Abstract
Selective adenosine receptor agonists and antagonists have marked effects on the outcome of cerebral ischemia, and adenosine receptors are expressed on astrocytes. In this study we examined the effects of various adenosine receptor agonists on the production of nitric oxide and the induction of iNOS in astrocytes activated by LPS/IFN-γ and TNF-α/IL-lβ and on the production of TNF-α. Treatment of the cells with the A2A receptor agonist CGS 21680 inhibited both NO production and iNOS expression induced by stimulation with either LPS/IFN-γ or TNF-α/IL-lβ, whereas the A1 and A3 receptor agonists, CPA and Cl-IB-MECA, respectively, did not have significant inhibitory effects. The inhibitory effect of the A2A receptor agonist was antagonized by the specific A2A receptor antagonist CSC. The A2A agonist also exerted a small inhibitory effect on the production of TNF-α. Similar inhibitory effects on the production of NO were obtained by cyclic AMP-elevating reagents, such as forskolin and dibutyryl cyclic AMP. Our findings suggest that activation of the A2A receptor inhibits NO production and iNOS expression likely via increased cAMP.
Keywords: C6 cell, Nitric oxide, Adenosine agonist, Adenosine receptor, Tumor necrosis factor, Cytokine
1. Introduction
Reactive astrocytes play an important role in immune responses during infectious, degenerative, and autoimmune diseases of the CNS [1]. Reactive astrocytes are also capable of producing nitric oxide via activation of the inducible form of nitric oxide synthetase (iNOS) [2]. The increased levels of NO are implicated in a variety of pathological conditions including neural toxicity in viral and bacterial infections, in autoimmune diseases of the CNS (Murphy et al., 1993), and in neurotoxicity in focal cerebral ischemia [3]. The induction of NO production has been reported to be regulated by LPS and combinations of inflammatory cytokines such as TNF-α/IL-lβ or IL-lβ/IFN-γ [3–5] and to be inhibited by β-adrenergic agonists, PGE, dexamethasone, and cytokines such as IL-4, TGF-β and IL-10 [6,7].
Selective adenosine receptor agonists and antagonists have marked effects on the outcome of models of cerebral ischemia, and astroglial adenosine receptors are potentially involved in these effects. There are four known subtypes of adenosine receptors, A1, A2A, A2B, and A3 [8]. Acute administration of the selective A1 agonists, ADAC and CPA [9], or chronic administration of the selective A3 agonist, IB-MECA [10], improved hippocampal cell survival following global ischemia in gerbils, and reduces lethality and impairment of behavior and learning. In both cases, the selective agonist treatment reduced the increased level of NOS observed in ischemic gerbil brains.
Functional adenosine receptors of all four subtypes are found on astrocytes. Activation of A1 receptors inhibited adenylyl cyclase in type-2 rat astrocytes [11], and in the same cultures activation of A2A receptors stimulated adenylate cyclase [12,13]. Recently, agonists selective for A3 receptors, such as IB-MECA and Cl-IB-MECA, were found to induce morphological changes in primary rat astroglial cultures [14] and to induce cell spreading and reorganization of the actin cytoskeleton in ADF human astrocytoma cells [15]. A2B receptor stimulation appeared to increase IL-6 in U373 human astrocytoma cells [16].
In this study we examined the effects of different adenosine agonists on the induction of iNOS and the production of NO in glial cells stimulated with LPS/IFN-γ or TNF-α/IL-lβ. For these studies we used C6 glial cells, which are used extensively to study glial cell function due to their similarity to primary glial cells [17]. We found that activation of the A2A receptors selectively inhibited NO production and iNOS induction, probably via an increase in cAMP levels.
2. Materials and methods
2.1. Materials
Monoclonal anti-iNOS antibody was obtained from Transduction Laboratories (Lexington, KY). Recombinant murine TNF-α, IFN-γ and IL-1β were obtained from Genzyme (Boston, MA). CPA, CPX, and CGS 21680 were obtained from Research Biochemicals International (Natick, MA). Cl-IB-MECA and CSC were synthesized as reported [18,19].
2.2. C6 glioma cell cultures
Cells (1 × 105 cells/ml) were seeded on tissue culture dishes (10 cm) and were grown in medium consisting of Dulbecco's modified Eagle's medium (DMEM) containing 10% heat-inactivated fetal calf serum, 2 mM glutamine, penicillin (50 U/ml), and streptomycin (0.05 mg/ml). Cells were split once a week by short exposure to 0.25% trypsin. C6 cells were treated with either LPS (1 μg/ml)/IFN-γ (100 U/ml) or TNF-α (30 ng/ml)/IL-1β (10 ng/ml) for 24 h in the presence or absence of adenosine ligands.
2.3. NO production
NO formation was detected by accumulation of nitrite in culture supernatants using the Griess reaction [20]. Briefly, 50 ml of culture medium were incubated with 50 ml of 1% sulfanilamide and 50 ml of 1% N-l-naphthylethylenediamine dihydrochloride in 2.5% H3PO4 at room temperature for 10 min. Optical density at 540 nm was measured, and the concentration of nitrite ions was quantified using NaNO2 as standard.
2.4. Measurement of TNF-α activity
Supernatants of cortical and cerebellar astrocytes were collected and levels of TNF-α were determined using a commercial ELISA kit. The detection level of TNF-α in this assay system was about 5 pg/ml.
2.5. Preparation of cell homogenates
Cells were washed and plates were placed on ice, scraped with a rubber policeman and centrifuged at 1400 rpm for 10 min. The supernatants were aspirated and the cell pellets were resuspended in 100 ml of lysis buffer (25 mM Tris-HCl, pH 7.4, 50 mM NaCl, 0.5% sodium deoxycholate; 2% NP-40; 0.2% SDS; 1 mM PMSF; 50 μg/ml aprotinin; 50 μM leupeptin; 0.5 mM Na3VO4) on ice for 15 min. The cell lysates were centrifuged for 15 min at 14000 rpm in an Eppendorf microcentrifuge, supernatants were removed and 2 × sample buffer was added.
2.6. Immunoblot analysis
Lysates (20 μg protein) were resolved by SDS-PAGE (10%) and were transferred to nitrocellulose membranes. The membranes were blocked with 5% dry milk in phosphate-buffered saline and subsequently stained with the primary antibody. Specific reactive bands were detected using a goat anti-rabbit or goat anti-mouse IgG conjugated to horseradish peroxidase (BioRad, Hercules, CA) and the immunoreactive bands were visualized by the ECL Western blotting detection kit (Amersham, Arlington Heights, IL).
3. Results
3.1. NO production
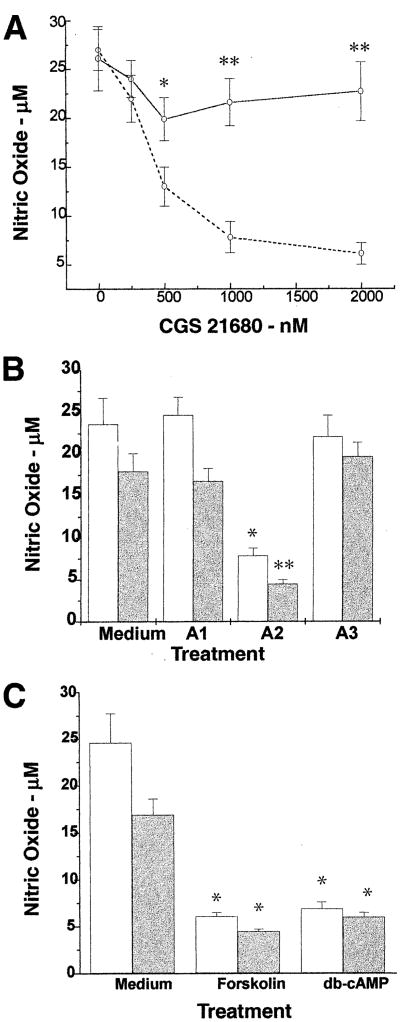
As already reported, we found that C6 glioma cells produced increased levels of NO in response to LPS/IFN-γ [21]. Pre-treatment of the cells for 1 h with the A2A receptor agonist CGS 21680 and LPS/IFN-γ exerted a dose-dependent inhibitory effect on NO production with an IC50 of 0.3 μM (Fig. 1A), whereas CGS 21680 itself had no effect on the production of NO by C6 cells. A lesser inhibitory effect were observed in cells treated concomitantly with the A2A agonist and LPS/IFN-γ (data not shown). We also examined the effects of CGS 21680 on NO production in C6 cells stimulated with TNF-α/IL-lβ and found similar inhibitory effects (Fig. 1B).
Fig. 1.
Effects of adenosine receptor agonists and cAMP-elevating agents on NO production in C6 glioma cells. C6 cells were treated with LPS/IFN-γ and different concentrations of COS 21680 in the absence (broken line) and presence (solid line) of the A2A antagonist CSC at a concentration of 10 μM, **P < 0.001, *P < 0.01. A: C6 cells were treated with either LPS/IFN-γ (open bars) or TNF-α/IL-lβ (shaded bars) for 24 h in the absence (labeled medium) or presence of A1 (CPA, 1 μM), A2A (CGS 21680, 10 μM), or A3 (Cl-IB-MECA, 1 μM) agonists, **P < 0.001, *P < 0.003 (B), or in the presence of cAMP-elevating agents, forskolin (10 μM) and db-cAMP (100 μM), *P < 0.004 (C). Supernatants were collected for the determination of . Results represent the means±S.D. of three separate experiments.
The inhibitory effect of the A2A agonist on NO production in cells stimulated with either stimuli was partially blocked in the presence of 10 μM of the specific A2A antagonist, CSC (Fig. 1A). The inhibitory effect was selective to the A2A receptor agonist, since the A1 and A3 receptor agonists, CPA (1 or 5 μM) and Cl-IB-MECA (1 or 10 μM), respectively, did not have significant inhibitory effects on NO production in cells stimulated with either LPS/IFN-γ or TNF-α/IL-lβ (Fig. 1B).
Since A2A agonists have been shown to increase cAMP levels in various cell types, including astrocytes [13,20], we examined the effects of cAMP-elevating agents on NO production in our system. As presented in Fig. 1C, forskolin and db-cAMP inhibited NO production in cells stimulated by both stimuli.
3.2. iNOS induction
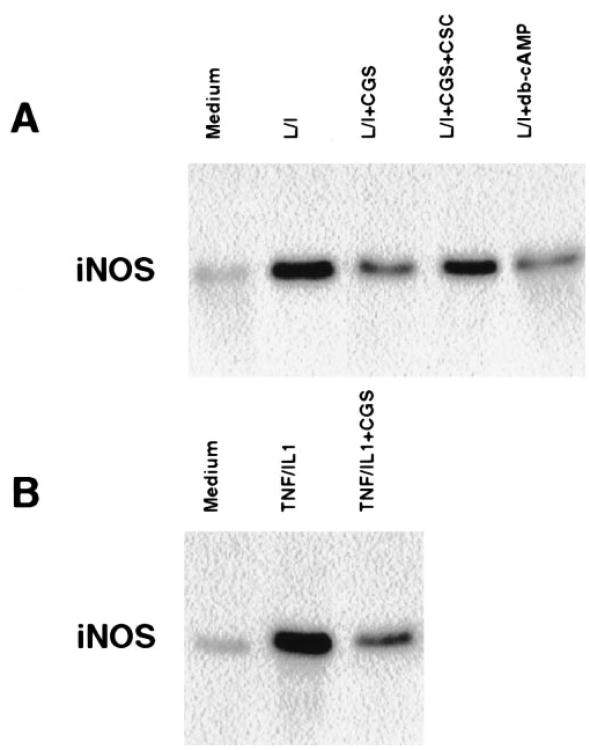
The inhibition of NO secretion in C6 cells by the A2A agonist CGS 21680 could be due to inhibition of the induction of iNOS protein. To explore this possibility, we pre-treated cells with CGS 21680 for 1 h followed by treatment with either LPS/IFN-γ or TNF-α/IL-1β for an additional 24 h. Whole cell lysates were analyzed by Western blotting using an anti-iNOS specific antibody. Fig. 2A shows that cells treated with LPS/IFN-γ for 24 h exhibited a pronounced increase in the expression of the iNOS protein. Pre-treatment of the cells with CGS 21680 reduced the expression of iNOS by 70%, and this inhibition was blocked by the receptor antagonist CSC. iNOS induction was also inhibited by db-cAMP. A similar inhibitory effect was observed in cells stimulated with TNF-α/IL-lβ (Fig. 2B). Neither CPA nor Cl-IB-MECA (1 μM) had a significant inhibitory effect on iNOS expression (data not shown).
Fig. 2.
Effect of the A2A receptor agonist CGS 21680 and db-cAMP on iNOS induction. Cells were stimulated with either LPS/IFN-γ (A) or with TNF-α/IL-lβ (B) for 24 h in the absence and presence of CGS 21680 (10 μM), CSC (10 μM) or db-cAMP (100 μM). Whole cell lysates were resolved by SDS-PAGE and proteins were immunoblotted with anti-iNOS antibody. The results are of one representative experiment out of three.
3.3. TNF-α production
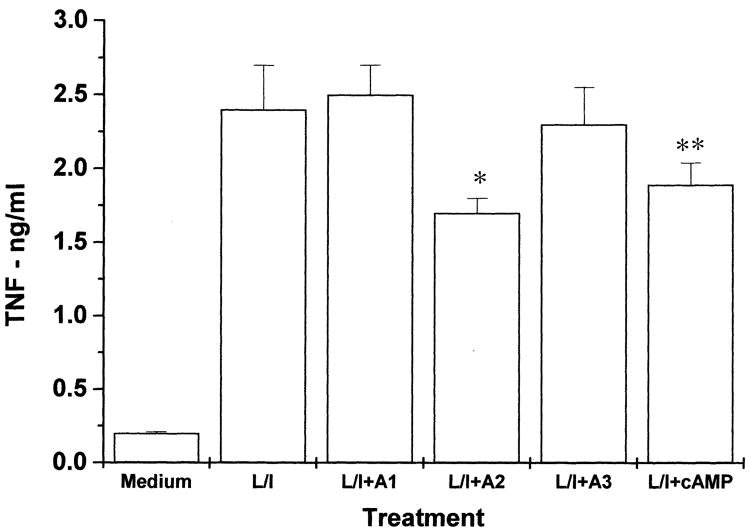
LPS-treated astrocytes are known to secrete cytokines such as IL-6 and TNF-α [22]. Since TNF-α is a possible mediator of LPS effects on the production of NO, we examined whether the inhibitory effect of the A2A agonist CGS 21680 is mediated by inhibiting the secretion of this cytokine in LPS-treated astrocytes. Fig. 3 shows that treatment of the cells with LPS induced a large increase in TNF-α secretion following 48 h of treatment. Treatment of the cells with CGS 21680 induced a small inhibitory effect on TNF-α secretion. A similar small inhibitory effect was induced by db-cAMP. The A1 and A3 selective agonists, CPA and Cl-IB-MECA, did not have significant effects on secretion of TNF-α (Fig. 3) or IL-6 (data not shown).
Fig. 3.
Effect of adenosine agonists selective for A1 (CPA, 1 μM), A2A (CGS 21680, 10 μM), or A3 (Cl-IB-MECA, 1 μM) receptors and db-cAMP (100 μM) on TNF-α secretion in LPS/IFN-γ-treated cells. C6 glioma cells were treated with LPS/IFN-γ in the presence and absence of different adenosine agonists and db-cAMP, and TNF-α levels were determined following 48 h using an ELISA kit. The results represent means ±S.D. from three separate experiments. *P < 0.002, **P < 0.01.
4. Discussion
During the course of brain trauma and inflammation there is induction of iNOS by astrocytes. These cells can produce high levels of NO, which play a role in host defense in the CNS [2]. Since NO mediates neuronal cell injury, the regulation of its production in the CNS must be well controlled. At least three cytokines are known to inhibit the induction of NO in astrocytes and microglial cells, TGF-β, IL-10 and IL-4 [6,7,23]. In addition, increases in intracellular cAMP have been reported to inhibit the induction of NO by LPS stimulation [24].
We found that the A2A agonist CGS 21680 inhibited NO production in astrocytes stimulated by either LPS/IFN-γ or TNF-α/IL-1β. In contrast, activation of the A1 and A3 adenosine receptors did not alter the production of NO or the induction of iNOS by the different stimuli. The inhibition of NO production by the A2A agonist was due to inhibition of iNOS protein expression. The inhibitory effect was mediated through activation of the A2A receptor since CGS 21680 is a selective agonist for A2A adenosine receptors vs. the other three subtypes. Moreover, the inhibitory effect was blocked by CSC, a selective A2A antagonist, which further supports the specific involvement of the A2A receptor in C6 cells.
The inhibitory effect of CGS 21680 on NO production was due to the inhibition of iNOS expression by stimulated astrocytes. The expression of iNOS in glial cells is induced by LPS, IFN-γ, TNF-α, IL-1 and phorbol esters [7], and activation of this enzyme leads to the production of high amounts of NO for prolonged periods of times. The expression of iNOS has been reported to be negatively regulated by β-adrenergic agonists, IL-4, IL-10, TGF-β, dexamethasone, and cAMP, and by NO itself [24].
The mechanism by which the A2A agonist inhibits NO production and iNOS induction is probably associated with an increased level of cAMP. Indeed, elevation of cAMP by either forskolin or db-cAMP inhibited NO production [24], and factors which activate adenylyl cyclase such as endothelin have been reported to inhibit NO production in cultured astrocytes [25]. The effect of the A2A agonist does not seem to be mediated by a decrease in TNF-α secretion, since the secretion of this cytokine was affected to a small degree by treatment with the A2A agonist. The effects of adenosine agonists on iNOS induction and NO production in the brain have not been described so far. Interestingly, activation of the A2A adenosine receptor has been reported to increase NO production in vascular smooth muscle cells [26]. However, in these cells, in contrast to glial cells, increases in cAMP induce activation of NO production.
Adenosine has been shown to be secreted during ischemic conditions in the brain. The roles of the different adenosine receptors have been associated with either neuronal protection or neurotoxicity depending on the concentrations of the adenosine and on the specific receptor being activated. Our results indicate that activation of the A2A adenosine receptor could inhibit NO production by astrocytes during inflammatory conditions in the CNS. In addition, since iNOS has also been also implicated in cerebral ischemic damage [27], activation of the A2A receptor may play a role in protecting against neural toxicity during ischemic conditions in the CNS.
Abbreviations
- db-cAMP
dibutyryl 3′,5′-cyclic AMP
- ADAC
N6-[4-[[[4-[[[(2-aminoethyl)amino]carbonyl]methyl]anilino]carbonyl]methyl]-phenyl]adenosine
- ADO
adenosine
- CGS 21680
2-[4-[(2-carboxyethyl)phenyl]ethylamino]-5′-N-ethylcarboxamidoadenosine
- Cl-IB-MECA
N6-(3-iodobenzyl)-2-chloroadenosine-5′-N-methyluronamide
- CNS
central nervous system
- CPA
N6-cyclopentyladenosine
- CPX
8-cyclopentyl-1,3-dipropylxanthine
- CSC
8-chlorostyrylcaffeine
- IB-MECA
N6-(3-iodobenzyl)adenosine-5′-N-methyluronamide
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- NO
nitric oxide
- iNOS
inducible nitric oxide synthetase
- PGE
prostaglandin E
- PMSF
phenylmethylsulfonylfluoride
- SDS-PAGE
sodium dodecyl-sulfate polyacrylamide gel electrophoresis
- TGF
transforming growth factor
- TNF
tumor necrosis factor
- Tris
tris(hydroxymethyl)amino-methane
References
- 1.Eddleston M, Micke L. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simmons ML, Murphy S. J Neurochem. 1992;59:97–905. doi: 10.1111/j.1471-4159.1992.tb08328.x. [DOI] [PubMed] [Google Scholar]
- 3.Minc-Golomb D, Schwartz JP. Trends Neurosci. 1993;16:323–328. doi: 10.1016/0166-2236(93)90109-y. [DOI] [PubMed] [Google Scholar]
- 4.Hooper DC, Ohnishi ST, Kean R, Numagami Y, Dietzschold B, Koprowski H. Proc Natl Acad Sci USA. 1995;92:5312–5316. doi: 10.1073/pnas.92.12.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SK, Murphy S. J Neurosci Res. 1994;39:405–411. doi: 10.1002/jnr.490390407. [DOI] [PubMed] [Google Scholar]
- 6.Hu S, Sheng WS, Peterson PK, Chao CC. Glia. 1995;15:491–494. doi: 10.1002/glia.440150412. [DOI] [PubMed] [Google Scholar]
- 7.Simmons ML, Murphy S. Eur J Neurosci. 1993;5:825–831. doi: 10.1111/j.1460-9568.1993.tb00934.x. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson KA, Suzuki F. Drug Dev Res. 1997;39:289–300. doi: 10.1002/(sici)1098-2299(199611/12)39:3/4<289::aid-ddr8>3.0.co;2-n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Lubitz DKJE, Beenhakker M, Lin RC, Carter MF, Paul LA, Bischofberger N, Jacobson KA. Eur J Pharmacol. 1996;302:43–48. doi: 10.1016/0014-2999(96)00101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Lubitz DKJE, Lin RCS, Popik P, Carter MF, Jacobson KA. Eur J Pharmacol. 1994;263:59–67. doi: 10.1016/0014-2999(94)90523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogata T, Nakamura Y, Schubert P. Eur J Neurosci. 1996;8:1124–1131. doi: 10.1111/j.1460-9568.1996.tb01280.x. [DOI] [PubMed] [Google Scholar]
- 12.Peakman MC, Hill SJ. Br J Pharmacol. 1994;1ll:191–198. doi: 10.1111/j.1476-5381.1994.tb14043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy MG, Moak CM, Byczko Z, MacDonald WF. J Neurosci Res. 1991;30:631–640. doi: 10.1002/jnr.490300406. [DOI] [PubMed] [Google Scholar]
- 14.Abbracchio MP, Ceruti S, Brambilla R, Franceschi C, Malorni W, Jacobson KA, von Lubitz DKJE, Cattabeni F. Ann NY Acad Sci. 1997;825:11–22. doi: 10.1111/j.1749-6632.1997.tb48410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbracchio MP, Rainaldi G, Giammarioli AM, Ceruti S, Brambilla R, Cattabeni F, Barbieri D, Franceschi C, Jacobson KA, Malorni W. Biochem Biophys Res Commun. 1997;241:297–304. doi: 10.1006/bbrc.1997.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiebich BL, Biber K, Gyufko K, Berger M, Bauer J, van Caulker D. J Neurochem. 1996;66:1426–1431. doi: 10.1046/j.1471-4159.1996.66041426.x. [DOI] [PubMed] [Google Scholar]
- 17.Mangoura D, Sakellaridis N, Jones J, Vernadakis A. Neurochem Res. 1989;14:941–947. doi: 10.1007/BF00965927. [DOI] [PubMed] [Google Scholar]
- 18.Kim HO, Ji Xd, Siddiqi SM, Olah ME, Stiles GL, Jacobson KA. J Med Chem. 1994;37:3614–3621. doi: 10.1021/jm00047a018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson KA, Nikodijevic O, Padgett W, Gallo-Rodriguez C, Maillard M, Daly JW. FEBS Lett. 1993;323:141–144. doi: 10.1016/0014-5793(93)81466-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drapier JC, Hibbs JB. Eur J Immunol. 1988;18:1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- 21.Brodie C, Goldreich G, Haiman T, Kazimirsky G. J Neuroimmunol. 1998;81:20–30. doi: 10.1016/s0165-5728(97)00154-9. [DOI] [PubMed] [Google Scholar]
- 22.Frohman EM, van den Noort S, Gupta S. J Clin Immunol. 1989;9:1–9. doi: 10.1007/BF00917121. [DOI] [PubMed] [Google Scholar]
- 23.Chao CC, Molitor TW, Hu S. J Immunol. 1993;151:1473–1481. [PubMed] [Google Scholar]
- 24.Pahan K, Namboodiri AMS, Sheikh FG, Smith BT, Singh I. J Biol Chem. 1997;272:7786–7791. doi: 10.1074/jbc.272.12.7786. [DOI] [PubMed] [Google Scholar]
- 25.Oda H, Toshihiko M, Nomura YJ. Neurochemistry. 1997;69:669–674. doi: 10.1046/j.1471-4159.1997.69020669.x. [DOI] [PubMed] [Google Scholar]
- 26.Dubey RK, Gillespie EK, Jackson EK. Hypertension. 1998;31:296–302. doi: 10.1161/01.hyp.31.1.296. [DOI] [PubMed] [Google Scholar]
- 27.Jeffery SR, Snyder SH. Annu Rev Cell Dev Biol. 1995;11:417–440. doi: 10.1146/annurev.cb.11.110195.002221. [DOI] [PubMed] [Google Scholar]