Abstract
Background.
Autism spectrum disorder (ASD) is a complex group of neurodevelopmental disorders. Studies conducted among Africans living outside the continent indicate that African children are more likely than Caucasian children to have a late diagnosis of ASD. There is a dearth of information on this topic among children with ASD living in Africa.
Methods.
To determine the pattern of impairments and age at diagnosis in ASD, sixty Nigerian children with a diagnosis of ASD were recruited from a neurodevelopmental clinic. DSM-V criteria were used to make a diagnosis of ASD, while a symptom checklist for ASD was used to determine the pattern of impairments in ASD.
Results.
Ages of the children ranged from 2 to 17 years with a mean age of 9.45 ± 4.33 years with the majority of them (75%) being 12 years or younger. All the children (100%) with ASD exhibited poor eye contact, difficulty in mixing with other children and inability to consistently respond to his/her name. More than a half of them (55%) lack verbal communication. Impairments that were uncommon are in the areas of object attachment (20.0%), odd postures (26.7%) and inappropriate facial expression (30.0%). Mean age at the observation of ASD features was 17.0 ± 6.7 months. Mean age at diagnosis of ASD was 9.00 ± 4.30 years. The mean time lag from a parental concern of ASD features to seeking specialist care was 85 months and to diagnosis was 91 months.
Conclusions.
Core symptoms/impairments of ASD are present in Nigerian children but a late diagnosis is common.
Key words: Africa, ASD, diagnosis, identification, impairment, Nigeria
Introduction
Autism spectrum disorder (ASD) is a complex lifelong, disabling, neurodevelopmental group of disorders that afflict a child in an early developmental period. Although impairments in ASD affect virtually all aspects of the child's functioning (APA, 2013), ASD is defined by qualitative impairments in social and communication skills, as well as a restricted, rigid and obsessive pattern of interest and behavior (WHO, 1992; APA, 2013). ASD comprises a group of heterogenous disorders with features ranging from mild to severe forms of symptoms (APA, 2013). A child with ASD has difficulty in interacting and communicating with others. Typically, individuals with autism retreat into isolated activities or get fixated on a word or object. Core symptoms of autism are often accompanied by abnormalities in cognitive functioning, learning, attention and sensory processing. Symptoms are typically present before the age of 3 years (APA, 2013); symptoms could manifest during infancy while, in other situations, development would be normal in the first 1 year or more (Yeargin-Allsop et al. 2003; Klin et al. 2004). Compared with other childhood medical conditions, ASD is grossly neglected and less researched among African children living in the continent.
Fraught with uncertainties, ASD remains a disorder of obscure etiology. At present, there appears to be no definite biological marker for ASD. Of the three core areas of impairments, social skill impairment, such as avoidance or poor eye contact, difficulty in mixing with peers and lack of emotional reciprocity, remains the hallmark feature of the most severe and commonest form of ASD termed autistic disorder (APA, 1994). Across the globe, there are reports of a rapidly growing number of children being diagnosed with ASD, which has led to increased attention on impairments and diagnosis of the spectrum of disorders especially in the Western countries (Yeargin-Allsop et al. 2003; Chakrabarti & Fombonne, 2005; Mandell et al. 2005; MMWR, 2012). Despite the increase in the reported prevalence of the disorders, ASD remains poorly recognized, under-diagnosed and less researched (Mandell et al. 2005; Wiggins et al. 2006).
A search of literature reveals that most studies on ASD emanate from the West, while the topic is disproportionately neglected in African countries in spite of evidence that most children of the world live in the highly disadvantaged low- and middle-income countries (LMICs) of Africa (UNDP, 2004). Early intervention has been consistently associated with improved outcome for which early age at diagnosis is pivotal. Although the average age at diagnosis for ASD range from 3 to 6 years (Chakrabarti & Fombonne, 2001; Mandell et al. 2005), there were reports of a reduction in the mean age at diagnosis and that diagnosis of ASD can be made accurately in the first 2 years of life (Howlin & Moore, 1997; Charman & Baird, 2002; Yeargin-Allsop et al. 2003; Klin et al. 2004). This observation could be as a result of improved public and physician level of awareness with the inexhaustive and ever-expanding body of knowledge about ASD in the Western World.
Studies conducted among Africans living outside the African continent indicate that African children are more likely than Caucasian children to have a late diagnosis of ASD (Mandell et al. 2002, 2007, 2009). Often, African children receive wrong psychiatric diagnoses and spend longer duration in search of help before receiving definitive diagnoses (Mandell et al. 2007; Bello-Mojeed et al. 2010, 2012; Bakare & Munir, 2011a, b). While most available data on this topic emanated from the West, there is a dearth of information on the pattern of impairments and diagnosis of ASD among affected children living in African the continent. Early diagnosis with active early intervention has been identified to improve outcome in affected children. This study assessed the pattern of autistic impairments and age at diagnosis of ASD in a clinical population of Nigerian children with the spectrum of disorders.
Methods
Location
This study was conducted at the neurodevelopmental disorder clinic of the Child and Adolescent Mental Health Service Unit of Federal Neuro-Psychiatric Hospital (FNPH), Lagos, Nigeria. The child clinic is located within the community and serves as a walk-in as well as formal referral facility to children with varying mental health problems. Formal referrals are usually received from schools, general physicians/private hospitals and other specialist hospitals while informal referrals originate from family/relatives, friends and neighbors.
For this clinic, there is no waiting list for a client to get the first appointment. All cases presenting at the clinic for the first time are seen same day and scheduled for follow-up clinic appointment within the duration of few days to 2 weeks based on the clinical assessment of the individual client. This Clinic is the largest Child facility, in term of structure and personnel that renders services to children with neurodevelopmental disorders in Nigeria; serves majorly children in Lagos and gets the referral from all parts of the country with diversity in ethnic representation and socio-economic status.
Ethical approval
Ethical approval was obtained from the ethical and research committee of FNPH, Lagos. Written consent was obtained from all mothers of children recruited for the study after explanations on the aims and objectives of the study were given to them.
Participant and sampling method
Study participants comprise 60 children with ASD attending the study location at the time of the study. DSM-V criteria were used to make a diagnosis of ASD, while a symptom checklist was used to determine the pattern of impairments in each child with ASD. Children with ASD that met inclusion criteria were consecutively recruited into the study after obtaining written consent from their mothers.
Material and procedure
A socio-demographic questionnaire and a symptom checklist for Autism were used for data collection.
Socio-demographic and clinical questionnaire
The socio-demographic and clinical questionnaire was used to obtain data on the socio-demographic characteristics and clinical data of the study participants. Items in the socio-demographic questionnaire include the child's age, gender, birth order and level of education. Information was also obtained on child's age at the parental observation of ASD symptoms, at contact with specialist facility and at diagnosis.
Symptom checklist for autism
The Symptom Checklist for examining the pattern of impairments in ASD was developed using items in the DSM-IV (Autistic Disorder, Aspergers and Pervasive Developmental Disorders NOS), DSM-V and ICD-10 criteria. It consists of 20 symptoms of ASD. The checklist assesses for presence or absence of varying symptoms of autism in the three core areas of impairments (social, communication and behavioral). Present in the Checklist is seven symptoms on the social impairment domain, five symptoms on the communication domain and eight symptoms on the behavioral domain. Responses on the Checklist were rated as either Yes (1 point) or No (0 points). The checklist was administered to mothers of children with autism who responded to the questions on behalf of their children. The Autism Symptom checklist was used to assess for the pattern of autistic impairments in the affected children. Two weeks test–retest reliability for the checklist was 0.96 with a Cronbach alpha of 0.91 (p < 0.001).
The study instruments were translated into Yoruba by a Yoruba-speaking psychiatrist and a linguist. The back translation was performed independently by another Yoruba speaking Psychiatrist and a Linguist. The back translation was then compared with the original translation by an independent panel and confirmed to be satisfactory before use.
The diagnosis of ASD was made based on DSM-V criteria. Two stages were involved in making a clinical diagnosis. The first stage involved comprehensive clinical assessment on every child with a previous diagnosis of ASD and any new patient suspected to have the spectrum of disorder; carried out by the first author who is a child psychiatrist with training in child development. The second stage comprised making or re-confirming a clinical diagnosis of ASD; the diagnosis of autism was made in any child who met the DSM-V criteria for the disorder before being recruited as study participants.
The mother completed the socio-demographic and other instruments. The literate mothers self-completed the socio-demographic questionnaire on behalf of the child. For the illiterate mothers, the questionnaires were read out to them, while the interviewer ticked off the items for them.
Data analysis
The data collected were analyzed using the Statistical Package for Social Sciences (SPSS 16) software. Frequencies, proportions, means and s.d. were calculated. Group comparison of categorical variables was by chi-square (χ2). The entire tests were two-tailed and the level of significance was set at p < 0.05.
Results
Sixty-two mothers of children with ASD were approached for recruitment of their children in the study but 60 children with a diagnosis of ASD participated in the study giving a response rate of 96.8%. Two mothers declined participation in the study due to a challenging behavior exhibited by one of the children difficult to manage by the mother who needed respite, while the second mother had to leave the clinic early to attend to her younger children at home. There was no difference in the socio-demographic characteristics of the children excluded and the sample recruited for the study.
Socio-demographic characteristics of children with ASD
Table 1 shows the socio-demographic characteristics of children with ASD. Their ages ranged from 2 to 17 years with a mean age of 9.45 ± 4.33 years. The majority of the children (75%) were 12 years or younger and there was a preponderance (70%) of males. Most children were from monogamous (95.0%) Christian (78.3%) family, reside in an urban setting (66.7%) and from a middle socio-economic group (51.7%).
Table 1.
Socio-demographic characteristics of children with ASD
(n = 60)
| Variables | Frequency (%) |
|---|---|
| Age range | Mean (s.d.) |
| 2–17 years | 9.45 (4.33) |
| Sex | |
| Males | 42 (70.0) |
| Females | 18 (30.0) |
| Family type | |
| Monogamous | 57 (95.0) |
| Polygamous | 3 (5.0) |
| Residence | |
| Urban | 40 (66.7) |
| Semi-urban | 17 (28.3) |
| Rural | 5 (5.0) |
| Socio-economic class | |
| High | 9 (15.0) |
| Middle | 31 (51.7) |
| Low | 20 (33.3) |
| Educational level | |
| None/out of school | 25 (41.7) |
| Nursery/play group | 24 (40.0) |
| Primary | 10 (16.6) |
| Secondary | 1 (1.7) |
| Birth order | |
| 1st child | 23 (38.3) |
| Middle child | 19 (31.7) |
| Last child | 18 (30.0) |
Health-contact and identification characteristics in ASD
Table 2 shows that about a half of the cases (51.7%) were referred from schools while the least proportions (16.7%) originated from informal sources including family, friends and neighbors. By the time each child attained the age of 2 years, all mothers (100%) of the children had observed an abnormality suggestive of ASD in their children but only 38.3% of them had contacted a child specialist clinic by the time the children were 6 years of age. Slightly above 33.3% of the children had a diagnosis of ASD made in the first 6 years of life (Table 2).
Table 2.
Health-contact and identification characteristics of children with ASD (n = 60)
| Variables | Frequency (%) |
|---|---|
| Referral source | |
| Parents/family | 2 (3.3) |
| Friends/neighbors | 8 (13.3) |
| General practitioners/private hospitals | 5 (8.3) |
| Specialist hospitals | 14 (23.3) |
| Schools | 31 (51.7) |
| Age (months) abnormality observed | (Mean ± s.d. = 17.02 ± 6.78) |
| 0–12 months | 25 (41.7) |
| 13–24 months | 35 (58.3) |
| >24 months | 0 (0.0) |
| Age (years) at contact with specialist clinic | (Mean ± s.d. = 8.13 ± 3.98) |
| 6 years and below | 23 (38.3) |
| 7–12 years | 27 (45.0) |
| 13 years and above | 10 (16.7) |
| Age (years) at diagnosis | (Mean ± s.d. = 9.0 ± 4.30) |
| 6 years and below | 20 (33.3) |
| 7–12 years | 26 (43.3) |
| 13 years and above | 14 (23.3) |
| Parent heard of ASD before diagnosis | |
| Yes | 2 (3.3) |
| No | 58 (96.7) |
Pattern of impairments in ASD
All the children with ASD (100%) exhibited poor eye contact, difficulty in mixing with other children and inability to consistently respond to his/her name. More than a half of them (55%) lack verbal communication (Table 3). The proportions of impairments that were not common are in the areas of object attachment (20.0%), odd postures (26.7%) and inappropriate facial expression (30.0%).
Table 3.
Pattern of autistic impairment in children with ASD
| Impairments | % |
|---|---|
| Social interactions impairment | |
| 1. Avoidance of/poor/no eye contact | 100.0 |
| 2. Inappropriate facial expression | 30.0 |
| 3. Sustained odd body postures | 26.7 |
| 4. Inability to indicate needs by gesture | 93.3 |
| 5. Difficulty in mixing with other children | 100.0 |
| 6. Lack emotional reciprocity | 95.0 |
| 7. Inappropriate object attachment | 23.3 |
| Communication impairment | |
| 8. Lack of speech development | 55.0 |
| 9. Inability to initiate/sustain conversation | 43.3 |
| 10. Stereotype and repetitive use of speech/action | 48.3 |
| 11. No consistent response to name | 100.0 |
| 12. Lack varied play | 91.7 |
| Behavioral impairment | |
| 13. Motor mannerism | 66.7 |
| 14. Resists changes in routines | 83.3 |
| 15. Extreme distress for no apparent reason | 76.7 |
| 16. Crying tantrum | 78.3 |
| 17. Apparent insensitivity to pain | 60.0 |
| 18. No fear of real danger | 46.7 |
| 19. Acting aggressively | 83.3 |
| 20. Preoccupation with an interest of abnormal intensity | 20.0 |
Age at diagnosis in ASD
Table 2 shows a significantly low mean age at the parental concern in ASD (17.0 ± 6.7 months). The mean age at diagnosis of ASD was 9.0 ± 4.3 years. The mean time lag from parental concern of features of ASD to seeking specialist care is 85 months and to diagnosis is 91 months.
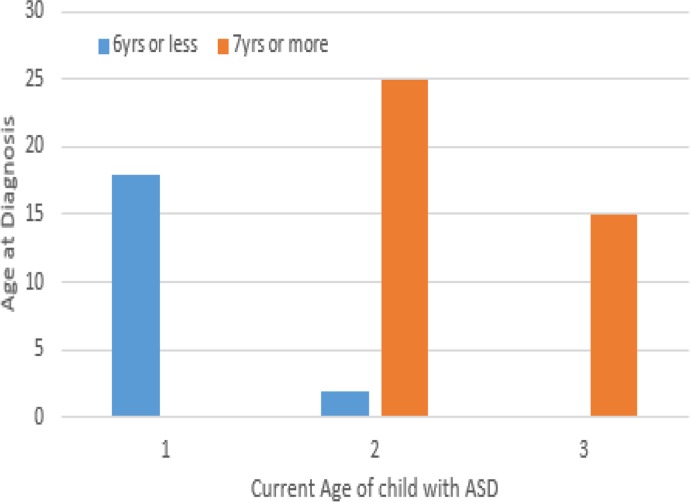
Figures 1 and 2 illustrate the distribution of the age at diagnosis with current age of child and age at child's contact with the specialist clinic respectively. Relationships between age at diagnosis and socio-demographic characteristics are shown in Table 4. Compared to children with speech development, about three-quarter of those that were non-verbal (75.1%) received their first diagnosis at older age/adolescence, but the difference was not statistically significant (χ2 = 2.727, p = 0.110). Similarly, no statistically significant relationship was found between age at diagnosis and other symptoms of ASD in the studied sample.
Fig. 1.
Bar chart showing age at contact with child specialist clinic (horizontal axis) against the age of diagnosis (vertical axis) at preschool and older/adolescent age group. On the vertical axis, (1) represents age range 6 years and below, (2) represents age range 7–12 years and (3) represents age range 13 years and above.
Fig. 2.
Bar chart showing current age of child with ASD (horizontal axis) against the age of diagnosis (vertical axis) at preschool and older/adolescent age group. On the vertical axis, (1) represents current age range 6 years and below, (2) represents current age range 7–12 years and (3) represents current age range 13 years and above.
Table 4.
Associations between age at diagnosis and socio-demographic characteristics
| Age at diagnosis <7 years (preschool) | Age at diagnosis at ≥7 years (older/adolescents) | Total | ||
|---|---|---|---|---|
| Variables | Frequency (%) | Frequency (%) | Frequency (%) | Difference |
| Age at 1st contact | ||||
| 6 years and below | 20 (87.0) | 3 (13.0) | 23 | χ2 = 58.570, df = 2 |
| 7–12 years | 0 (0.0) | 27 (100.0) | 27 | p < 0.001 |
| 13 years and above | 0 (0.0) | 10 (100.0) | 10 | |
| Current age range | ||||
| 6 years and below | 18 (100) | 0 (0.0) | 18 | χ2 = 51.667, df = 2 |
| 7–12 years | 2 (7.4) | 25 (92.6) | 27 | p < 0.001 |
| 13 years and above | 0 (0.0) | 15 (100.0) | 15 | |
| Gender | ||||
| Males | 16 (38.1) | 26 (61.9) | 42 | χ2 = 1.429, df = 1 |
| Females | 4 (22.2) | 14 (77.8) | 18 | p = 0.232a |
| Educational level | ||||
| None/out of school | 8 (32.0) | 17 (88.0) | 25 | χ2 = 1.906, df = 2 |
| Nursery/play group | 10 (41.7) | 14 (58.3) | 24 | p = 0.386 |
| Primary/junior college | 2 (18.2) | 9 (81.8) | 11 | |
| Socio-economic class | ||||
| High | 4 (44.4) | 5 (55.6) | 9 | χ2 = 0.598, df = 2 |
| Middle | 10 (32.3) | 21 (67.7) | 31 | p = 0.226 |
| Low | 6 (30.0) | 14 (70.0) | 20 | |
| Residence | ||||
| Urban | 17 (42.5) | 23 (57.5) | 40 | χ2 = 4.538, df = 1 |
| Rural & semi-urban | 3 (15.0) | 17 (85.0) | 20 | p = 0.044c |
| Autism awareness | ||||
| No | 18 (31.0) | 40 (69.0) | 58 | χ2 = 4.069,df = 1 |
| Yes | 2 (100.0) | 0 (0.0) | 2 | p = 0.107a |
Fisher's exact value.
Discussion
This study examined the pattern of impairments and age at diagnosis of ASD among a group of clinical population of children in Nigeria. The age range of 12 years and below for the majority of children with ASD (75%), seen in our study is similar to the findings of other studies carried out in child psychiatric clinics in Nigeria (Omigbodun, 2004; Aina et al. 2008). The finding of children in their early years of life is not unexpected as ASD is a medical condition of early onset. Also of note is the preponderance of males (70%) which is in keeping with findings of previous studies on a higher male to female ratio in autism occurrence (Chakrabarti & Fombonne, 2001; Yeargin-Allsop et al. 2003).
Although, most sampled children with ASD were referred through the school system, signifying the importance of the school system in the identification of children with ASD. However, the schools appear to lack basic skills required for early ASD identification as they were referred late and mostly had not been diagnosed with ASD prior to contact with the specialist center. An important finding in this study is a significantly low level of educational achievement among children with ASD. Only 1.7% proportion of those children with ASD was in the first year of a junior secondary school, on completion of a 6-year formal primary school education. In addition to the explanation of language impairment in especially the severe form of ASD, this finding is most likely a reflection of the challenges encountered in accessing appropriate education for this special group of disadvantaged African children (Omigbodun, 2004; Bello-Mojeed et al. 2010; Bakare & Munir, 2011a). In a study carried out in a child psychiatric clinic in Ibadan, Nigeria, Omigbodun (2004) found that 27.6% of the children in the study were out of school due to lack of suitable schools to meet their educational needs and children with autism were significantly associated with that problem. An interaction between severity of symptoms, associated intellectual impairment and ingrained stigma in Africa could also influence the observation of a low level of educational attainment among children with autism in our study (Aina et al. 2008; Bello-Mojeed et al. 2010, 2013, 2016; Bakare & Munir, 2011b). The previous report made in the country where the study was conducted, suggests that these children are often kept away from the stigmatizing community. Schools and the teachers present within may be reluctant to accept them due to a lack of knowledge about this disorder (Omigbodun, 2004; Bakare et al. 2009; Bello-Mojeed et al. 2010, 2013). Additionally, the lack of skills to tackle the disabilities associated with this disorder may also prevent mainstream schools from attending to the educational needs of children with ASD (Omigbodun, 2004; Bello-Mojeed et al. 2010, 2013).
The pattern of impairment reported in our study builds on previous evidence on impairments in three major areas of functioning in ASD (Kanner, 1943; Wing, 1969; Bartak & Rutter, 1974; Marchant et al. 1974; Chakrabarti & Fombonne, 2001; Yeargin-Allsop et al. 2003). The observation of more impairment in the social domain in this study is similar to the findings of studies conducted outside the African continent (Lord, 1995; Cox et al. 1999; Stone et al. 1999). Though social impairment is more specific to ASD and appears before the age of 3 years they could escape recognition in the context of the poor level of knowledge about features of ASD as reported earlier in Nigeria (Bakare et al. 2009; Bello-Mojeed et al. 2010, 2013).
Similar to our finding, previous studies reported a lack of ‘eye-to-eye gaze’ to be an important characteristic of ASD (Sorosky et al. 1968; Wing, 1969). Affected children often show a marked delay in both verbal and non-verbal communication skills as reported in the current study. The finding of a 55% rate of non-verbal communication among the children with ASD in the study location supports earlier reports of overrepresentation of non-verbal cases of ASD in Africa (Lotter, 1980; Belhadj et al. 2006; Mankoski et al. 2006; Bello-Mojeed et al. 2010, 2012; Bakare & Munir, 2011a). It has been suggested that about one-third to a half of individuals with autism failed to develop sufficient speech required for their daily communication needs (Noens et al. 2006). While many of them failed to acquire meaningful speech, ‘echolalia’, in which the child displays mechanical repetition of words or phrases spoken out by other people, often accompanied by pronominal reversal such as the use of ‘You’ instead of ‘I’ could be characteristic of their communication pattern. Many of the features found in our study, such as ‘difficulty in mixing with peers, gaze avoidance, difficulty in changing routine and lack varied play’, fit into Kanner's description of ‘infantile autism’. Assessment and subsequent diagnosis may be delayed in such children till attainment of school-age period (Yeargin-Allsop et al. 2003) when difficulties with peer interactions become apparent.
Although all mothers of children with the disorder had observed an abnormality suggestive of ASD in their children before the age of 2 years, the diagnosis of ASD was not made until a mean age of 9 years. A significantly high mean age of 9 years for ASD diagnosis found in this study demonstrates late diagnosis of the disorder among Nigerian children. Our report of maternal concern about features suggestive of ASD in their children before the attainment of age 2 years is similar to reports of studies emanating from the Western culture (Frith & Soares, 1993; Wiggins et al. 2006). On the other hand, the observation from the current study that children with ASD were not evaluated by a qualified professional until a mean age of 8.13 years, despite the presence of parental concern before the age of 2 years, is in contrast to research findings from outside Africa. Wiggins et al. (2006) in a study conducted among children with ASD in Atlanta reported a mean age of 4 years for evaluation by a qualified professional after parental concern in the age range from 12 to 21 months. Among possible factors, tortuous pathway to care in ASD, effect of perceived stigma on the affected child and parents, scarcity of ASD-specific intervention facilities in Africa could account for the marked delay in accessing professionals help (Omigbodun, 2004; Bello-Mojeed et al. 2010, 2012; Bakare & Munir, 2011a, b).
The finding of a mean age of 9 years for ASD diagnosis in our study is significantly higher than research findings of mean ages of 3.0–6.0 years among children with ASD from the West (Chakrabarti & Fombonne, 2001; Mandell et al. 2002, 2005; Yeargin-Allsopp et al. 2003). In a study conducted in the USA, Yeargin-Allsopp et al. (2003) found the mean age at first diagnosis of ASD in the studied children to be 3.9 years, while in a subsequent study in Pennsylvania Yeargin-Allsopp et al. (2003) observed that the average age at diagnosis for autistic disorder was 3.1 years. These findings suggest that over time, there appears to be a reduction in the age at which children with autism get diagnosed in the developed countries (Howlin & Moore, 1997; Yeargin-Allsop et al. 2003). On the contrary, the current study found a significant delay of about 7 years between the average time of parental observation of autistic features and diagnosis. The time lag between parental observation and diagnosis of ASD represents a vital period for institution of early intervention, which was lost by the child, family and the therapist. In the Western world, several programs are put in place to enhance early diagnosis and intervention in ASD. Comparatively, children with ASD living in an African country such as Nigeria are at a disadvantage not only in term of late identification, but also have to battle with the scarcity of ASD specific intervention facilities in the continent (Bello-Mojeed & Bakare, 2013; Bello-Mojeed et al. 2016). Research findings such as from the current study are important in informing policy makers of the urgent need to attend to the specific educational, social and medical needs of Nigerian children with ASD.
Late age of presentation at ASD child specialist center and place of residence were observed to be significantly associated with age at which the sampled population of Nigerian children receives a diagnosis of ASD. Children who lived in the rural and sub-urban areas were found to be more likely to receive a diagnosis of ASD later than their counterparts in the urban areas. This finding is in support of previous studies indicating a scarcity of specific intervention for children with ASD in rural areas (Palmer et al. 2005). In contrast to the Western countries, children with ASD in sub-Saharan African settings are seriously underserved, especially in the resource-disadvantaged rural communities. The very few available child health care facilities are concentrated in the urban areas with associated bottleneck of poor access.
This study has a limitation of being hospital-based, which precludes generalization and parents’ responses on behalf of the children relied on the memory recall of the participating mothers, which may not be foolproof. Also, the study did not make use of standardized ASD instrument, which may make it non-feasible to compare findings of the study to similar previous studies. In the diagnosis of ASD in Nigeria and other sub-Saharan regions, there are major difficulties in importing standardized instrument from Western countries to African children as some of the contents in such tools are culturally biased. Additionally, there are immense disparities in access to the available Western autism diagnostic tools. For example validated diagnostic tools such as ADI-R (Autism Diagnostic Interview-Revised) and ADOS (Autism Diagnostic Observation Scale), are very costly, require extensive training, lengthy to administer and culturally biased, all of which create huge obstacles to the access and use of such tools, especially in LMICs of Africa where most studies in ASD are self-sponsored. Despite the limitation, a considerable sample size was obtained and ASD Diagnosis made with Diagnostic Manual of Mental Disorders, Fifth Edition. Our study ensures a comprehensive psychiatric assessment and confirmation of the presence of core autistic symptoms and pattern of impairments were carried out.
To the best of the authors’ knowledge, this is the first study to address age at diagnosis and pattern of impairment in children with ASD in Nigeria. The implications of our study are significant. This study points to the importance of early recognition of ASD, appropriate specialist evaluation for definitive diagnosis and an increased need for improved knowledge among parents and the public in Nigeria. Educational intervention programs to improve the level of awareness and aid early recognition of ASD should be implemented. Social skills education and communication skills, especially in a naturalistic environment, should be an integral component of intervention programs. There is a need for community studies in this less researched area of childhood neurodevelopmental disorders. More studies are needed to assess for severity of autistic impairments and its relationship to the burden of care in African family caregivers. Findings of such studies should help in sensitizing policy makers to the importance of giving better attention to ASD and substantiating the need to give quality and evidence-based support to individuals and families with a diagnosis of ASD in Nigeria.
Conclusions
Core symptoms/impairments of ASD are present in Nigerian children but a late presentation and evaluation at specialist facilities with a significant delay from the time of parental symptom observation to a diagnosis of ASD are common findings. Early screening for ASD, prompt access to needed specialist evaluation for definitive diagnosis, improved education/knowledge about ASD among parents and the public should be integral components of management of ASD in Nigerian children. Factors associated with late age at diagnosis of ASD should form an essential component of interventions and support services in the management of children with ASD in sub-Saharan Africa.
Acknowledgement
A sincere gratitude to the staff at the Child and Adolescent Centre, Oshodi annex of the Federal Neuro-Psychiatric Hospital, Lagos, especially the Records and Nursing Departments.
Authors contribution
All Authors are involved with the conception of the study. M. A. B. designed the study, did statistical analysis and wrote the initial draft of the manuscript. O. O. O. was involved in the study design and revision of the manuscript. M. O. B. and A. O. A. were involved in the revision of the manuscript. All authors read and approved the final version of the manuscript.
Declaration of Interest
None.
References
- Aina OF, Ogun OC, Ladapo HTO, Lesi FEA, Famuyiwa OO (2008). Clinical neuro-psychiatric correlates and EEG findings among children with developmental disorders in Lagos, Nigeria. African Journal of Psychiatry 11, 123–127. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders, 4th edn (DSM-IV). American Psychiatric Publishing: Washington, DC. [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th edn (DSM-V). American Psychiatric Publishing: Washington, DC. [Google Scholar]
- Bakare MO, Ebigbo PO, Agomoh AO, Eaton J, Onwukwe JU, Onyeama GM, Okonkwo KO, Igwe MN, Orovighwo AO, Aguocha CM (2009). Knowledge about childhood autism and opinion among healthcare workers on availability of facilities and law caring for needs and rights of children with childhood autism and other developmental disorders in Nigeria. BMC Pediatrics 9, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakare MO, Munir KM (2011a). Autism spectrum disorders in Africa In A Comprehensive Book on Autism Spectrum Disorders (ed. Mohammadi M.-R.), Chapter 10; ISBN: 978-953-307-494-8, InTech (http://www.intechopen.com/books/a-comprehensive-book-on-autism-spectrum-disorders/autism-spectrum-disorders-in-africa). [Google Scholar]
- Bakare MO, Munir KM (2011b). Excess of non-verbal cases of autism spectrum disorders presenting to orthodox practice in Africa – a trend possibly resulting from late diagnosis and intervention. South African Journal of Psychiatry 17, 118–120. [PMC free article] [PubMed] [Google Scholar]
- Bartak L, Rutter M (1974). Use of personal pronouns by autistic children. Journal of Autism and Childhood Schizophrenia 4, 217–222. [DOI] [PubMed] [Google Scholar]
- Belhadj A, Mrad R, Halayem MB (2006). A clinic and para-clinic study of Tunisian population of children with autism. About 63 Cases. La Tunisie Médicale 84, 763–767. [PubMed] [Google Scholar]
- Bello-Mojeed M, Ani C, Lagunju I, Omigbodun O (2016). Feasibility of parent-mediated behavioural intervention for behavioural problems in children with Autism Spectrum Disorder in Nigeria: a pilot study. Child and Adolescent Psychiatry and Mental Health 10, 28. doi: 10.1186/SI3034-016-0117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Mojeed MA, Bakare MO (2013). Improving treatment of children with autism spectrum disorder in low- and middle- income countries: the role of non-specialist care providers. PLoS Medicine 10, e1001573 Doi: 10.1371/journal.pmed.10001572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Mojeed MA, Bakare MO, Munir KM (2012). Identification of Autism Spectrum Disorders (ASD) in Africa: Need for Shifting Research and Public Health Focus; The Comprehensive Guide to Autism (http://www.springerreference.com/docs/edit/chapterdbid/331260.html).
- Bello-Mojeed MA, Ogun OC, Omigbodun OO, Adewuya OA (2010). Late identification of autistic disorder in Nigeria: an illustration with 2 case reports. Nigerian Journal of Psychiatry 9, 31–35. [Google Scholar]
- Bello-Mojeed MA, Omigbodun OO, Ogun OC, Adewuya OA, Adedokun B (2013). The relationship between the pattern of impairments and in autism spectrum disorder and maternal psychosocial burden of care. OA Autism 1, 4. [Google Scholar]
- Chakrabarti S, Fombonne E (2001). Pervasive developmental disorders in preschool children. Journal of the American Medical Association 285, 3093–3099. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Fombonne E (2005). Pervasive developmental disorders in preschool children: confirmation of high prevalence. American Journal of Psychiatry 162, 1133–1141. [DOI] [PubMed] [Google Scholar]
- Charman T, Baird G (2002). Practitioner review: diagnosis of autism spectrum disorders in 2 and 3 year-old children. Journal of Child Psychology and Psychiatry, and Allied Disciplines 43, 289–305. [DOI] [PubMed] [Google Scholar]
- Cox A, Klein K, Charman T, Baird G, Baron-Cohen S, Swettenham J, Drew A, Wheelwright S (1999). Autism spectrum disorders at 20 and 42 months of age: stability of clinical and ADI–R diagnosis. Journal of Child Psychology and Psychiatry 40, 719–732. [PubMed] [Google Scholar]
- Frith U, Soares I (1993). Research into earliest detectable signs of autism: what parents say. Communication 27, 17–18. [Google Scholar]
- Howlin P, Moore A (1997). Diagnosis of autism: a survey of over 1200 patients in the UK. Autism 1, 135–162. [Google Scholar]
- Kanner L (1943). Autistic disturbances of affective contact. Nervous Child 2, 217. [PubMed] [Google Scholar]
- Klin A, Chawarska K, Paul R, Rubin E, Morgan T, Wiesner L, Volkmar F (2004). Autism in a 15-month old child. American Journal of Psychiatry 161, 1981–1988. [DOI] [PubMed] [Google Scholar]
- Lord C (1995). Follow-up of two-year-olds referred for possible autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines 36, 1365–1382. [DOI] [PubMed] [Google Scholar]
- Lotter V (1980) Cross cultural perspectives on childhood autism. Journal of Tropical Pediatrics 26, 131–133. [DOI] [PubMed] [Google Scholar]
- Mandell D, Listerud J, Levy S, Pinto-Martin J (2002). Race differences in the age at diagnosis among medicaid-eligible children with autism. Journal of American Academy of Child and Adolescent Psychiatry 41, 1447–1453. [DOI] [PubMed] [Google Scholar]
- Mandell D, Novak M, Zubritsky C (2005). Factors associated with the age of diagnosis among children with autism spectrum disorders. Pediatrics 116, 1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell DS, Ittenbach RF, Levy SE, Pinto-Martin JA (2007). Disparities in diagnoses received prior to the diagnosis of autism spectrum disorder. Journal of Autism and Developmental Disorders 37, 1795–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell DS, Wiggins LD, Carpenter LA, Daniels J, DiGuiseppi C, Durkin MS, Giarelli E, Morrier MJ, Nicholas JS, Printo-Martin JA, Shattuck PT, Thomas KC, Kirby RS, Yeargin-Allsopp M (2009). Racial/ethnic disparities in the identification of children with autism spectrum disorders. American Journal of Public Health 99, 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankoski RE, Collins M, Ndosi NK, Mgalla EH, Sarwatt VV, Folstein SE (2006). Etiologies of autism in a case-series from Tanzania. Journal of Autism and Developmental Disorders 36, 1039–1051. [DOI] [PubMed] [Google Scholar]
- Marchant R, Howlin P, Yule W, Rutter M (1974). Graded change in the treatment of the behaviour of autistic children. Journal of Child Psychology and Psychiatry 15, 221–227. [DOI] [PubMed] [Google Scholar]
- MMWR (2012). Prevalence of Autism Spectrum Disorders- Autism and Disabilities Monitoring Network, 14 sites, United States, 2008, 61, SS-3 (http://www.cdc.gov/mmwr/pdf/ss/ss6103.pdf). [PubMed]
- Noens I, van Berkelaer-Onnes I, Verpoorten R, van Duijn G (2006). The ComFor: an instrument for the indication of augmentative communication in people with autism and intellectual disability. Journal of Intellectual Disability Research 50, 621–632. [DOI] [PubMed] [Google Scholar]
- Omigbodun OO (2004). Psychosocial issues in child and adolescent psychiatric clinic population in Nigeria. Social Psychiatry and Psychiatric Epidemiology 39, 667–672. [DOI] [PubMed] [Google Scholar]
- Palmer RF, Blandard S, Jean CR, Mandell DS (2005). School districts resources and identification of children with autistic disorder. American Journal of Public Health 95, 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorosky AD, Omitz EM, Brown NB, Ritvo ER (1968). Systematic observations of autistic behavior. Archives of General Psychiatry 18, 439–449. [DOI] [PubMed] [Google Scholar]
- Stone W, Lee E, Ashford L, Brissie J, Hepburn S, Coorod E, Weiss B (1999). Can autism be diagnosed accurately in children under 3 years? Journal of Child Psychology and Psychiatry, and Allied Disciplines 40, 219–226. [PubMed] [Google Scholar]
- UNDP (2004). World Development report. United Nations Development Programme (http://hdr.undp.org/en/media/hdr04_complete.pdf).
- Wiggins LD, Baio J, Rice C (2006). Examination of the time between first evaluation and first autism spectrum diagnosis in a population-based sample. Journal of Developmental & Behavioral Pediatrics 27, S79–S87. [DOI] [PubMed] [Google Scholar]
- Wing L (1969). The handicaps of autistic children – a comparative study. Journal of Child Psychology and Psychiatry, and Allied Disciplines 10, 1–40. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1992). International Classification of Diseases, 10th edn (ICD – 10). WHO: Geneva. [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurka T, Doernberg N, Boyle C, Murphy C (2003). Prevalence of autism in a US metropolitan area. Journal of the American Medical Association 289, 49–55. [DOI] [PubMed] [Google Scholar]