaw. J Bone Oncol. 2014;3:90–5. doi: 10.1016/j.jbo.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen M, Allen C, Nickoloff JA, et al. Synthetic lethality: exploiting the addiction of cancer to DNA repair. Blood. 2011;117:6074–82. doi: 10.1182/blood-2011-01-313734. [DOI] [PubMed] [Google Scholar]
- Talar-Wojnarowska R, Gąsiorowska A, Olakowski M, et al. Analysis of XRCC2 and XRCC3 gene polymorphisms in pancreatic cancer. Biomed Rep. 2016;4:236–40. doi: 10.3892/br.2015.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebbs RS, Zhao Y, Tucker JD, et al. Correction of chromosomal instability and sensitivity to diverse mutagens by a cloned cDNA of the XRCC3 DNA repair gene. Proc Natl Acad Sci USA. 1995;92:6354–8. doi: 10.1073/pnas.92.14.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MJ, Zhu Y, Guo XJ, et al. Genetic variability of genes involved in DNA repair influence treatment outcome in osteosarcoma. Genet Mol Res. 2015;14:11652–7. doi: 10.4238/2015.September.28.17. [DOI] [PubMed] [Google Scholar]
- Yang L, An Y, Wang G, et al. Association between XRCC3 Thr241Met polymorphism and risk of osteosarcoma in a Chinese population. Int J Clin Exp Pathol. 2015;8:11670–4. [PMC free article] [PubMed] [Google Scholar]
- Yarmish G, Klein MJ, Landa J, et al. Imaging characteristics of primary osteosarcoma: nonconventional subtypes. Radiographics. 2010;30:1653–72. doi: 10.1148/rg.306105524. [DOI] [PubMed] [Google Scholar]
- Yeh CC, Sung FC, Tang R, et al. Polymorphisms of the XRCC1, XRCC3, & XPD genes, and colorectal cancer risk: a case-control study in Taiwan. BMC Cancer. 2005;5:12. doi: 10.1186/1471-2407-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye J, Li B, et al. DNA repair gene XRCC3 Thr241Met polymorphism and glioma risk: a meta-analysis. Int J Clin Exp Med. 2013;6:438–43. [PMC free article] [PubMed] [Google Scholar]


