Abstract
Infection, relapse and GVHD can complicate allogeneic hematopoietic stem cell transplantation (allo-HSCT). While the effect of poor immune recovery on infection risk is well-established, there are limited data on the effect on relapse and survival, especially following T cell-depletion (TCD). To characterize the pattern of immune reconstitution in the first year after transplant and its effects on survival and relapse, we performed a retrospective study in 375 recipients of a myeloablative TCD allo-HSCT for hematologic malignancies. We noted that different subsets recover sequentially, CD8+ T cells first, followed by total CD4+ and naïve CD4+ T cells, indicating thymic recovery during the first year after HSCT. In the multivariate model, a fully HLA-matched donor and recovery of T cell function, assessed by PHA response at 6 months, were the only factor independently associated with OS and EFS. In conclusion, T cell recovery is a predictor of outcome after TCD allo-HSCT.
Keywords: immune reconstitution, allogeneic hematopoietic stem cell transplant, T cell depletion
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an established treatment for hematologic malignancies. However, it is associated with significant adverse effects including infection, relapse, and graft versus host disease (GVHD). One variable that may affect these outcomes is the recovery of the immune system after transplantation.[1-7] Deficiencies in post-transplant T-cell reconstitution, and in particular of CD4+ T cells, correlate with an increased risk of infections.[1,2] Several groups have shown that early recovery of absolute lymphocyte count (ALC) after unmodified or partially T-cell depleted (TCD) allo-HSCT is associated with improved overall survival (OS), decreased relapse and lower transplant-related mortality (TRM).[4-7] There are, however, incomplete data regarding the effect of the quantitative and functional recovery of T cells on relapse and survival.[8-10] Data on immune reconstitution following TCD allo-HSCT are significantly more limited than in the unmodified setting.[1,11-14]
TCD allo-HSCT lowers the incidence and severity of both acute and chronic GVHD without increased relapse or decreased survival when used in selected patient populations.[13,15-22] This approach is now being investigated in a multicenter randomized phase 3 trial (BMT CTN 1301, NCT NCT02345850). However, immune recovery after TCD-HSCT is delayed because of the paucity of T cells administered in the graft. Thus, understanding the delayed immune reconstitution in recipients of TCD grafts is potentially critical to improving outcomes in these patients. To that end, we characterized the pattern of immune recovery in the first year after TCD allo-HSCT and its effects on post-transplant morbidity and mortality, in a retrospective study in 375 patients transplanted for hematologic malignancies.
Materials and Methods
Patients
Three hundred and seventy-five patients with hematologic malignancies underwent an allogeneic TCD-HSCT at Memorial Sloan Kettering Cancer Center (MSKCC) from January 1997 through December 2005. Patients transplanted after 2005 were excluded because they received palifermin, for which pre-clinical data suggest a positive effect on immune recovery.[23-26] One additional patient was excluded because he received post-transplant interleukin-7, which enhances immune reconstitution.[27] All patients received grafts from an HLA-identical or single HLA-mismatched (A, B, C, DR) related or unrelated donor. HLA matching was established for all patients by DNA sequence-specific oligonucleotide typing for HLA-A, -B, and DR-B1 loci, and additionally for C and DQ-B1 loci for unrelated donors. Written informed consent for treatment was obtained from all patients and donors. Approval for this retrospective review was obtained under a waiver of authorization from the Institutional Review and Privacy Board.
Transplant procedure and supportive care
Patients received myeloablative cytoreduction with either total body irradiation (TBI) or chemotherapy-based regimens. Either of two TBI-based regimens were used: TBI 1375 or 1500 cGy, followed by 2 daily doses of thiotepa (5 mg/kg/day) and, either 2 daily doses of cyclophosphamide (60 mg/kg/day, n=218) starting after thiotepa (TBI/THIO/CY), or 5 daily doses of fludarabine (25 mg/m2/day, n=109) beginning the first day of thiotepa (TBI/THIO/FLU).[13,15] The chemotherapy-based regimen (n=48) consisted of busulfan (0.8 mg/kg/dose) every 6 hours for 10 doses, melphalan (70 mg/m2/day) for 2 doses and fludarabine (25 mg/m2/day) for 5 doses (BU/MEL/FLU).[28] The three conditioning regimens did not change over the time period of the study and continue in use at our center. Both the TBI/THIO/CY and BU/MEL/FLU regimens are included in the ongoing BMT CTN 1301 randomized trial (NCT NCT02345850). T cells were removed from bone marrow grafts (n=190) or granulocyte colony stimulating factor (G-CSF)-mobilized peripheral blood stem cells (PBSC, n=153) as previously described.[13,15,29] T cells were removed from bone marrow grafts by sequential soybean lectin agglutination and sheep red blood cell (sRBC)-rosette depletion.[15,29] Positive selection of CD34+ cells was performed by using Isolex 300i Magnetic Cell Separator (Baxter, Deerfield, IL) and subsequent sheep RBC rosette depletion,[13] or using the CliniMACS CD34 Reagent System (Miltenyi Biotech, Gladbach, Germany).[21,30] In addition, 32 patients received combined T cell depleted BM and PBSC allografts, on a protocol in which both products were being administered. Equine anti-thymocyte globulin (ATG, 15 mg/kg/dose × 2 equine) or rabbit ATG (2.5 mg/kg/dose × 2) provided graft rejection prophylaxis.[13,15,31] Patients who received mismatched grafts received 3 doses of ATG. Four patients received alemtuzumab instead of ATG prior to transplant. Recipients of HLA-matched related donors treated with TBI, thiotepa, and fludarabine (n=53) and 21 patients under the age of 24 treated with TBI, thiotepa, and cyclophosphamide did not receive ATG rejection prophylaxis. All patients received ex vivo T cell depleted grafts and no post transplant GVHD prophylaxis was given to any patient. All patients received supportive care and prophylaxis against opportunistic infections according to standard guidelines. Briefly, patients were monitored weekly using the pp65 antigenemia assay from engraftment until day 100,[32] and on a similar schedule by EBV PCR. Pre-emptive therapy was used for CMV (antivirals) and EBV (rituximab). There was no monitoring for fungal infections and all patients received mold active anti-fungal prophylaxis.
Acute GVHD (aGVHD) was diagnosed clinically, confirmed pathologically by biopsy whenever possible, and classified according to standard criteria.[33] Patients with late aGVHD were scored as having aGVHD, per consensus guidelines.[34] Chronic GVHD (cGVHD) was classified as limited or extensive by the criteria of Sullivan,[35] because the retrospective nature of the study precluded accurate scoring of chronic GVHD severity per consensus criteria in all patients. Patients who engrafted were evaluable for aGVHD, and patients surviving at least 100 days were evaluable for cGVHD. Cause of death was determined using a standard algorithm .[36]
Immune recovery monitoring
Data were collected for ALC at days +30, +60 and +90 after transplant. If the patient was not available on those specific days, the ALC within one day of those time points was used. Circulating lymphocyte subsets (total CD4+, CD4+45RA+ and CD8+ T cells, NK cells) and T cell proliferative responses (measured by 3HTdR incorporation) to the mitogen phytohemagglutinin (PHA) were measured at varying time points for 12 months after transplant, as previously published.[1] Immune recovery data were censored at time of donor lymphocyte infusion (DLI) or second transplant.
Statistical analysis
Immunologic data were collected longitudinally, and early measurements were used as predictors of survival time. Other baseline factors included age, diagnosis (ALL vs. others), conditioning regimen (TBI-based or chemotherapy only), HLA matching status (identical or not), ATG (yes vs. no), remission status (first vs. others), transplant type (PBSC vs. others), donor sex match status, and year of transplant (1997-2001 vs. 2002-2005). Four outcomes were evaluated: OS was defined from the date of HSCT to the date of death or last follow-up; event-free survival (EFS) was defined from the date of HSCT to the earliest date of relapse/progression, death, or last follow-up; time to relapse/progression was the time interval between the date of HSCT and the date of relapse/progression, and the patients without relapse/progression were censored at the last available follow-up; time to non-relapse mortality (NRM) was defined as the same time interval as EFS, and patients who relapsed were censored at the date of relapse.
The association between baseline factors and each immune recovery variable measured over time was investigated using a mixed effects model. The mixed effects model contained subject-specific and time-random effects, with linear and quadratic functions of time, and baseline factor fixed effects. Baseline factors included here are all baseline factors used in outcome analyses plus patient sex. The slopes of the baseline factors within the model were estimated along with the 95% confidence intervals (CI) and tested in the mixed effects models.
Landmark survival analyses at 3 months and 6 months were performed to examine the association between each survival outcome and immune reconstitution factors (total CD4, CD8, NK, naïve CD4 and PHA) plus baseline factors. Landmark survival analyses at 1 month were performed for OS, EFS, relapse and NRM associations with total CD4, CD8 and NK. The effect of ALC recovery on each survival outcome was examined at landmark times of 30, 60 and 90 days. The immune reconstitution value was recorded at the closest time point prior to the landmark. The values measured after relapse/progression were not used in EFS, relapse or NRM outcome analyses. Univariate analysis was first performed on each immune reconstitution factor or baseline factor using the Cox regression. Multivariable Cox regression started with all baseline and immune reconstitution factors in the model, and used stepwise selection with significance level < 0.01. For the landmark analyses of OS and EFS at 6 months, we plotted a smooth nonparametric curve showing the 75th quantile of the survival distribution as a function of the immune recovery [37]. A test with p value < 0.01 was considered statistically significant. Statistical analyses were performed in software packages SAS 9.2 (SAS Institute Inc., Cary, NC, USA), and R version 2.13 (The R Foundation for Statistical Computing).
Results
Patient characteristics and transplant outcomes
Pre-transplant characteristics of the 375 patients are detailed in Table 1. The median age was 40 years (range 2-68). Diseases transplanted included ALL (n=85), AML (n=150), CML (n=55), MDS (n=36) and NHL (n=49). Immune recovery data was available for 354 patients. The other 21 patients were not evaluable because of primary (n=5) or early secondary (n=3) graft failure or early death (n=13). Sixty-seven patients were censored at the time of unselected DLI (n=64) or EBV-specific cytotoxic lymphocytes infusion (n=3). Reasons for DLI included disease (morphologic relapse or minimal residual disease, n=36), infection including EBV lymphoproliferative disorder (n=22), mixed chimerism (n=5), and other (n=4). The median time to first DLI was 293 days (range 47-1617 days). Thirty-four patients were censored at the time of second transplant (n=21) or stem cell “boost” (n=13). Reasons for second transplant included disease (n=14) or graft failure (n=7); while reasons for a stem cell boost included graft failure (n= 10), disease (n=2), or low cell dose (n=1). The median time to second transplant or boost was 217 days (range 5-4345 days).
Table 1. Patient Characteristics.
| Patient Characteristics | |
|---|---|
| Age (years) | |
| Median [Range] | 40 [2-68] |
| <18 | 69 |
| <18-30 | 64 |
| 30-49 | 150 |
| >50 | 92 |
| Disease | |
| AML | 150 |
| ALL | 85 |
| CML | 55 |
| NHL | 49 |
| MDS | 36 |
| Donor | |
| Matched Related | 202 |
| Mismatched Related | 36 |
| Matched Unrelated | 77 |
| Mismatched unrelated | 60 |
| Status at HSCT | |
| 1st complete remission, 1st chronic phase, untreated MDS without increased blasts | 178 |
| active relapse, refractory disease, progressive disease | 22 |
| Other | 175 |
| Conditioning Regimen | |
| TBI/Thiotepa/Cyclophosphamide | 218 |
| TBI/Thiotepa/Fludarabine | 109 |
| Busulfan/Melphalan/Fludarabine | 48 |
| Stem Cell Source | |
| Bone Marrow | 190 |
| Peripheral Blood Stem Cells | 153 |
| Bone Marrow & Peripheral Blood Stem Cells | 32 |
AML: acute myelogenous leukemia; ALL: acute lymphoblastic leukemia; CML: chronic myelegenous leukemia; NHL: non-Hodgkin lymphoma; MDS: Myelodysplastic syndrome; HSCT: hematopoietic stem cell transplantation; TBI: total body irradiation.
At one year, the cumulative incidence of grade II-IV and III-IV aGVHD in 363 evaluable patients was 0.177 (95%CI: 0.13, 0.216) and 0.100 (95%CI: 0.069, 0.132), respectively. The cumulative incidence of cGVHD and extensive cGVHD at 1 year in 304 evaluable patients was 0.093 (95%CI: 0.060, 0.127) and 0.047 (95%CI: 0.027, 0.071), respectively. The non-relapse mortality (NRM) at 1 year was 0.296 (95%CI: 0.250, 0.342). Non-relapse causes of death included infection (n=64), GVHD (n=35), organ failure (n=17), non-engraftment (n=9), secondary malignancy (n=6), and other causes (n=11).
The pace of recovery after allo-HSCT differs for different T cell subsets
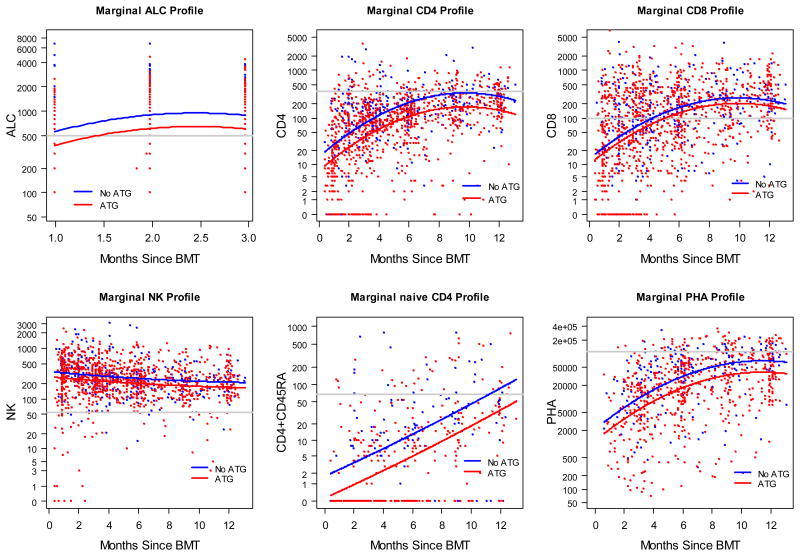
The pattern of immune recovery is depicted in Figure 1. There was a rapid normalization of the ALC with engraftment and, subsequently, a small increase during the first 3 months post transplant. The pattern of recovery of NK cells mirrored the recovery of ALC. These findings are consistent with other studies in the unmodified setting that show early ALC values represent mostly NK cells.[4,38,39]
Figure 1. Immune recovery for the first 12 months after allo-HSCT with and without ATG.
While we observed increases in CD4+ T cells, CD8+ T cells, naïve CD4+ T cells, and PHA responses over time, we noted differences between subsets. This included more rapid recovery to a normal range in CD8+ T cells compared with CD4+ T cells, resulting in pertubation of the expected CD4+: CD8+ ratio. At 6 months, median CD8+ T cell count was 132/mm3 (range 0-3643/mm3), which is above the lower limit of normal (LLN, 98/mm3). In contrast, median CD4+ T cell count was 135/mm3 (range 1-1771/mm3) at 6 months and 252/mm3 (range 10-1151/mm3) at 12 months, both below the LLN (359/mm3). In patients who had immune reconstitution parameters measured at 12 months, 28% (33/119) achieved CD4+ T cell counts in the normal range, while 79% (94/119) had normal CD8+ T cell counts (median 269/mm3). Furthermore, the recovery of naïve CD4+ T cells trailed that of total CD4+ T cells, consistent with delayed thymic recovery. The median naïve CD4+ T cell count was 3/mm3 (range 0-177/mm3) at 6 months and 40/mm3 (range 0-748/mm3, LLN 67/mm3) at 12 months. However, by 12 months the proportion of patients with normal naïve CD4+T cell counts (30%, 13/43) and normal total CD4+ T cell counts were nearly identical. There was a modest correlation between these patients (Spearman correlation coefficient 0.586, P<.001). Finally, functional T cell responses, assessed by response to PHA, paralleled CD4+ T cell recovery. At 12 months, median response to PHA was 55,512 cpm (range 503-253691 cpm, limits of normal differed over time), with 38% (44/117) of patients achieving a PHA response in the normal range. Although median CD4+ and CD8+ counts appeared to decrease near the end of the observation period, this likely reflects continued immune recovery monitoring of patients with delayed recovery at 12 months post transplant and beyond.
Effect of transplant factors on immune recovery and clinical outcomes
The effect of transplant factors on immune recovery is presented in Table 2. In multivariate analysis, receiving an HLA identical graft was associated with improved recovery of ALC, CD8+ T cells, and NK cells. Increased age was associated with decreased recovery of T cell function as measured by PHA response (slope on log (PHA) -0.17, P<.001). Surprisingly, age did not otherwise strongly affect immune reconstitution, including recovery of naïve CD4+ T cells. Diagnosis and remission status did not have an effect on immune reconstitution. ATG had an adverse effect on recovery of ALC, CD4+ T cells, naïve T cells, and PHA response (Figure 1, Table 2).
Table 2. Effect of baseline factors on immune reconstitution.
| Univariate Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Slope | 95%CI | P-value | Slope | 95%CI | P-value | |
| log(ALC) n =324 | ||||||
|
| ||||||
| Age (10-year increment) | 0.04 | -0.02 , 0.09 | .195 | |||
| Sex (Male) | 0.11 | -0.09 , 0.31 | .275 | |||
| ATG use | -0.38 | -0.61 , -0.16 | .001 | |||
| HLA Identical | 0.43 | 0.22 , 0.65 | <.001 | 0.43 | 0.22 , 0.65 | <.001 |
| Regimen (chemo only) | 0.14 | -0.14 , 0.43 | .332 | |||
| 1st Remission | -0.02 | -0.22 , 0.19 | .865 | |||
| Diagnosis (ALL) | -0.19 | -0.41 , 0.04 | .103 | |||
| PBSC only | 0.16 | -0.04, 0.36 | .111 | |||
| Donor Sex (Male) | 0.17 | -0.02, 0.37 | .079 | |||
|
| ||||||
| log(CD4), n = 315 | ||||||
|
| ||||||
| Age (10-year increment) | -0.01 | -0.08 , 0.06 | .698 | |||
| Sex (Male) | 0.28 | 0.03 , 0.52 | .027 | |||
| ATG use | -0.66 | -0.94 , -0.39 | <.001 | -0.66 | -0.94 , -0.39 | <.001 |
| HLA Identical | 0.43 | 0.15 , 0.71 | .003 | |||
| Regimen (chemo only) | 0.07 | -0.30 , 0.44 | .698 | |||
| 1st Remission | 0.25 | 0 , 0.51 | .052 | |||
| Diagnosis (ALL) | -0.07 | -0.35 , 0.21 | .622 | |||
| PBSC only | 0.14 | -0.11, 0.38 | .275 | |||
| Donor Sex (Male) | -0.05 | -0.30, 0.19 | .666 | |||
|
| ||||||
| log(CD8), n = 314 | ||||||
|
| ||||||
| Age (10-year increment) | -0.01 | -0.10 , 0.07 | .746 | |||
| Sex (Male) | 0.27 | -0.04 , 0.58 | .085 | |||
| ATG use | -0.26 | -0.62 , 0.09 | .149 | |||
| HLA Identical | 0.50 | 0.15 , 0.85 | .006 | 0.50 | 0.15 , 0.85 | .006 |
| Regimen (chemo only) | -0.08 | -0.54 , 0.39 | .745 | |||
| 1st Remission | -0.10 | -0.43 , 0.22 | .527 | |||
| Diagnosis (ALL) | -0.03 | -0.38 , 0.32 | .854 | |||
| PBSC only | -0.40 | -0.70, -0.09 | .011 | |||
| Donor Sex (Male) | -0.24 | -0.54, 0.07 | .132 | |||
|
| ||||||
| log(NK), n = 314 | ||||||
|
| ||||||
| Age (10-year increment) | 0.05 | 0 , 0.09 | .059 | |||
| Sex (Male) | -0.06 | -0.22 , 0.11 | .508 | |||
| ATG use | -0.23 | -0.42 , -0.04 | .019 | |||
| HLA Identical | 0.28 | 0.08 , 0.47 | .005 | 0.28 | 0.08 , 0.47 | .005 |
| Regimen (chemo only) | 0.17 | -0.08 , 0.42 | .190 | |||
| 1st Remission | 0.04 | -0.13 , 0.22 | .640 | |||
| Diagnosis (ALL) | -0.15 | -0.34 , 0.04 | .118 | |||
| PBSC only | 0.16 | 0, 0.33 | .055 | |||
| Donor Sex (Male) | 0.14 | -0.02, 0.3 | .097 | |||
|
| ||||||
| log(CD45), n= 187 | ||||||
|
| ||||||
| Age (10-year increment) | -0.10 | -0.20 , 0.01 | .066 | |||
| Sex (Male) | 0.23 | -0.17 , 0.63 | .266 | |||
| ATG use | -0.86 | -1.30 , -0.42 | <.001 | -0.86 | -1.30 , -0.42 | <.001 |
| HLA Identical | 0.38 | -0.03 , 0.80 | .072 | |||
| Regimen (chemo only) | -0.19 | -0.72 , 0.35 | .488 | |||
| 1st Remission | 0.12 | -0.30 , 0.54 | .585 | |||
| Diagnosis (ALL) | 0.18 | -0.25 , 0.60 | .419 | |||
| PBSC only | 0.08 | -0.32, 0.48 | .699 | |||
| Donor Sex (Male) | 0.08 | -0.32, 0.47 | .711 | |||
|
| ||||||
| log(PHA), n =258 | ||||||
|
| ||||||
| Age (10-year increment) | -0.17 | -0.26 , -0.08 | <.001 | -0.16 | -0.25 , -0.07 | .001 |
| Sex (Male) | 0.00 | -0.34 , 0.33 | .980 | |||
| ATG use | -0.58 | -0.95 , -0.21 | .002 | -0.53 | -0.89 , -0.16 | .005 |
| HLA Identical | 0.32 | -0.06 , 0.71 | .100 | |||
| Regimen (chemo only) | 0.04 | -0.45 , 0.52 | .883 | |||
| 1st Remission | 0.26 | -0.09 , 0.60 | .144 | |||
| Diagnosis (ALL) | 0.20 | -0.17 , 0.57 | .285 | |||
| PBSC only | 0.02 | -0.31, 0.35 | .917 | |||
| Donor Sex (Male) | 0.07 | -0.26, 0.40 | .678 | |||
The factors without estimates and p value were not significant in multivariable analysis.
ALC: absolute lymphocyte count; ATG: antithymocyte globulin; ALL: acute lymphoblastic leukemia; PBSC: peripheral blood stem cells; NK: natural killer; PHA: phytohemagglutinin.
The effect of transplant factors on outcomes is presented in Table 3. The following factors were significantly associated with improved OS, NRM and EFS for patients alive at 1 month: not receiving ATG (p=0.003, p=0.001, and p=0.001) and having an HLA identical match (p<0.001 for all). At 3 months, not receiving ATG was associated with improved EFS, (P=.007) and having an HLA identical match was associated with improved OS as well as EFS (P<.001 in both). In addition, a TBI-based preparative regimen was associated with improved EFS at 3 months, although P=.046. At 6 months, HLA match (P<.001, P<.001) still predicted OS and EFS. In the multivariate model, a fully HLA-matched donor was the only factor independently associated with OS and EFS in landmark analyses at 1, 3 and 6 months and NRM in a landmark analysis at 1 month.
Table 3a. Landmark analysis of effect of baseline factors and immune recovery at 1 month on survival outcomes, NRM and relapse.
| 1 Month | Total N | Univariate Analysis | Multivariable Analysis* | ||
|---|---|---|---|---|---|
|
| |||||
| OS | |||||
|
| |||||
| HR of Parameter (95%CI) | P-value | HR of Parameter (95%CI) | P-value | ||
|
| |||||
| Baseline Factor | |||||
| Age (10-year increment) | 355 | 1.07 (0.98, 1.17) | .129 | ||
| ATG (yes) | 355 | 1.86 (1.23, 2.80) | .003 | ||
| HLA identical (yes) | 355 | 0.51 (0.37, 0.69) | <.001 | 0.51 (0.37, 0.69) | <.001 |
| Regimen (chemo only) | 355 | 1.22 (0.80, 1.85) | .354 | ||
| 1st Remission (yes) | 355 | 0.74 (0.54, 1.03) | .073 | ||
| ALL | 355 | 1.24 (0.89, 1.72) | .210 | ||
| PBSC only | 355 | 0.81 (0.60, 1.10) | .183 | ||
| Donor Sex match (yes) | 355 | 0.96 (0.72, 1.29) | .806 | ||
| Transplant Year (2002-2005) | 355 | 0.77 (0.57, 1.04) | .087 | ||
| Immune recovery parameter | |||||
| ALC | 323 | 0.88 (0.66, 1.17) | .364 | ||
| log(CD4) | 193 | 0.94 (0.84, 1.05) | .253 | ||
| log(CD8) | 193 | 0.95 (0.87, 1.04) | .267 | ||
| log(NK) | 192 | 0.85 (0.74, 0.98) | .025 | ||
|
| |||||
| NRM | |||||
|
| |||||
| HR of Parameter (95%CI) | P-value | HR of Parameter (95%CI) | P-value | ||
|
| |||||
| Baseline Factor | |||||
| Age (10-year increment) | 353 | 1.12 (1.00, 1.25) | .042 | ||
| ATG (yes) | 353 | 2.77 (1.56, 4.93) | .001 | ||
| HLA identical (yes) | 353 | 0.45 (0.31, 0.65) | <.001 | 0.45 (0.31, 0.65) | <.001 |
| Regimen (chemo only) | 353 | 1.06 (0.62, 1.82) | .838 | ||
| 1st Remission (yes) | 353 | 0.71 (0.48, 1.06) | .095 | ||
| ALL | 353 | 1.09 (0.72, 1.65) | .676 | ||
| PBSC only | 353 | 0.81 (0.56, 1.17) | .257 | ||
| Donor Sex match (yes) | 353 | 0.99 (0.69, 1.41) | .942 | ||
| Transplant Year (2002-2005) | 353 | 0.64 (0.44, 0.93) | .018 | ||
| Immune recovery parameter | |||||
| ALC | 322 | 0.74 (0.49, 1.12) | .158 | ||
| log(CD4) | 193 | 0.92 (0.81, 1.05) | .216 | ||
| log(CD8) | 193 | 0.93 (0.83, 1.04) | .183 | ||
| log(NK) | 192 | 0.81 (0.7, 0.95) | .008 | ||
|
| |||||
| EFS | |||||
|
| |||||
| HR of Parameter (95%CI) | P-value | HR of Parameter (95%CI) | P-value | ||
|
| |||||
| Baseline Factor | |||||
| Age (10-year increment) | 353 | 1.04 (0.96, 1.13) | .353 | ||
| ATG (yes) | 353 | 1.94 (1.29, 2.91) | .001 | ||
| HLA identical (yes) | 353 | 0.50 (0.37, 0.68) | <.001 | 0.50 (0.37, 0.68) | <.001 |
| Regimen (chemo only) | 353 | 1.34 (0.90, 2.01) | .149 | ||
| 1st Remission (yes) | 353 | 0.74 (0.54, 1.01) | .059 | ||
| ALL | 353 | 1.21 (0.87, 1.67) | .258 | ||
| PBSC only | 353 | 0.80 (0.59, 1.08) | .147 | ||
| Donor Sex match (yes) | 353 | 0.95 (0.71, 1.27) | .735 | ||
| Transplant Year (2002-2005) | 353 | 0.80 (0.59, 1.07) | .132 | ||
| Immune recovery parameter | |||||
| ALC | 322 | 0.79 (0.58, 1.08) | .144 | ||
| log(CD4) | 193 | 0.97 (0.87, 1.07) | .505 | ||
| log(CD8) | 193 | 0.99 (0.90, 1.08) | .755 | ||
| log(NK) | 192 | 0.84 (0.74, 0.96) | .011 | ||
|
| |||||
| Relapse | |||||
|
| |||||
| HR of Parameter (95%CI) | P-value | HR of Parameter (95%CI) | P-value | ||
|
| |||||
| Baseline Factor | |||||
| Age (10-year increment) | 353 | 0.90 (0.78, 1.04) | .169 | ||
| ATG (yes) | 353 | 1.11 (0.61, 2.01) | .742 | ||
| HLA identical (yes) | 353 | 0.63 (0.37, 1.09) | .101 | ||
| Regimen (chemo only) | 353 | 2.04 (1.10, 3.77) | .023 | ||
| 1st Remission (yes) | 353 | 0.78 (0.45, 1.34) | .364 | ||
| ALL | 353 | 1.42 (0.82, 2.47) | .210 | ||
| PBSC only | 353 | 0.83 (0.49, 1.40) | .486 | ||
| Donor Sex match (yes) | 353 | 0.80 (0.48, 1.32) | .385 | ||
| Transplant Year (2002-2005) | 353 | 1.29 (0.78, 2.15) | .319 | ||
| Immune recovery parameter | |||||
| ALC | 322 | 0.92 (0.58, 1.44) | .708 | ||
| log(CD4) | 193 | 1.07 (0.90, 1.27) | .457 | ||
| log(CD8) | 193 | 1.11 (0.96, 1.29) | .168 | ||
| log(NK) | 192 | 0.93 (0.71, 1.21) | .583 | ||
The factors without estimates and p value were not significant in multivariable analysis.
ALC: absolute lymphocyte count; ATG: antithymocyte globulin; ALL: acute lymphoblastic leukemia; PBSC: peripheral blood stem cells; NK: natural killer; PHA: phytohemagglutinin.
Baseline transplant factors including HLA matching status and preparative regimen type also influenced relapse rate, but did not reach statistical significance level (p≥0.01 for all factors). For example, at 3 and 6 months post allo-HSCT, having an HLA match (P=.020, P=.013) and receiving a TBI- based preparative regimen (P=.016, P=.027) was associated with a decreased relapse rate. In the multivariate model, having an HLA-matched donor was associated with a decreased relapse rate at 6 months (P=.013). Receipt of a TBI-based regimen was independently predictive of decreased relapse at 3 months (P<.001).
Immune Recovery predicts increased EFS and OS
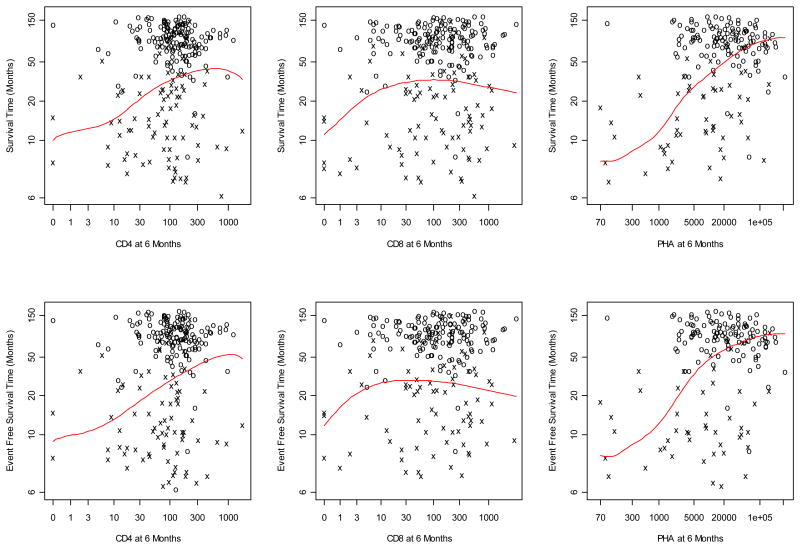
We also examined the impact of early recovery of immune parameters on survival outcomes and relapse. Higher NK cell counts at 1 month was associated with improved NRM after 1 month (p=0.008). The effects of CD4+ and CD8+ T cell counts on EFS and OS at 1, 3 and 6 months by landmark analysis are depicted in Table 3 and Figure 2. Total CD4+ T cells at 6 months were significantly associated in a univariate analysis with improved EFS (p=0.001) and OS (p=0.002) after 6 months. However, the effect of these parameters did not appear to be independent as they were not predictive of EFS or OS in a multivariate model. There was no effect of either CD4+ or CD8+ T cell recovery on relapse at 3 or 6 months. Because there were a small number of immune reconstitution data points at 12 months, biased towards patients with poorer outcomes, we did not examine the effect of immune recovery on survival outcomes at this time point.
Figure 2. Landmark analysis of immune reconstitution at 6 months on OS and EFS.
Furthermore, in univariate analysis, T cell response to the mitogen PHA at 6 months predicted improved EFS (P<.001) and OS (P<.001) (Table 3). The PHA response was independent of other factors, predicting improved EFS (P=.002) and OS (P<.001) at 6 months in a multivariate model. In contrast, the earlier PHA response measured at 3 months did not predict OS or EFS.
Discussion
Our results represent the most extensive analysis to date of immune recovery following TCD allo-HSCT. We focused on TCD allo-HSCT, because delayed immune reconstitution is one of the potential limitations of this approach and a source of morbidity and mortality. Recognizing the limitations of a retrospective study, we illustrate the pattern and timing of reconstitution of T cell subsets and function over the first year after TCD-HSCT in a cohort of 375 patients. Additionally, we provide evidence T cell function is associated with improved OS and EFS.
T cell subsets recover to different degrees and with different kinetics. Notably, CD8+ T cells reach the normal range within the 1st year in a majority of patients, while this only occurs in 28% of patients for CD4+ T cells. It is particularly interesting that, while naïve CD4+ T cell recovery is not robust in the first 6 months, there is clear generation of naïve CD4+ T cells at later time points in many patients. In fact, the percentage of patients who reach normal naïve CD4+ T cell counts at 12 months is nearly identical to the percentage of those who reach normal total CD4+ T cell counts. Thus, early reconstitution of T cells likely results from peripheral expansion either of residual host CD4+ T cells or of the limited CD4+ T cells in the graft. At later time points, reconstitution of T cells using thymic pathways becomes more prominent as the production of naïve CD4+ T cells increases. However, the modest correlation between patients with normal naïve CD4+ T cell counts and those with normal total CD4+ T cell counts suggests that peripheral expansion remains an important contributor to immune reconstitution at 1 year post allo-HSCT. While we do not have data on CD4 or CD8 subsets, beyond naïve CD4 T cells, in this cohort, we did examine T cell subsets in our phase 1 study of recombinant human IL-7, and showed that the main subset present early after TCD-HCT was effector memory T cells.[27] In that study, the percent of regulatory T cells was within the normal range and was not affected by treatment with IL-7, which is not surprising as they don't express CD127. While that represents a very small group of patients (n=12) that requires prospective validation in a larger dataset, it is consistent with the notion that early recovery is driven by peripheral expansion. In addition, we were not able to examine B cell recovery or T cell chimerism, both parameters that would enhance the overall analysis.
Another important finding is the wide variability in immune recovery, implying that factors aside from TCD affect T cell recovery. For example, CD4+ T cell counts ranged between 0-1771/mm3 at 6 months and 0-2960/mm3 at 12 months. While studies in the non-TCD setting have suggested that patient age, CD4+ transplant dose, donor type, graft source, administration of ATG, CMV reactivation, and the development of GVHD are important,[8,9,40,41] we examined factors influencing immune recovery after fully TCD allo-HSCT. We identified ATG as a factor that impaired immune recovery, suggesting that we could potentially improve outcomes if we focus on strategies to perform TCD allo-HSCT without ATG.[13] However, we cannot exclude potential bias in this finding because patients who did not require ATG were either young or received an HLA-matched graft. In addition, receiving an HLA-matched graft predicted improved recovery of all immune reconstitution parameters, while lower patient age predicted improved T cell function post TCD allo-HSCT. Although GVHD impacts immune recovery in non-TCD transplants, there were only a small number of GVHD events in the present study.
In this large patient population, we demonstrate that recovery of T cell function as measured by response to the mitogen PHA is independently predictive of OS and EFS at 6 months post transplant. Our data confirm and significantly expand on a prior report of 69 patients receiving conventional allo-HSCT, which showed an improvement in OS with CD4+ T cell recovery at 3 months.[8] This is the first description of the effect of immune recovery on OS and EFS after TCD allo-HSCT. In this population of patients who receive a fully TCD allo-HSCT, we did not confirm the impact of ALC at day 30 on OS and EFS that has been seen in conventional and partially TCD studies.[4-7] This finding may result from a lower NK cell content administered in the graft with TCD. We also did not demonstrate an independent effect of T cell count recovery in the multivariate analysis.
Our study also seeks to assess the effect of immune recovery on disease relapse over the first year after TCD allo-HSCT. While no effect on relapse was seen in our study, our ability to detect such a difference was limited by the small number of these events observed. Our results are in agreement with a study in which neither CD4+ T cell nor CD8+ T cell recovery affected relapse in recipients of conventional grafts.[42] Interestingly, this study demonstrated an NK cell count >150 cells/uL at 12 months (but not earlier) is associated with a decreased risk of relapse. The discrepancy between this finding and our study may be related to the use of GVHD prophylaxis in the Bühlmann paper.
There have been other studies that demonstrate the impact of immune reconstitution after allo-HSCT. A previous report in 71 patients from MSKCC showed that CD4+ T cell recovery can mitigate opportunistic infections.[1] Berger et al [9] also reported that early CD4+ T cell recovery after conventional BMT leads to decreased infections, resulting in reduced TRM. No difference in TRM was noted for CD8+ and CD56+ cell counts. Pao et al[43] also reported that higher levels of circulating naïve CD4+ T cells correlated with an improved response to pneumococcal vaccination.
There are limitations to our study. First, it is retrospective and our findings would be strengthened if confirmed in a prospective study. There also several additional factors that are known to impact post-transplant outcomes that we were not able to include in the analysis. These include the Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI), which we recently reported is also predictive in TCD all-HSCT,[44] and the disease risk index (DRI). Other factors, such as ABO have been shown to have at best a modest effect in a recent CIBMTR analysis of over 10,000 patients.[45] The dose of ATG has been shown in some series to affect outcomes. Those reports have all been with unmodified grafts. In our series, the dose of ATG was standard and the main effect was seen in the presence or absence of ATG.
Despite these limitations, the study provides a clear picture of the pattern of immune recovery after TCD allo-HSCT. Important findings include a description of the kinetics of naïve CD4+ T cell recovery and the demonstration that recovery of T cell function, assessed by PHA response at 6 months, independently predicts improved OS and EFS and may therefore provide a marker for patients whose outcomes could improve with investigational agents for the promotion of immune reconstitution. While the main immune parameters measured, CD4 and CD8 T cells, were not independently associated with outcomes in the multivariable analysis, their analysis and correlations with risk of infection and response to vaccines for example could still indicate their clinical utility.
Given recent increased interest in TCD in the setting of the ongoing randomized trial,[17,18,30,46] and the risk of poor immune recovery associated with TCD, it will become increasingly important to identify patients at risk for delayed immune recovery and develop agents for the promotion of the immune system after TCD. Our demonstration of naïve T cell recovery post TCD allo-HSCT implies that patients can have thymic recovery following myeloablative allo-HSCT. Thus, preserving the thymus, perhaps with KGF or androgen ablation, or directly effecting T cells with agents such as IL-7,[27] are worthwhile strategies to pursue for promoting immune reconstitution. Future plans include further assessing the close relationship between immune recovery and the development of infections including how the development of infection affects the immune system. In addition, it will be critical to further dissect the relationship between T cell numbers, function and T cell receptor diversity.[14]
Supplementary Material
Table 3b. Landmark analysis of effect of baseline factors and immune recovery at 3 months on survival outcomes and relapse.
| 3 Months | Total N | Univariate Analysis | Multivariable Analysisa | ||
|---|---|---|---|---|---|
|
| |||||
| OS | |||||
|
| |||||
| HR of Parameter (95%CI) | P-value | HR of Parameter (95%CI) | P-value | ||
|
| |||||
| Baseline Factor | |||||
| Age (10-year increment) | 314 | 1.08 (0.98, 1.19) | .132 | ||
| ATG (yes) | 314 | 1.71 (1.09, 2.68) | .019 | ||
| HLA identical (yes) | 314 | 0.44 (0.31, 0.62) | <.001 | 0.44 (0.31, 0.62) | <.001 |
| Regimen (chemo only) | 314 | 1.44 (0.92, 2.26) | .110 | ||
| 1st Remission (yes) | 314 | 0.79 (0.55, 1.14) | .214 | ||
| ALL | 314 | 1.40 (0.97, 2.02) | .075 | ||
| PBSC only | 314 | 0.85 (0.60, 1.20) | .347 | ||
| Donor Sex match (yes) | 314 | 0.93 (0.67, 1.30) | .678 | ||
| Transplant Year (2002-2005) | 314 | 0.83 (0.59, 1.16) | .271 | ||
| Immune recovery parameter | |||||
| log(CD4) | 252 | 0.89 (0.82, 0.98) | .012 | ||
| log(CD8) | 252 | 0.91 (0.84, 0.98) | .014 | ||
| log(NK) | 251 | 0.93 (0.68, 1.26) | .637 | ||
| log(CD45) | 114 | Na | |||
| log(PHA) | 89 | 0.94 (0.79, 1.12) | .473 | ||
|
| |||||
| EFS | |||||
|
| |||||
| HR of Parameter (95%CI) | P value | HR of Parameter (95%CI) | P value | ||
|
| |||||
| Baseline Factor | |||||
| Age (10-year increment) | 304 | 1.06 (0.96, 1.18) | .232 | ||
| ATG (yes) | 304 | 1.90 (1.19, 3.02) | .007 | ||
| HLA identical | 304 | 0.41 (0.29, 0.58) | <.001 | 0.41 (0.29, 0.58) | <.001 |
| Regimen (chemo only) | 304 | 1.58 (1.01, 2.47) | .046 | ||
| 1st Remission | 304 | 0.79 (0.55, 1.14) | .205 | ||
| ALL | 304 | 1.28 (0.88, 1.86) | .205 | ||
| PBSC only | 304 | 0.80 (0.57, 1.14) | .214 | ||
| Donor Sex match (yes) | 304 | 0.84 (0.60, 1.17) | .294 | ||
| Transplant Year (2002-2005) | 304 | 0.84 (0.60, 1.19) | .330 | ||
| Immune recovery parameter | |||||
| log(CD4) | 243 | 0.93 (0.85, 1.02) | .128 | ||
| log(CD8) | 243 | 0.93 (0.86, 1.01) | .083 | ||
| log(NK) | 242 | 0.87 (0.64, 1.19) | .399 | ||
| log(CD45) | 111 | Na | |||
| log(PHA) | 85 | 0.92 (0.78, 1.09) | .350 | ||
|
| |||||
| Relapse | |||||
|
| |||||
| HR of Parameter (95%CI) | P value | HR of Parameter (95%CI) | P value | ||
|
| |||||
| Baseline Factor | |||||
| Age (10-year increment) | 304 | 0.93 (0.79, 1.10) | .385 | ||
| ATG (yes) | 304 | 1.14 (0.58, 2.24) | .700 | ||
| HLA identical | 304 | 0.49 (0.27, 0.90) | .020 | ||
| Regimen (chemo only) | 304 | 2.29 (1.17, 4.94) | .016 | ||
| 1st Remission | 304 | 0.82 (0.45, 1.52) | .536 | ||
| ALL | 304 | 1.43 (0.76, 2.66) | .264 | ||
| PBSC only | 304 | 0.81 (0.45, 1.45) | .472 | ||
| Donor Sex match (yes) | 304 | 0.51 (0.28, 0.93) | .028 | ||
| Transplant Year (2002-2005) | 304 | 1.49 (0.83, 2.65) | .180 | ||
| Immune recovery parameter | |||||
| log(CD4) | 243 | 1.06 (0.91, 1.23) | .447 | ||
| log(CD8) | 243 | 1.02 (0.89, 1.16) | .777 | ||
| log(NK) | 242 | 0.85 (0.66, 1.10) | .226 | ||
| log(CD45) | 111 | Na | |||
| log(PHA) | 85 | 0.94 (0.72, 1.23) | .662 | ||
The factors without estimates and p value were not significant in multivariable analysis.
ALC: absolute lymphocyte count; ATG: antithymocyte globulin; ALL: acute lymphoblastic leukemia; PBSC: peripheral blood stem cells; NK: natural killer; PHA: phytohemagglutinin.
Table 3c. Landmark analysis of effect of baseline factors and immune recovery at 6 months on survival outcomes and relapse.
| 6 Months | Total N | Univariate Analysis | Multivariable Analysisa | ||
|---|---|---|---|---|---|
|
| |||||
| OS | |||||
|
| |||||
| HR of Parameter (95%CI) | P-value | HR of Parameter (95%CI) | P-value | ||
|
| |||||
| Baseline Factor | |||||
| Age (10-year increment) | 272 | 1.07 (0.95, 1.21) | .255 | ||
| ATG (yes) | 272 | 1.52 (0.91, 2.53) | .111 | ||
| HLA identical (yes) | 272 | 0.45 (0.29, 0.68) | <.001 | 0.48 (0.27, 0.82) | .008 |
| Regimen (chemo only) | 272 | 1.37 (0.79, 2.37) | .267 | ||
| 1st Remission (yes) | 272 | 0.95 (0.62, 1.44) | .805 | ||
| ALL | 272 | 1.43 (0.92, 2.22) | .110 | ||
| PBSC only | 272 | 0.96 (0.64, 1.44) | .843 | ||
| Donor Sex match (yes) | 272 | 0.88 (0.59, 1.31) | .528 | ||
| Transplant Year (2002-2005) | 272 | 1.10 (0.74, 1.65) | .640 | ||
| Immune recovery parameter | |||||
| log(CD4) | 238 | 0.76 (0.65, 0.90) | .002 | ||
| log(CD8) | 238 | 0.91 (0.79, 1.04) | .149 | ||
| log(NK) | 238 | 1.10 (0.83, 1.46) | .505 | ||
| log(CD45) | 121 | 0.86 (0.71, 1.04) | .118 | ||
| log(PHA) | 179 | 0.70 (0.61, 0.80) | <.001 | 0.74 (0.65, 0.86) | <.001 |
|
| |||||
| EFS | |||||
|
| |||||
| HR of Parameter (95%CI) | P-value | HR of Parameter (95%CI) | P-value | ||
|
| |||||
| Baseline Factor | |||||
| Age (10-year increment) | 254 | 1.03 (0.91, 1.17) | .647 | ||
| ATG (yes) | 254 | 1.56 (0.91, 2.69) | .106 | ||
| HLA identical | 254 | 0.37 (0.24, 0.56) | <.001 | 0.37 (0.21, 0.67) | .001 |
| Regimen (chemo only) | 254 | 1.54 (0.87, 2.74) | .137 | ||
| 1st Remission | 254 | 0.85 (0.54, 1.32) | .464 | ||
| ALL | 254 | 1.41 (0.88, 2.24) | .150 | ||
| PBSC only | 254 | 0.99 (0.64, 1.52) | .957 | ||
| Donor Sex match (yes) | 254 | 0.97 (0.64, 1.47) | .869 | ||
| Transplant Year (2002-2005) | 254 | 1.13 (0.74, 1.73) | .567 | ||
| Immune recovery parameter | |||||
| log(CD4) | 222 | 0.76 (0.64, 0.90) | .001 | ||
| log(CD8) | 222 | 0.94 (0.81, 1.08) | .374 | ||
| log(NK) | 222 | 1.02 (0.76, 1.37) | .897 | ||
| log(CD45) | 115 | 0.81 (0.65, 1.00) | .046 | ||
| log(PHA) | 164 | 0.73 (0.63, 0.85) | <.001 | 0.80 (0.69, 0.92) | .002 |
|
| |||||
| Relapse | |||||
|
| |||||
| HR of Parameter (95%CI) | P-value | HR of Parameter (95%CI) | P-value | ||
|
| |||||
| Baseline Factor | |||||
| Age (10-year increment) | 254 | 0.91 (0.73, 1.13) | .380 | ||
| ATG (yes) | 254 | 0.87 (0.38, 1.97) | .731 | ||
| HLA identical | 254 | 0.38 (0.18, 0.82) | .013 | ||
| Regimen (chemo only) | 254 | 2.63 (1.12, 6.22) | .027 | ||
| 1st Remission | 254 | 0.86 (0.39, 1.91) | .717 | ||
| ALL | 254 | 1.41 (0.62, 3.21) | .411 | ||
| PBSC only | 254 | 0.99 (0.47, 2.11) | .982 | ||
| Donor Sex match (yes) | 254 | 0.51 (0.24, 1.10) | .087 | ||
| Transplant Year (2002-2005) | 254 | 1.82 (0.83, 3.98) | .132 | ||
| Immune recovery parameter | |||||
| log(CD4) | 222 | 0.91 (0.65, 1.28) | .603 | ||
| log(CD8) | 222 | 1.06 (0.81, 1.39) | .646 | ||
| log(NK) | 222 | 1.14 (0.67, 1.92) | .632 | ||
| log(CD45) | 115 | 0.82 (0.55, 1.23) | .339 | ||
| log(PHA) | 164 | 1.02 (0.75, 1.38) | .903 | ||
The factors without estimates and p value were not significant in multivariable analysis.
ALC: absolute lymphocyte count; ATG: antithymocyte globulin; ALL: acute lymphoblastic leukemia; PBSC: peripheral blood stem cells; NK: natural killer; PHA: phytohemagglutinin.
Acknowledgments
We gratefully acknowledge the expert care provided to these patients by the fellows, housestaff and nurses of Memorial Sloan Kettering Cancer Center.
Financial disclosures: This research was supported in part by National Institutes of Health award number P01 CA23766 and NIH/NCI Cancer Center Support Grant P30 CA008748. Support was also received from NIH U01HL069315 (JDG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Support was also received from the Translational and Integrative Medicine Research Fund of Memorial Sloan-Kettering Cancer Center (JDG), Cycle for Survival (MAP), The New York Community Trust (MAP), and When Everyone Survives (MAP).
Footnotes
Conflict of Interest Disclosures: The authors report no conflicts of interest.
References
- 1.Small TN, Papadopoulos EB, Boulad F, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93:467–480. [PubMed] [Google Scholar]
- 2.Maury S, Mary JY, Rabian C, et al. Prolonged immune deficiency following allogeneic stem cell transplantation: risk factors and complications in adult patients. Br J Haematol. 2001;115:630–641. doi: 10.1046/j.1365-2141.2001.03135.x. [DOI] [PubMed] [Google Scholar]
- 3.Maraninchi D, Gluckman E, Blaise D, et al. Impact of T-cell depletion on outcome of allogeneic bone-marrow transplantation for standard-risk leukaemias. Lancet. 1987;2:175–178. doi: 10.1016/s0140-6736(87)90763-x. [DOI] [PubMed] [Google Scholar]
- 4.Savani BN, Mielke S, Rezvani K, et al. Absolute lymphocyte count on day 30 is a surrogate for robust hematopoietic recovery and strongly predicts outcome after T cell-depleted allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:1216–1223. doi: 10.1016/j.bbmt.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powles R, Singhal S, Treleaven J, Kulkarni S, Horton C, Mehta J. Identification of patients who may benefit from prophylactic immunotherapy after bone marrow transplantation for acute myeloid leukemia on the basis of lymphocyte recovery early after transplantation. Blood. 1998;91:3481–3486. [PubMed] [Google Scholar]
- 6.Kim DH, Kim JG, Sohn SK, et al. Clinical impact of early absolute lymphocyte count after allogeneic stem cell transplantation. Br J Haematol. 2004;125:217–224. doi: 10.1111/j.1365-2141.2004.04891.x. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti S, Brown J, Guttridge M, Pamphilon DH, Lankester A, Marks DI. Early lymphocyte recovery is an important determinant of outcome following allogeneic transplantation with CD34+ selected graft and limited T-cell addback. Bone Marrow Transplant. 2003;32:23–30. doi: 10.1038/sj.bmt.1704082. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, Sohn SK, Won DI, Lee NY, Suh JS, Lee KB. Rapid helper T-cell recovery above 200 × 10 6/l at 3 months correlates to successful transplant outcomes after allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;37:1119–1128. doi: 10.1038/sj.bmt.1705381. [DOI] [PubMed] [Google Scholar]
- 9.Berger M, Figari O, Bruno B, et al. Lymphocyte subsets recovery following allogeneic bone marrow transplantation (BMT): CD4+ cell count and transplant-related mortality. Bone Marrow Transplant. 2008;41:55–62. doi: 10.1038/sj.bmt.1705870. [DOI] [PubMed] [Google Scholar]
- 10.Kanda J, Chiou LW, Szabolcs P, et al. Immune recovery in adult patients after myeloablative dual umbilical cord blood, matched sibling, and matched unrelated donor hematopoietic cell transplantation Biol Blood Marrow. Transplant. 2012;18:1664–1676. doi: 10.1016/j.bbmt.2012.06.005. e1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Small TN, Avigan D, Dupont B, et al. Immune reconstitution following T-cell depleted bone marrow transplantation: effect of age and posttransplant graft rejection prophylaxis. Biol Blood Marrow Transplant. 1997;3:65–75. [PubMed] [Google Scholar]
- 12.Jakubowski AA, Small TN, Kernan NA, et al. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2011;17:1335–1342. doi: 10.1016/j.bbmt.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakubowski AA, Small TN, Young JW, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110:4552–4559. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Heijst JW, Ceberio I, Lipuma LB, et al. Quantitative assessment of T cell repertoire recovery after hematopoietic stem cell transplantation. Nat Med. 2013;19:372–377. doi: 10.1038/nm.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91:1083–1090. [PubMed] [Google Scholar]
- 16.Perales MA, Jenq R, Goldberg JD, et al. Second-line age-adjusted International Prognostic Index in patients with advanced non-Hodgkin lymphoma after T-cell depleted allogeneic hematopoietic SCT. Bone Marrow Transplant. 2010;45:1408–1416. doi: 10.1038/bmt.2009.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devine SM, Carter S, Soiffer RJ, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17:1343–1351. doi: 10.1016/j.bbmt.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasquini MC, Devine S, Mendizabal A, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30:3194–3201. doi: 10.1200/JCO.2012.41.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayraktar UD, de Lima M, Saliba RM, et al. Ex vivo T cell-depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2013;19:898–903. doi: 10.1016/j.bbmt.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg JD, Linker A, Kuk D, et al. T Cell-Depleted Stem Cell Transplantation for Adults with High-Risk Acute Lymphoblastic Leukemia: Long-Term Survival for Patients in First Complete Remission with a Decreased Risk of Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2013;19:208–213. doi: 10.1016/j.bbmt.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hobbs GS, Hamdi A, Hilden PD, et al. Comparison of outcomes at two institutions of patients with ALL receiving ex vivo T-cell-depleted or unmodified allografts. Bone Marrow Transplant. 2015;50:493–498. doi: 10.1038/bmt.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamari R, Chung SS, Papadopoulos EB, et al. CD34-Selected Hematopoietic Stem Cell Transplants Conditioned with Myeloablative Regimens and Antithymocyte Globulin for Advanced Myelodysplastic Syndrome: Limited Graft-versus-Host Disease without Increased Relapse. Biol Blood Marrow Transplant. 2015;21:2106–2114. doi: 10.1016/j.bbmt.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alpdogan O, Hubbard VM, Smith OM, et al. Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood. 2006;107:2453–2460. doi: 10.1182/blood-2005-07-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenq RR, King CG, Volk C, et al. Keratinocyte growth factor enhances DNA plasmid tumor vaccine responses after murine allogeneic bone marrow transplantation. Blood. 2009;113:1574–1580. doi: 10.1182/blood-2008-05-155697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly RM, Highfill SL, Panoskaltsis-Mortari A, et al. Keratinocyte growth factor and androgen blockade work in concert to protect against conditioning regimen-induced thymic epithelial damage and enhance T-cell reconstitution after murine bone marrow transplantation. Blood. 2008;111:5734–5744. doi: 10.1182/blood-2008-01-136531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly RM, Goren EM, Taylor PA, et al. Short-term inhibition of p53 combined with keratinocyte growth factor improves thymic epithelial cell recovery and enhances T-cell reconstitution after murine bone marrow transplantation. Blood. 2010;115:1088–1097. doi: 10.1182/blood-2009-05-223198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perales MA, Goldberg JD, Yuan J, et al. Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogeneic stem cell transplantation. Blood. 2012;120:4882–4891. doi: 10.1182/blood-2012-06-437236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castro-Malaspina H, Jabubowski AA, Papadopoulos EB, et al. Transplantation in remission improves the disease-free survival of patients with advanced myelodysplastic syndromes treated with myeloablative T cell-depleted stem cell transplants from HLA-identical siblings. Biol Blood Marrow Transplant. 2008;14:458–468. doi: 10.1016/j.bbmt.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kernan NA, Flomenberg N, Collins NH, O'Reilly RJ, Dupont B. Quantitation of T lymphocytes in human bone marrow by a limiting dilution assay. Transplantation. 1985;40:317–322. doi: 10.1097/00007890-198509000-00019. [DOI] [PubMed] [Google Scholar]
- 30.Keever-Taylor CA, Devine SM, Soiffer RJ, et al. Characteristics of CliniMACS(R) System CD34-enriched T cell-depleted grafts in a multicenter trial for acute myeloid leukemia-Blood and Marrow Transplant Clinical Trials Network (BMT CTN) protocol 0303. Biol Blood Marrow Transplant. 2012;18:690–697. doi: 10.1016/j.bbmt.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kernan NA, Bordignon C, Heller G, et al. Graft failure after T-cell-depleted human leukocyte antigen identical marrow transplants for leukemia: I. Analysis of risk factors and results of secondary transplants. Blood. 1989;74:2227–2236. [PubMed] [Google Scholar]
- 32.Almyroudis NG, Jakubowski A, Jaffe D, et al. Predictors for persistent cytomegalovirus reactivation after T-cell-depleted allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2007;9:286–294. doi: 10.1111/j.1399-3062.2007.00235.x. [DOI] [PubMed] [Google Scholar]
- 33.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 34.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan KM. Chronic graft-versus-host disease. Cancer Treat Res. 1990;50:79–98. doi: 10.1007/978-1-4613-1493-6_5. [DOI] [PubMed] [Google Scholar]
- 36.Copelan E, Casper JT, Carter SL, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. 2007;13:1469–1476. doi: 10.1016/j.bbmt.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 37.Bowman AW, Azzalini A. Applied Smoothing Techniques for Data Analysis: The Kernel Approach with S-Plus Illustrations. Oxford: Oxford University Press; 1997. [Google Scholar]
- 38.Niederwieser D, Gastl G, Rumpold H, Marth C, Kraft D, Huber C. Rapid reappearance of large granular lymphocytes (LGL) with concomitant reconstitution of natural killer (NK) activity after human bone marrow transplantation (BMT) Br J Haematol. 1987;65:301–305. doi: 10.1111/j.1365-2141.1987.tb06857.x. [DOI] [PubMed] [Google Scholar]
- 39.Roberts MM, To LB, Gillis D, et al. Immune reconstitution following peripheral blood stem cell transplantation, autologous bone marrow transplantation and allogeneic bone marrow transplantation. Bone Marrow Transplant. 1993;12:469–475. [PubMed] [Google Scholar]
- 40.Heining C, Spyridonidis A, Bernhardt E, et al. Lymphocyte reconstitution following allogeneic hematopoietic stem cell transplantation: a retrospective study including 148 patients. Bone Marrow Transplant. 2007;39:613–622. doi: 10.1038/sj.bmt.1705648. [DOI] [PubMed] [Google Scholar]
- 41.Oshrine BR, Li Y, Teachey DT, Heimall J, Barrett DM, Bunin N. Immunologic recovery in children after alternative donor allogeneic transplantation for hematologic malignancies: comparison of recipients of partially T cell-depleted peripheral blood stem cells and umbilical cord blood. Biol Blood Marrow Transplant. 2013;19:1581–1589. doi: 10.1016/j.bbmt.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buhlmann L, Buser AS, Cantoni N, et al. Lymphocyte subset recovery and outcome after T-cell replete allogeneic hematopoietic SCT. Bone Marrow Transplant. 2011;46:1357–1362. doi: 10.1038/bmt.2010.306. [DOI] [PubMed] [Google Scholar]
- 43.Pao M, Papadopoulos EB, Chou J, et al. Response to pneumococcal (PNCRM7) and haemophilus influenzae conjugate vaccines (HIB) in pediatric and adult recipients of an allogeneic hematopoietic cell transplantation (alloHCT) Biol Blood Marrow Transplant. 2008;14:1022–1030. doi: 10.1016/j.bbmt.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barba P, Ratan R, Cho C, et al. The Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) Predicts Outcomes in Patients with Acute Myeloid Leukemia and Myelodysplastic Syndromes Receiving CD34+ Selected Grafts for Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016 doi: 10.1016/j.bbmt.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kollman C, Spellman SR, Zhang MJ, et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood. 2016;127:260–267. doi: 10.1182/blood-2015-08-663823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ho VT. Ex Vivo T Cell Depletion of Allogeneic PBSC as Acute and Chronic GVHD Prophylaxis after Myeloablative HCT: Time to Reconsider? Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2011.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.