Abstract
The ability to inhibit prepotent but context- or goal-inappropriate responses is essential for adaptive self-regulation of behavior. Deficits in response inhibition, a key component of impulsivity, have been implicated as a core dysfunction in a range of neuropsychiatric disorders such as ADHD and addictions. Identification of genetically transmitted variation in the neural underpinnings of response inhibition can help to elucidate etiological pathways to these disorders and establish the links between genes, brain, and behavior. However, little is known about genetic influences on the neural mechanisms of response inhibition during adolescence, a developmental period characterized by weak self-regulation of behavior. Here we investigated heritability of ERPs elicited in a Go/No-Go task in a large sample of adolescent twins assessed longitudinally at ages 12, 14, and 16. Genetic analyses showed significant heritability of inhibition-related frontal N2 and P3 components at all three ages, with 50 to 60% of inter-individual variability being attributable to genetic factors. These genetic influences included both common genetic factors active at different ages and novel genetic influences emerging during development. Finally, individual differences in the rate of developmental changes from age 12 to age 16 were significantly influenced by genetic factors. In conclusion, the present study provides the first evidence for genetic influences on neural correlates of response inhibition during adolescence and suggests that ERPs elicited in the Go/No-Go task can serve as intermediate neurophysiological phenotypes (endophenotypes) for the study of disinhibition and impulse control disorders.
Keywords: response inhibition, no-go, heritability, brain, twins
1. Introduction
1.1. ERP correlates of response inhibition and their functional significance
Response inhibition (RI), or ability to voluntarily suppress a prepared but context- or goal-inappropriate action plays a critical role in the self-regulation of adaptive goal-directed behavior. Response inhibition is considered an essential facet of executive functioning and cognitive control (Chambers et al., 2009; Dalley et al., 2011), and weak response inhibition constitutes a key component of impulsivity. The neural dynamics underlying response inhibition has been extensively studied using brain event-related potential (ERPs),which represent a direct real-time measure of neuronal activity and permit the discrimination between consecutive stages of cognitive processing that unfold at the time scale of tens to hundreds of milliseconds. Most ERP studies of response inhibition employ Go/No-Go tasks in which participants are instructed to respond to Go stimuli and to withhold their responses to No-Go stimuli, as well as stop-signal tasks in which participants have to stop the response triggered by a Go stimulus if a stop stimulus is presented shortly after the Go stimulus.
There are two ERP components that have been consistently associated with response inhibition. The first is the midline frontal N2 component which is observed at 200–270 ms post-stimulus in successful No-Go trials (Eimer, 1993; Falkenstein et al., 1999; Jodo and Kayama, 1992; Kirmizi-Alsan et al., 2006; Nieuwenhuis et al., 2004; Nieuwenhuis et al., 2003; van Boxtel et al., 2001). The frontal No-Go N2 component, interpreted as a correlate of inhibition in earlier studies, more recently has been viewed as manifestation of a conflict between incompatible response tendencies. The conflict account of N2 suggests that N2 reflects detection of a conflict caused by the competition between the execution and the inhibition of a single response (Donkers and van Boxtel, 2004; Gomez et al., 2007; Nieuwenhuis et al., 2003; Smith et al., 2010).
The second ERP component associated with successful withholding of a response is the No-Go P3 wave peaking in the time window of 250–500 ms which is substantially enhanced in frontal and central areas on No-Go trial relative to Go trials. This effect, labeled as “No-Go anteriorization” of P3 (Fallgatter and Strik, 1999; Pfefferbaum et al., 1985; Roberts et al., 1994), has been proposed as a robust topographical marker of the activation of frontal circuitry related to response inhibition (Fallgatter et al., 1997). In contrast to N2, there is stronger evidence linking the No-Go P3 to response inhibition (Bekker et al., 2004; Bruin and Wijers, 2002; Donkers and van Boxtel, 2004; Smith et al., 2008). Although studies comparing neural correlates of withholding of overt and covert responses suggested that No-Go P3 is related to both cognitive and motor inhibition, the P3 effect was greatly enhanced when motor inhibition was required. Thus, No-Go N2 and P3 represent distinct and temporally dissociable processes related to response inhibition: the N2 is implicated in the detection of conflict between a prepared response and the need to inhibit it, whereas No-Go P3 is seen as a manifestation of motor inhibition (Randall and Smith, 2011; Smith et al., 2008).
1.2. Neuroanatomical substrates of response inhibition: ERP and fMRI studies
Human lesion studies have shown that the medial prefrontal cortex, including the anterior cingulate and the supplementary motor areas, plays a key role in both response preparation and inhibition (Verfaellie and Heilman, 1987). Studies using source localization methods as well as studies combining fMRI and ERP measurement in the same Go/No-Go tasks have consistently associated the No-Go N2 component with the activation of the anterior cingulate cortex (ACC)(Garavan et al., 2002; Mathalon et al., 2003; Swainson et al., 2003; van Veen and Carter, 2002a, b). The sources for No-Go P3 were found in the ACC, premotor areas, and the dorsolateral prefrontal cortex (Dias et al., 2003; Fallgatter et al., 2002; Kiefer et al., 1998). More recently, neural substrates of N2 and P3 were further clarified by multimodal imaging studies capitalizing on high temporal resolution of EEG and high spatial resolution of fMRI. A study using separate ERP and fMRI recordings in the same subjects showed strong correlations between No-Go N2 and activation in the caudal part of the ACC and executive control regions in the dorsolateral prefrontal cortex (Mathalon et al., 2003). In a study with simultaneous EEG and fMRI recording during a stop-signal task (Huster et al., 2011), N2/P3 ERP complex produced by successful response inhibition was associated with activation in a functional network of regions known from previous fMRI studies to be involved in response inhibition, most notably, the anterior midcingulate cortex, pre-supplementary motor area, and the anterior insula. A subsequent review (Huster et al., 2013) of source localization and imaging studies provided a more detailed account of separate brain regions implicated in N2 and P3 generation. Converging evidence suggests that N2 reflects activity in the medial and lateral prefrontal cortices, with the medial source located in the anterior midcingulate region, in line with ERP source localization studies consistently implicating a generator in the ACC (Huster et al., 2013). Furthermore, in another study N2, but not P3, correlated with morphological variation in the anterior cingulate region (Huster et al., 2014). Frontocentral P3 showed associations with a broader network of regions including medial frontal and precentral regions, including the pre-SMA, anterior insulae, and temporoparietal regions. Interestingly, both N2 and P3 correlated with activation of the midcingular region, although N2 sources were located more anteriorly than P3 sources (Huster et al., 2013).
1.3. Developmental changes in the neural correlates of response inhibition
Structural and functional imaging data suggest that brain regions supporting cognitive control, most notably, the anterior cingulate (ACC) and dorsolateral prefrontal cortex (DLPFC), continue to develop during adolescence(Luna et al., 2010; Rubia, 2013). Consistent with this evidence, ERP studies of response inhibition indicate substantial differences in inhibition-related brain activity between children and adults. A comparison of children at age 9 and young adults with respect to ERPs in a Go/No-Go task (Jonkman et al., 2003) found a well-pronounced N2 component that was larger in children compared with adults, however, the No-Go P3 was absent in children. These results were corroborated in a subsequent study with an extended age range (Jonkman, 2006), a cross-sectional study comparing children at age 6–7 and 9–10 years and young adults suggested a decrease of N2 amplitude and increase of P3 with age. Convergent results were obtained in another cross-sectional study of children and adults: the N2 component assessed in an auditory Go/No-Go task diminished with age, while No-Go P3 showed a substantial increase (Johnstone et al., 2005). From the conflict monitoring perspective, the decrease in N2 amplitude may reflect a decrease in the level of experienced conflict, perhaps due to more efficient processing in the ACC (Jonkman, 2006). It is important to note that the same developmental pattern was observed in studies using visual and auditory stimuli, suggesting that No-Go ERP components and their developmental changes represent general, modality-independent neural processing underlying response inhibition (Johnstone et al., 2005; Jonkman, 2006). However, another study of children of 5–16 years of age reported an increase in both N2 and P3 components with age (Lewis et al., 2006). This discrepancy may be related to task differences (the latter study involved using negative emotion induction) as well as small sample sizes in these studies. Overall, these studies suggest that neurophysiological mechanisms of response inhibition continue to develop during adolescence and even young adulthood, and suggest that No-Go N2 and P3 potentials may have distinct developmental trajectories.
Findings from developmental ERP studies are generally consistent with functional magnetic resonance imaging (fMRI) studies of response inhibition. These studies suggest that functional activation underlying inhibitory control in lateral and medial frontal regions increases with age (Rubia, 2011; Rubia et al., 2000; Rubia et al., 2006) and provide evidence for increasing inter-regional connectivity within task-relevant fronto-striatal and fronto-parieto-temporal networks (Rubia, 2013; Rubia et al., 2007).
1.4. Neural correlates of response inhibition, Individual differences, and psychopathology
Deficits in response inhibition in adolescence are associated with increased risk for maladaptive behaviors and psychopathology, most notably, a range of “externalizing” neuropsychiatric disorders characterized by poor inhibitory control and impulsivity, such as ADHD, conduct and antisocial disorders, and substance abuse (Gorenstein and Newman, 1980; Young et al., 2000). In particular, an influential theory of attention deficit and hyperactivity disorder (ADHD) posits that response inhibition deficit plays a central role in the disorder etiology, whereas deficits in working memory, attention, and emotional regulation can be secondary to inhibition deficit (Barkley, 1997). Deficits in inhibitory control can be a common factor underlying a constellation of impulsive, disruptive, and reckless behaviors, demonstrated in many epidemiological studies and labeled as behavioral disinhibition (Gorenstein and Newman, 1980; Iacono et al., 2008; Young et al., 2009; Young et al., 2000), externalizing factor (Krueger et al., 2002; Krueger et al., 2007), or psychological dysregulation (Tarter et al., 2003).
It is reasonable to expect that this spectrum of disinhibited behaviors is mediated by abnormalities in the neural mechanisms of RI, and increased prevalence of these behaviors during adolescence may be related to immaturity of the neurophysiological substrates of response inhibition due to their late development. This perspective on adolescent disinhibitory psychopathology is supported by evidence for abnormal No-Go N2 and P3 components in externalizing spectrum disorders. Reduced No-Go P3 has been reported in both children and adult individuals with ADHD (Brandeis et al., 2002; Cheung et al., 2015; Fallgatter et al., 2004; Overtoom et al., 1998) (Helenius et al., 2011; McLoughlin et al., 2010; Tye et al., 2013; Wild-Wall et al., 2009; Woltering et al., 2013). Abnormal No-Go ERP components have been found in children with externalizing disorders: the clinical sample had larger N2 magnitudes and smaller frontal P3 magnitudes compared with controls (Woltering et al., 2011). A study of juvenile delinquents with antisocial personality characteristics reported amplitude reduction of both N2 and P3 No-Go components (Guan et al., 2015). A study of adult psychopaths (Kiehl et al., 2000) showed a marked reduction of the N2 component and a lack of No-Go P3 effect, with psychopaths showing the opposite direction of the Go versus No-Go difference compared to controls. These results of ERP studies converge with findings from fMRI studies of response inhibition showing reduced activation of inhibition-related network in adolescent substance users (Norman et al., 2011; Whelan et al., 2012).
Furthermore, behavioral correlates of altered neural mechanisms of response inhibition as indicated by No-Go ERPs are not limited to externalizing-spectrum behaviors: inhibitory deficits may be associated with a broader range of behaviors characterized by poor self-control such as overeating, as evidenced by the finding of reduced No-Go P3 component (Reyes et al., 2015) and its diminished anteriorization (Kamijo et al., 2012) in obese children, which was paralleled by poorer inhibitory performance. However, the opposite extreme, i.e. overactive response inhibition-related neural activation is not necessarily beneficial: an ERP study in children suggested that increased N2 (linked to the anterior cingulate cortical activity in that study) might be a biomarker inhibited social behavior and increased risk for later anxiety problems (Lamm et al., 2014). Taken together, these data suggest a possibility that an intermediate level of inhibition-related activity may be most beneficial for adaptive behavior because it provides an optimal combination of control and flexibility of response production and suppression. The relationships between neural mechanisms of response inhibition and individual differences in normal-range behavior remains unclear due to the scarcity of studies, especially those using developmental designs.
Finally, emerging evidence suggests that abnormalities in ERP correlates of response inhibition may be associated with familial and, presumably, genetic risk for addictive disorders. Kamarajan et al. (2005a) reported significantly reduced No-Go P3 along with the relatively less anteriorized topography in alcoholics. Further analysis of ERPs in family members of alcoholic probands revealed a similar deficit in offspring of alcoholics, suggesting that reduced No-Go P3 can be an endophenotypic marker of risk for the development of alcoholism and related disorders (Kamarajan et al., 2005b).
In summary, No-Go P3 showed more consistent impairments in clinical and at-risk populations, whereas findings for N2 have been more variable.
1.5. Response inhibition-related ERPs as intermediate phenotypes linking genes and behavior
Numerous twin, family, and adoption studies demonstrated that individual differences in personality, behavior, and psychopathology are strongly influenced by genetic factors. However, efforts to identify specific genes so far yielded modest results; furthermore, when such genes are identified, the mechanisms by which they influence behavioral phenotypes remain largely unknown. It has been suggested that these issues can be addressed by shifting the focus of genetic studies from complex (and likely heterogeneous) behavioral phenotypes to brain-based intermediate phenotypes (Vogel, 1981; Vogel et al., 1979), sometimes referred to as “endophenotypes” (Gottesman and Gould, 2003). By focusing on neurobiological processes that are more proximal to immediate effects of genes than complex behavioral phenotypes, this approach may both facilitate the identification of relevant genes and, most importantly, clarify the mechanisms and pathways mediating the effects of these genes on behavior. One important attribute of such an intermediate phenotype is heritability.
We have previously demonstrated significant heritability of No-Go N2 and P3 components in adults and proposed that these neural markers of response inhibition can serve as endophenotypes for genetic studies of disorders characterized by neurobehavioral disinhibition (Anokhin et al., 2004). However, little is known about heritability of the neural correlates of response inhibition during adolescence. This is a significant gap in knowledge because this developmental period is characterized by relative immaturity of the prefrontal cortex and increased risk for impulse control disorders. Therefore, understanding the developmental course of the neural mechanisms of response inhibition and their genetic end environmental determinants can provide important knowledge about the etiological mechanisms of these disorders.
1.6. Integration of genetic and developmental approaches and aims of the present study
Investigation of genetic influences during a period of active development presents a number of challenges due to complex interplay between developmental and genetic factors. First, the role of genetic factors may change with development (change of heritability). Furthermore, the structure of genetic influences may also change with development: some genes influencing inhibition may be expressed at one but not another developmental stage (change in genetic architecture). Consequently, even when genetic influences are of the same magnitude at different ages, the nature of these genetic influences (i.e. the set of genes influencing the phenotype) may be different: some genes may be active only at earlier stages of development and then “turned off”, while others may become active at later developmental stages. Finally, genetic factors can affect not only the overall expression of a phenotype at different points in development, but also the developmental process itself, resulting in genetically determined individual differences in developmental trajectories (heritability of change).
This complex set of interrelated problems can be approached only by the integration of genetic and developmental approaches within a longitudinal twin design, which is both developmentally and genetically informative. Here we use data from an ongoing population-based longitudinal study of adolescent twins to address a number of fundamental questions: Do neural correlates of response inhibition show developmental changes during adolescence? Do individual differences in these neural phenotypes show developmental stability despite population-level changes in mean values? Are these individual differences heritable? Do same or different genetic factors influence ERP traits at different ages? Does heritability vary significantly as a function of age (change of heritability)? Are individual differences in the rate of development influenced by genes (heritability of change)?
2. Method
2.1. Participants
Subjects were adolescent twins participating in a longitudinal study of Genetics, Neurocognition, and Adolescent Substance Abuse (GNASA). All participants were recruited from the local population using a state birth records database, therefore, the sample is largely representative of the general population. Exclusion criteria were minimal and included a history of serious head trauma and health conditions precluding a laboratory visit or the ability to perform the experimental tasks (e.g. severe visual impairment or mental retardation). The first assessment (baseline) was conducted at age 12, and follow up assessments were conducted at ages 14 and 16. The Continuous Performance Task (CPT) described below was administered to a total of 743 subjects at age 12, 614 subjects at age 14, and 404 subjects at age 16 (for a more detailed description of the sample, see Table 1). Zygosity was determined using a set of 160 DNA markers, an interview administered to the twins’ parents, and research assistants’ ratings of twins’ physical similarity. The study was approved by Washington University Institutional Review Board, and written informed assent and consent were obtained from adolescent participants and their parents, respectively, after complete description of the study to the subjects and their parents.
Table 1.
Sample characteristics across longitudinal waves.
| Assessment wave | Total n | % female | Age (M±SD) | Complete MZ pairs | Complete DZ pairs |
|---|---|---|---|---|---|
| 1 | 743 | 47.8 | 12.58±.31 | 157 | 179 |
| 2 | 614 | 48.5 | 14.61±.28 | 142 | 153 |
| 3 | 404 | 47.1 | 16.61±.29 | 91 | 108 |
2.2. Materials and procedure
Participants were administered a slightly modified Go/No-Go version of the Continuous Performance Test (CPT) that has been shown to be a reliable measure of response inhibition (Fallgatter et al., 1997; Fallgatter et al., 1999; Fallgatter and Strik, 1999). A series of letters was presented sequentially, one at a time, for 0.2 s with inter-stimulus interval of 2 s (Fig. 1). The subjects were instructed to respond as quickly as possible to a letter X preceded by a letter O (O-X combination) by pressing a button on a response pad with the right index finger, but to withhold their response when the letter O was followed by any other letter than X (O-non-X combination). Response speed and accuracy were equally emphasized. A total of 400 letters were presented, of which 80 (20%) were cue stimuli (the letter “O”), 40 (10%) were Go stimuli (X following O), 40 (10%) were No-Go stimuli (any letter other than X following O), and the remaining 240 stimuli were distracters (12 letters not including O or X). Thus, the O-X combination represented a Go trial, whereas any O-not-X combination such as O-Z, O-K, etc. represented a No-Go trial, and there were a total of 40 O-X (Go) and 40 O-non-X (No-Go) trials in the task presented in a pseudo-random order. The letter O served as a warning cue informing the subject that the next stimulus is likely to be a Go signal and thus triggered a motor preparation and response prepotency. A No-Go stimulus (any letter other than X) presented after O required withholding of the pre-activated response. It is important to note that equal probability of Go and No-Go stimuli ensured that Go versus No-Go differences are not confounded with oddball effects that are typically observed when stimuli differ in their frequency.
Fig. 1.
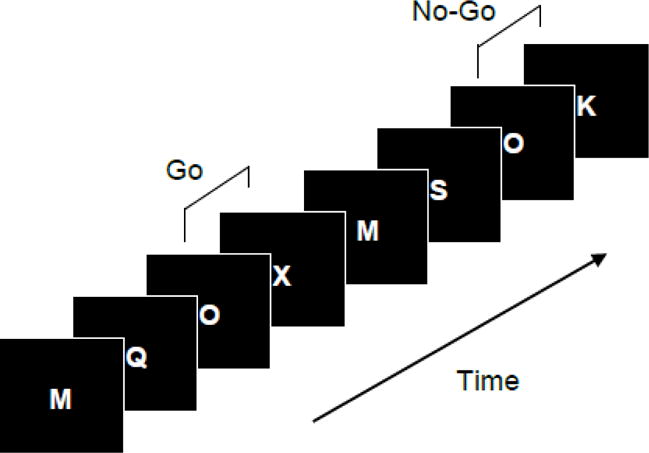
The Go/No-Go Continuous Performance Task (CPT). An X following O is a target stimulus requiring a speeded response, while any other letter following O is a No-Go stimulus. Since O is relatively rare (20%), it serves as a warning stimulus triggering motor preparation and thus creating a response prepotency.
2.3. EEG recording and ERP analysis
The EEG was recorded from 30 scalp locations according to the extended 10–20 system using an elastic cap with Ag/AgCl electrodes and a ground electrode on the forehead, with high- and low-pass filters set at 0.05 and 100 Hz, respectively. The left mastoid served as reference, and an averaged mastoid reference was digitally computed off-line using the right mastoid recording as a separate channel. Vertical electro-oculogram recording was used for eyeblink artifact correction using a regression-based procedure (Semlitsch et al., 1986). After screening for artifacts, EEG signals were subjected to 30 Hz low-pass filtering, and 1000 ms epochs time-locked to the response were extracted (from -200 to 900 ms relative to the stimulus onset), baseline-corrected using a 200 ms baseline, and averaged separately for correct Go and No-Go trials, i.e. correct button presses and withholding of the response, respectively (trials with missed responses to Go stimuli and false alarm responses to No-Go stimuli were excluded). The P3 peak was detected as the most positive voltage value within 300–500 ms window, and scored as absolute amplitude relative to baseline. The NoGo N2 peak was detected as the most negative voltage value within 190–360 ms window following the stimulus. N2 amplitude was scored as peak-to-peak difference between N2 and the amplitude of the immediately preceding positive trough as in previous studies (Nieuwenhuis et al., 2003). The use of different scoring methods for P3 and N2 was driven by the following considerations: P3 in the present task is a high-amplitude late component that can be reliably evaluated relative to the pre-stimulus baseline. In contrast, N2 is a smaller component overlapping with the ascending limb of the larger P3 component. Therefore, absolute N2 amplitude is strongly influenced by the rate of increase in P3 positivity, such that earlier onset and/or steeper increase in P3 lead to greater reduction of absolute N2 amplitude. Measuring N2 relative to the preceding positive trough mitigates this confounding as indicated by lower correlation between peak-to-peak N2 and No-Go P3 amplitudes (r= 0.11) relative to the correlation between absolute N2 amplitude and No-Go P3 amplitude (r= 0.43), i.e. a reduction from 18% to 1% of shared variance.
2.4. Genetic analysis and assessment of heritability
Genetic analyses of twin data were conducted by fitting linear structural equation models (SEM) using the Mx package (Neale et al., 2002), which is a standard approach in twin genetic research (Neale and Cardon, 1992; Rijsdijk and Sham, 2002). Since our data included longitudinal observations, we fit a trivariate Cholesky model (see Neale and Cardon, 1992) with measurements taken at ages 12, 14, and 16 entered as separate variables (Fig. 5A). This multivariate approach allows one to estimate heritability of ERP phenotypes at each age, to estimate the extent of genetic overlap across ages (i.e. to what extent the same genetic factors influence these phenotypes at different ages) and whether there are novel genetic influences at later ages, and to test for age differences in heritability.
Fig. 5.
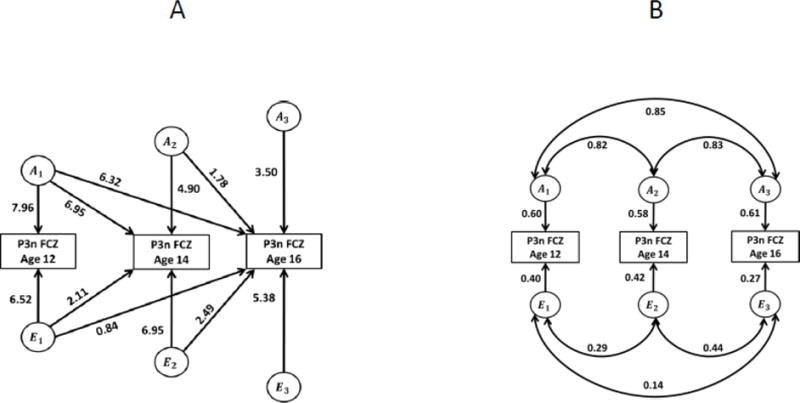
Path diagrams for structural equation models, No-Go P3 amplitude. A. Cholesky (triangular decomposition) model. Rectangles are observed (measured) ERP phenotypes, circles are latent factors (A, additive genetic; E, non-shared environmental). Arrows show causal paths from latent factors to phenotypes. Note that the paths from genetic factors at age 12 lead to the phenotype at all three ages, allowing for the possibility of genetic overlap across ages (i.e. continuity of genetic influences). Additional paths at ages 14 and 16 allow for the possibility of age-specific genetic influences (i.e. new genetic influences emerging in the course of development). Paths from the environmental factors have similar interpretation. B. Standardized solution showing proportions of variance attributable to genetic and environmental factors at each age, and the genetic and environmental correlations. Same models for other ERP phenotypes (N2 and Go P3) are presented in Supplementary Material.
Structural equation models of twin data assume that phenotypic variance arises from the following factors: additive genetic influences (A), non-additive genetic influences (D) including within-locus allelic interaction (dominance) and between-locus interaction (epistasis), environmental influences shared by family members (C), and individually unique (unshared) environmental influences (E). It is important to note that A, D, and C increase similarity within twin pairs, whereas E decreases it. When using only data from twin pairs reared together, it is only possible to test three of these four components simultaneously, and a decision regarding whether to test an ADE or an ACE model is made based upon the observed twin correlations (see Rijsdijk and Sham, 2002). Heritability, which can be estimated using these models, is the percentage of the total variance of the trait attributable to genetic factors. For a more detailed introduction into the genetic methods that can be applied to psychophysiological traits, we refer the reader to methodological and review papers (Anokhin, 2014; Boomsma et al., 2002; Posthuma et al., 2003; Rijsdijk and Sham, 2002; van Dongen et al., 2012).
Path coefficients were estimated using the method of maximum likelihood, and the goodness of model fit was indicated by −2 times the log likelihood (−2LL). As described elsewhere (Neale and Cardon, 1992; Sham, 1998), the fit of nested submodels was tested by dropping individual paths from the full model (e.g., genetic overlap across assessments, or novel genetic influences at later ages), with the significance of individual paths tested by comparing the fit of the restricted submodel with the fit of the more general model using a χ2 test with degrees of freedom corresponding to the difference in the degrees of freedom between two models (e.g., df=1 if only one parameter is dropped in the restricted model). If dropping a path significantly reduced the goodness of fit (the change in χ2 was significant), the path was retained in the model, otherwise the more parsimonious model was chosen (i.e. the one that accounted for the variance equally well, but with a fewer number of parameters). The fit of the nested submodels was also assessed through Akaike’s Information Criterion (AIC), where AIC= χ2 − 2df (see Neale and Cardon, 1992). Lower AIC values indicate better fit.
In the case of longitudinal data, structural equation modeling permits not only the assessment of heritability at each age, but also the estimation of the extent of the overlap between genetic factors influencing the trait at different ages. For example, genetic factors influencing an ERP variable at age 12 may also influence it at ages 14 and 16, which results in a significant genetic correlation across ages. Such genetic correlations, along with environmental correlations, give rise to cross-age phenotypic correlations, i.e. longitudinal stability. However, in addition to these age-invariant influences, there may be novel genetic influences emerging at later ages due to e.g. developmental changes in gene expression. These genetic factors contribute to the overall heritability of a trait but not to genetic correlation across ages. Such novel genetic (and environmental) influences may explain the fact that cross-age phenotypic correlation is far from perfect. The Cholesky models utilized in the present analyses assessed the significance of both genetic continuity and novel genetic influence across adolescence.
To test for significant differences in heritability across age, we compared the goodness of fit of alternative Cholesky models in which heritability was constrained to be equal or allowed to vary across ages. To examine genetic and environmental influences on the extent of developmental changes, we computed individual age difference scores for ERP variables by subtracting values at age 12 from the corresponding values at age 16 and used them as new phenotypes in genetic analysis. The main advantage of this approach is that it does not make any assumptions about the shape of the developmental trajectory (an alternative approach would be using a biometric growth modeling, however, with only three time points modeling the developmental trajectory could be inaccurate).
A detailed description of genetic model fitting and comparison of alternative models is presented in the Supplementary Material.
3. Results
3.1. Go versus No-Go differences
Averaged ERP waveforms elicited in Go and No-Go trials are shown in Fig. 2 for selected midline electrodes and in Supplementary Fig. 1 for all 30 scalp locations. Consistent with previous studies, we observed a striking difference between ERP responses to Go and No-Go stimuli. It is important to note that because Go and No-Go stimuli were equiprobable, the No-Go versus Go ERP differences in the present study are not confounded with the frequency (oddball) effect, as may be the case in Go/No-Go tasks using frequent Go and infrequent No-Go stimuli. No-Go trials produced a prominent frontal N2 and P3 components that were virtually absent in Go trials. The scalp distribution of ERP components (Fig. 2, 3, Supplementary Fig. 1) shows a distinctive “anteriorization” of the P3 component in the No-Go condition, consistent with previous studies (Fallgatter and Strik, 1999; Roberts et al., 1994). In contrast, Go stimuli elicited a P3 component peaking at the mid-parietal (Pz) location, with its morphology and scalp topography being very similar to a classical oddball P3.
Fig. 2.
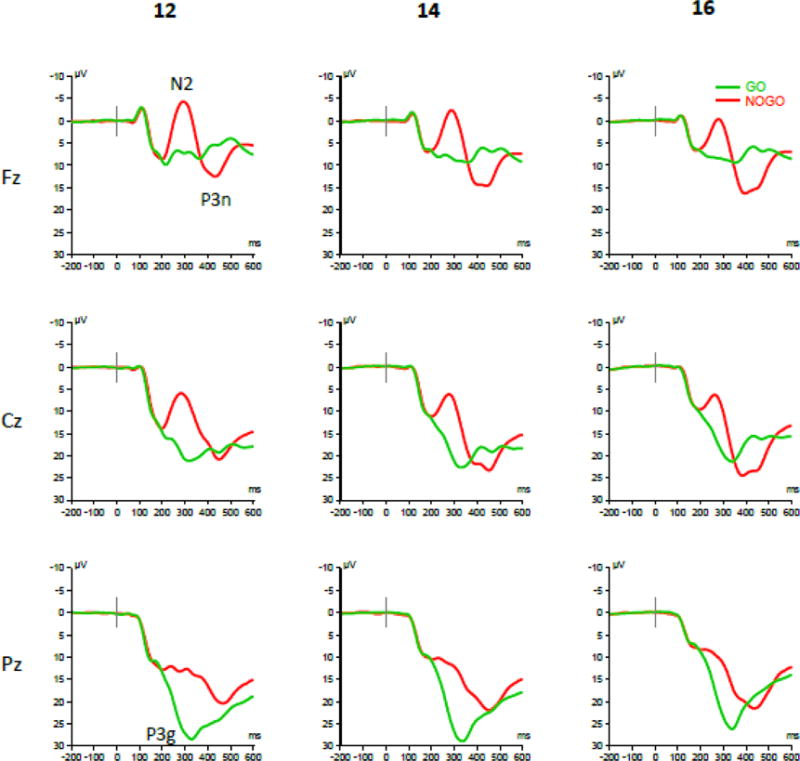
Event-related brain potentials (ERPs) elicited at midline frontal (Fz), central (Cz), and parietal (Pz) scalp locations at ages 12, 14, and 16. No-Go stimuli produced a prominent frontal N2 potential and “anteriorized” P3 potential (P3n), whereas Go stimuli elicited a P3 wave peaking in the mid-parietal area (P3g), similar to a classical oddball P3.
Fig. 3.
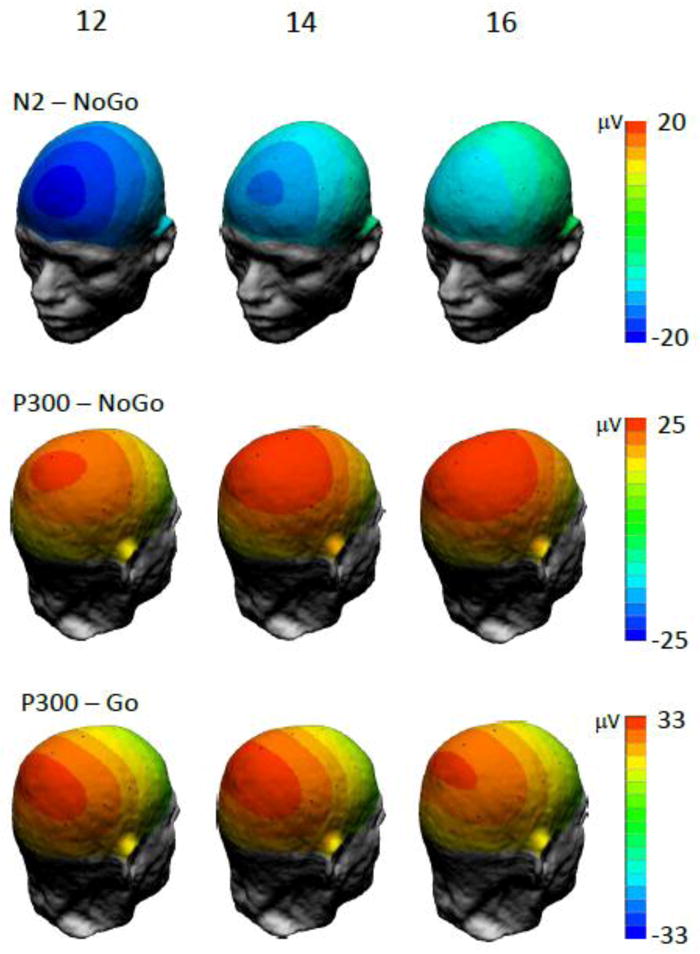
Scalp potential maps of ERPs in No-Go and Go conditions at ages 12, 14, and 16.
3.2. Developmental changes
Different ERP components showed distinct and highly significant patterns of age-related changes (Fig 4). The frontal No-Go N2 component decreased with age (F[1,363]=507.2, p<.001), whereas No-Go P3 increased with age (F[1,363]=31.1, p<.001). The parietal Go-P3 decreased with age (F[1,363]=115.3, p<.001).
Fig. 4.
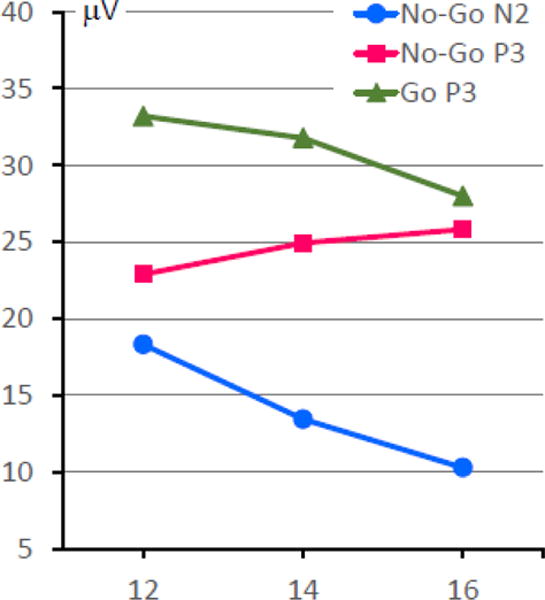
Age-related changes in the amplitudes of ERP components (all trends are significant, p<.001).
As expected, task performance (accuracy and speed, Mean±SD) showed substantial improvement with age: the rate of missed responses decreased from 12.1±13.9 to 2.5±4.9%, the rate of false alarms (responses in No-Go trials) dropped from 9.1±10.4 to 5.3±6.0%, and the mean reaction time in Go trials decreased from 362±78 to 324±54 ms (all effects of age were highly significant, p<.001).
3.3. Gender differences
Analysis of gender differences showed that girls had slightly larger N2 components (F[1,362]=4.39, p=.04. Follow-up analyses by age showed that gender differences were non-significant at age 12 but reached significance at ages 14 and 16 (p<.05), however, the effect size was small (Cohen’s d=.21 and .25, respectively). There was no significant main effect of gender on No-Go or Go P3.
3.4. Longitudinal stability of individual differences
All ERP components showed highly significant correlations between the same measures at different ages ranging from .53 to .71, indicating temporal stability of individual differences (Table 2). In other words, rank ordering of individuals remained relatively stable despite the systematic age-related trends in absolute values described above.
Table 2.
Longitudinal test-retest correlations for ERP amplitudes.
| Retest interval | n | No-Go N2 | No-Go P3 | Go P3 |
|---|---|---|---|---|
| 12 – 14 | 606 | .58 | .58 | .64 |
| 14 – 16 | 281 | .61 | .64 | .74 |
| 12 – 16 | 292 | .51 | .54 | .61 |
Note: all correlations are significant at p<.001 level.
3.5. Twin correlations and heritability
Twin-pair correlations for frontal No-Go N2 and P3 and parietal Go P3 amplitudes are shown in Table 3. The pattern of MZ and DZ correlations suggests 1) significant familiality for all ERP measures (rMZ was statistically significant in all cases), 2) significant genetic influences for all measures at all ages (rMZ>rDZ), and 3) the possibility of non-additive genetic influences, particularly at age 16 (rMZ>2 rDZ). Below we describe the results of fitting and comparing different models for each of the ERP variables. Path diagrams for different types of models are illustrated in Fig. 5 using No-Go P3 as an example (a full set of models and the corresponding estimated path coefficients is presented in Supplementary Fig. 2).
Table 3.
Twin correlations and heritability estimates (with 95% confidence intervals) for longitudinal measures of No-Go and Go ERP components.
| ERP phenotype | Age | rMZ | rDZ | Additive Genetic (Heritability) | Non-shared Environmental |
|---|---|---|---|---|---|
| N2, FCZ | 12 | 0.60* (0.49 – 0.69) | 0.33* (0.19 – 0.45) | 0.62* (0.53 – 0.69) | 0.38* (0.31 – 0.47) |
| 14 | 0.56* (0.43 – 0.66) | 0.26* (0.10 – 0.40) | 0.56* (0.45 – 0.65) | 0.44* (0.35 – 0.55) | |
| 16 | 0.52* (0.30 – 0.69) | 0.08 (−0.14 – 0.29) | 0.49* (0.33 – 0.63) | 0.51* (0.37 – 0.67) | |
| P3N, FCZ | 12 | 0.60* (0.48 – 0.69) | 0.28* (0.13 – 0.41) | 0.60* (0.50 – 0.68) | 0.40* (0.32 – 0.50) |
| 14 | 0.55* (0.42 – 0.65) | 0.32* (0.17 – 0.46) | 0.58* (0.47 – 0.67) | 0.42* (0.34 – 0.53) | |
| 16 | 0.64* (0.46 – 0.77) | 0.06 (−0.16 – 0.27) | 0.61* (0.45 – 0.73) | 0.39* (0.27 – 0.55) | |
| P3G, PZ | 12 | 0.60* (0.49 – 0.69) | 0.28* (0.13 – 0.41) | 0.62* (0.53 – 0.69) | 0.38* (0.31 – 0.47) |
| 14 | 0.66* (0.55 – 0.74) | 0.21* (0.06 – 0.37) | 0.63* (0.53 – 0.71) | 0.37* (0.29 – 0.47) | |
| 16 | 0.66* (0.47 – 0.78) | 0.22 (−0.00 – 0.42) | 0.63* (0.48 – 0.74) | 0.37* (0.26 – 0.52) |
N2, FCZ: amplitude of the No-Go N2 component at the fronto-central midline electrode (FCz); P3N, FCZ: amplitude of the No-Go P3 component at FCz; P3G, PZ: amplitude of the Go P3 component at the parietal midline electrode (Pz). rMZ and rDZ are intrapair correlations for MZ and DZ twin pairs, respectively (95% confidence intervals are shown in brackets);
p<.05;
a2 is the proportion of total phenotypic variance explained by genetic factors (heritability) and e2 is the proportion of variance due to non-shared environmental factors. Variance component estimates are based on the best-fitting model (see text and Supplementary Table 1).
A detailed description of genetic model fitting and comparison of alternative models is presented in the Supplementary Material, including longitudinal Cholesky models (Supplementary Table 1) and analysis of changes from age 12 to age 16 (Supplementary Table 2). In summary, for each of the three variables non-additive genetic influences were non-significant, and AE model including additive genetic and non-shared environmental factors was the best fitting one.
Maximum-likelihood estimation of model parameters showed that all three ERP components indicated significant heritability at each assessment, indicating that 50–60% of inter-individual variability can be attributed to genetic factors (Table 3 and Fig. 6).
Fig. 6.
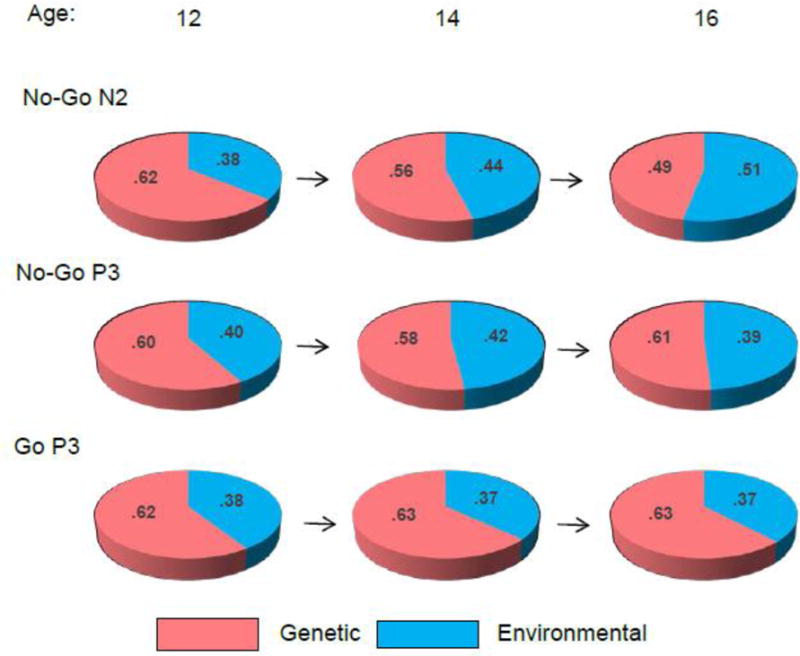
Heritability of ERP components at different ages. The proportions of variance attributable to genetic factors (heritability) is shown in red, the residual environmental variance including measurement error is shown in blue.
3.6. Testing for age-related changes in heritability and genetic architecture
To test for significance of the relatively minor differences in heritability estimates across age, we compared the goodness of fit of alternative models in which heritability was constrained to be equal or allowed to vary across age. These analyses showed that heritability can be equated across age without a significant decrement of model fit, indicating the lack of significant changes in the magnitude of heritability with age for any of the ERP components (Supplementary Table 1).
Analysis of genetic covariance showed a substantial overlap among genetic influences at different ages (Table 4). Cross-age genetic correlations ranged from .79 to .99, indicating a 63 to 98% overlap among genetic factors influencing ERP amplitudes at different ages (i.e. the largely same set of genes influences the ERP parameters at ages 12, 14, and 16). However, this overlap was not complete, and significant age-specific genetic influences were also detected for No-Go N2 and P3. For N2, novel (i.e. not shared with age 12) genetic influences emerged at age 14 and were transmitted to age 16 (the genetic path from age 14 to 16 could not be dropped without a significant decrement in model fit) but no new genetic influences were detected at age 16. For No-Go P3, novel genetic influences also emerged at age 14 but were not transmitted to age 16, and there were no new genetic influences at age 16. This pattern of results suggests age-limited genetic factors that operated at age 14 but not 12 or 16. In contrast to No-Go N2 and P3, the parietal Go P3 amplitude did not show evidence for age-specific genetic effects: a model with one common genetic factor for all three assessments fit the data well, whereas one with independent genetic factors for each occasion did not. Thus, a model with significant heritability but no new genetic influences was the most parsimonious one for Go P3 amplitude (see Supplementary Table 1). Correspondingly, genetic correlations among assessments were >.90 (Table 4).
Table 4.
Longitudinal genetic and environmental correlations across ages for peak amplitudes of ERP components (based on the trivariate AE models).
| Measure | Ages | rG | rE | Genetic overlap |
|---|---|---|---|---|
| No-Go N2, FCZ | 12 – 14 | 0.84* (0.74 – 0.94) | 0.18* (0.04 – 0.32) | 0.71* (0.55 – 0.88) |
| 14 – 16 | 0.88* (0.73 – 1.00) | 0.34* (0.15 – 0.51) | 0.77* (0.53 – 1.00) | |
| 12 – 16 | 0.79* (0.62 – 0.97) | 0.16 (−0.04 – 0.34) | 0.63* (0.39 – 0.93) | |
| No-Go P3, FCZ | 12 – 14 | 0.82* (0.71 – 0.91) | 0.29* (0.14 – 0.42) | 0.67* (0.51 – 0.83) |
| 14 – 16 | 0.83* (0.70 – 0.95) | 0.44* (0.26 – 0.59) | 0.69* (0.49 – 0.89) | |
| 12 – 16 | 0.85* (0.68 – 1.00) | 0.14 (−0.09 – 0.36) | 0.72* (0.46 – 1.00) | |
| Go P3, PZ | 12 – 14 | 0.93* (0.84 – 0.99) | 0.15 (−0.00 – 0.30) | 0.87* (0.70 – 0.99) |
| 14 – 16 | 0.91* (0.81 – 0.99) | 0.46* (0.28 – 0.61) | 0.83* (0.66 – 0.99) | |
| 12 – 16 | 0.99* (0.84 – 1.00) | 0.03 (−0.15 – 0.24) | 0.98* (0.72 – 1.00) |
Notes: rG and rE are genetic and environmental correlations, respectively, between different longitudinal assessments of the same ERP variable;
indicates significant at p<0.05; Genetic overlap is the proportion of genetic variance shared across ages.
3.7. Genetic influences on the rate of development (heritability of change)
The univariate genetic analysis of age difference scores indicating the amount of age-related change (Table 5) showed modest but significant heritability of all three ERP phenotypes, suggesting that individual differences in the rate of age-related changes of No-Go ERP components (Fig. 4) are influenced by genetic factors.
Table 5.
Genetic and environmental contributions to the rate of developmental changes in the amplitudes of No-Go and Go ERP components (difference scores, age 16 – age 12).
| ERP phenotype | rMZ | rDZ | Additive Genetic (Heritability) | Non-shared Environmental |
|---|---|---|---|---|
| N2, FCZ | 0.30* (0.09 – 0.49) | 0.10 (−0.11 – 0.30) | 0.29* (0.10 – 0.46) | 0.71* (0.54 – 0.90) |
| P3N, FCZ | 0.22* (0.001 – 0.42) | 0.09 (−0.12 – 0.29) | 0.21* (0.01 – 0.39) | 0.79* (0.61 – 0.99) |
| P3G, PZ | 0.40* (0.19 – 0.56) | −0.05 (−0.26 – 0.16) | 0.28* (0.09 – 0.45) | 0.72* (0.55 – 0.91) |
rMZ and rDZ are intrapair correlations for MZ and DZ twin pairs, respectively (95% confidence intervals are shown in brackets);
p<.05;
a2 is the proportion of total phenotypic variance explained by genetic factors (heritability) and e2 is the proportion of variance due to non-shared environmental factors. Variance component estimates are based on the best-fitting model.
4. Discussion
The present study provides the first evidence for heritability of brain activity related to response inhibition in adolescence. To our knowledge, it is also the first longitudinal study of developmental changes in inhibition-related ERPs during adolescence. In summary, the present analyses lead to the following conclusions: 1) neural correlates of response inhibition undergo significant developmental changes during adolescence, with distinct subprocesses indicated by N2 and P3 (conflict processing and motor inhibition) showing different developmental trajectories; 2) despite these changes, individual differences remain relatively stable over age; 3) neural correlates of response inhibition are strongly influenced by genetic factors throughout adolescence, with little change in heritability over age; 4) these genetic influences include both common genetic factors active at different ages and novel genetic influences emerging during development, and 5) individual differences in developmental trajectory (the rate of change) in inhibition-related brain activity are significantly influenced by genetic factors. Taken together, these results significantly extend our previous finding of heritability of response-inhibition-related ERPs (Anokhin et al., 2004) and provide novel insights into the interplay of genetic and developmental influences on the neural underpinnings of inhibitory control during a sensitive developmental period when deficits in inhibitory control may increase the risk for a range of maladaptive behaviors and psychopathology. Thus, response inhibition-related ERPs represent developmentally stable and highly heritable traits that can potentially serve as intermediate neurobiological phenotypes for genetic studies of developmental neuropsychiatric disorders.
4.2. Neural correlates of response inhibition undergo significant developmental changes over adolescence
Reduction in N2 and increase in P3 with age is consistent with a previous cross-sectional study (Johnstone et al., 2005; Jonkman, 2006). The divergence of developmental trajectories of No-Go N2 and P3 is consistent with the notion that these components reflect distinct cognitive processes. Accumulating evidence suggests that N2 reflects the detection of conflict associated with the need to suppress a pre-activated response by the anterior cingulate (Garavan et al., 2002; Mathalon et al., 2003; Swainson et al., 2003; van Veen and Carter, 2002a, b), whereas No-Go P3 reflects inhibition-related activity in a broader network of regions involving the ACC, supplementary motor area, dorsolateral prefrontal cortex, and the fronto-parietal network (Dias et al., 2003; Fallgatter et al., 2002; Kiefer et al., 1998). A study using EEG and fMRI (Garavan et al., 2002) revealed 2 systems related to RI: the ACC and a network including right prefrontal and parietal areas. Furthermore, it was shown that absent-minded subjects tended to rely more on the ACC system, whereas subjects low on the absentmindedness score showed a stronger and more selective use of the frontoparietal system. In light of these findings, it is reasonable to suggest that opposite developmental changes of N2 and P3 reflect a changing balance in the relative role of ACC and more extended inhibition network in the processing of No-Go stimuli, such that decreasing activation of the ACC is paralleled by increasing engagement of the dorsolateral prefrontal areas and the frontoparietal network. From the conflict monitoring perspective, the decrease in N2 amplitude may reflect a decrease in the level of experienced conflict (Jonkman, 2006), perhaps due to more efficient processing in the ACC.
Given the broader scalp distribution of No-Go P3 compared with N2, it is reasonable to suggest that this component reflects the activation of a network of regions involved in response inhibition with a “hub” in the ACC. A rapid integration of multiple distributed brain regions into a unified functional network would require mature long-range structural connectivity. However, developmental studies indicate a prolonged myelination of human association cortices, particularly the frontal cortex (Yakovlev and Lecours, 1967).
4.3. Individual differences show significant developmental stability
Despite the developmental changes described above, individual differences in ERPs remain relatively stable across ages, indicating that No-Go N2 and P3 are reliable indicators of neural processes related to response inhibition in adolescence. It is important to note that there is no contradiction between developmental changes and developmental stability of individual differences because the latter refers to the preservation of relative rank-ordering of individuals over age despite age-related changes in absolute values. However, this stability is not perfect and test-retest coefficients are lower than those obtained in adults using a 30-min retest (Fallgatter et al., 2001). Although this difference can be partially attributed to the difference in retest interval, there is another potential source of variability, namely, individual differences in the rate of developmental changes (see 4.6 below).
4.4. Neural correlates of response inhibition are heritable
Analysis of heritability yielded two major findings. First, both Go and No-Go ERP components showed significant heritability at all three ages, indicating that 49 to 63% of observed inter-individual variation can be attributed to genetic differences among individuals (Table 3). Second, there was no significant change in the magnitude of heritability over the ages studied. Interestingly, these heritability estimates are in the same range as those obtained in our previous study of young adult twins (age: 18–28, heritability estimates: 41 to 60%). Taken together, these studies suggest that neuroelectric indicators of response inhibition are strongly influenced by genetic factors in both adolescents and adults, and the overall strength of genetic influences does not change with age.
These results, as any finding of strong heritability, raise the issue of pre-determination versus changeability of a trait. It is important to underscore that high heritability does not necessarily imply that inhibitory control capacity is genetically pre-determined for each particular individual. First, the notion of heritability pertains to the population, not a single individual, and refers to genetic determination of inter-individual variance but not individual value of a trait. Second, heritability does not imply that individual values of the trait and the population mean value are unchangeable. In particular, individual values and, accordingly, the population mean value of a certain trait can be elevated in a given population due to e.g. enriched environment or training, but individual differences may still be highly heritable. In fact, there is evidence that response inhibition-related ERP can be modified by directed training. Children aged 8–12 with externalizing problems showed larger N2 and smaller P3 amplitudes relative to controls, but a 3-month cognitive behavioral therapy program resulted in modification of these ERP responses, moreover, greater clinical improvement after treatment was associated with a decrease of N2 amplitude (Woltering et al., 2011). Given the normative developmental decrease in N2 and increase in P3 amplitude, these results suggest a possibility of a developmental lag in the neural correlates of RI, which can be corrected by a therapy program but only in treatment-responsive individuals. In an fMRI study, neural activity related to response inhibition changes as a function of repeated task practice (Kelly et al., 2006). Taken together, these ERP and fMRI studies suggest that the neural circuitry subserving inhibitory control is amenable to training, despite high heritability at the population level.
4.5. Continuity and change in genetic influences
Multivariate genetic analyses showed a substantial overlap among genetic factors operating at different ages (Table 4), indicating that 63 to 98% of genetic influences on ERP amplitudes are common across age, i.e. the largely same set of genes influences the ERP parameters at ages 12, 14, and 16.
Although no significant age-related differences in overall heritability were observed, multivariate genetic analyses revealed significant changes in genetic architecture, and these changes differed among ERP variables. Despite a strong overlap of genetic influences on ERP components at different ages, significant age-specific genetic influences were identified for No-Go ERP components. For both N2 and P3, novel genetic influences emerged at age 14 and, in the case of N2, these new genetic factors remained active at age 16, whereas for No-Go P3 they were restricted to age 14 only. This pattern of results suggests the possibility of age-limited genetic influences, such that new genes become active between age 12 and 14 and are inactivated between 14 and 16. However, it cannot be ruled out that the overlap between novel genetic influences emerging at age 14 and genetic influences at age 16 were not detected due to smaller number of pairs at age 16 and thus lower statistical power.
It is important to note that novel genetic influences were detected for responses to No-Go but not Go stimuli, suggesting that the set of genes influencing the mechanisms of response inhibition changes in the course of prefrontal development, probably due to changes in gene expression. One can speculate that increasing environmental demands during a sensitive developmental period trigger the expression of new genes affecting prefrontally-mediated cognitive control functions. In contrast, genetic influences on the mostly parietal cortical activity related to the processing of Go stimuli (resembling classical oddball stimuli) may already be stabilized by age 12 and undergo little change during adolescent development.
4.6. Genetic influences on developmental changes in response inhibition
As described above, there are highly significant developmental changes in the neural markers of response inhibition at the group (population) level. However, the extent of these changes varies across individuals, suggesting individual differences in the rate of brain maturation. A genetic analysis of longitudinal difference scores indicated that the rate of developmental changes in the neural processes underlying response inhibition is significantly influenced by genetic factors, although the amount of this influence is modest relative to non-shared environmental influences (Table 5). This finding has important implications for the understanding of developmental psychopathology and substance abuse. In particular, a delayed maturation of the functional neural system subserving response inhibition may result in a mismatch between individual’s self-control capacity and challenges presented by changing social environment and roles such as increased stress and peer pressure, leading to elevated risk for maladaptive behaviors and psychopathology. Another mismatch may occur between the underdeveloped inhibitory control system and already developed reward/motivation system, leading to impulsive reward seeking such as increased risk-taking and delay discounting behaviors (Casey, 2015). It is reasonable to suggest that this behavioral pattern characteristic of adolescence is at least partially mediated by heritable differences in the rate of brain maturation, particularly prefrontal brain regions that are critical for inhibitory self-regulation of behavior.
4.8. Limitations and future directions
Although the present study provided important novel information about the development and genetics of brain activity related to response inhibition in adolescents, a number of important questions still remain unanswered, warranting future studies. First, estimates of heritability, while important, provide little information about specific genes involved. The present analysis suggests that there may be at least two distinct sets of genes: the first set may affect the overall level of inhibitory capacity, while the second set may influence developmental changes during mid-adolescence. An important aim for future genetic investigation of the ERP markers of response inhibition will be identification of genes influencing overall inhibitory control capacity and its developmental changes.
Next, the present analysis was limited to an age range of 12 to 16 only. It is important to determine whether developmental changes continue past the age of 16, and whether No-Go ERPs in young adults are influenced by the same genetic factors as in adolescents. The ongoing work in our laboratory will extend the present analyses to older ages including the important period of transition from adolescence to adulthood. Furthermore, our longitudinal analysis included three time points only, which limited our ability to evaluate the shape of developmental trajectories and fit advanced developmental models such as the growth curves model.
The present analyses focused on traditional peak amplitude measures of major ERP components that have been used in the majority of previous studies using Go/No-Go paradigms. However, these ERP components may represent relatively distinct temporally and spatially overlapping processes, and quantification of ERP components can be further improved by the application of methods permitting the isolation of these constituent processes such as the principal component analysis (PCA) and the independent component analysis (ICA). In particular, recent studies have demonstrated that PCA provides a more fine-grained characterization of separable neural processes involved in response inhibition compared to traditional peak measures (Barry and De Blasio, 2015), however, little is known about common and specific genetic influences on these characteristics.
Furthermore, averaged ERPs used in the present study provide a relatively limited insight into event-related neuronal dynamics. Analysis of brain oscillations suggests that ERPs related to response inhibition emerge largely due to phase-locking of neuronal oscillations in delta and theta frequency bands (Muller and Anokhin, 2012). It is reasonable to suggest that distinct developmental trajectories of N2 and P3 components described above can potentially be explained by differences in the underlying oscillatory processes, including their frequency, topographical distribution, and time course. Thus, the present analyses should be expanded by focusing on event-related brain oscillations and connectivity in order to better characterize the underlying neural dynamics and to refine phenotypes for genetic association studies.
Another important issue is further neuroanatomical validation of No-Go ERP and oscillatory EEG measures, which can provide cost-effective neural markers of response inhibition that can be used in large-scale genetic studies of psychopathology where fMRI assessments may be cost-prohibitive.
Finally, another interesting direction for future studies is the examination of relationships between brain correlates of response inhibition and behavior. It is important to establish whether genetically transmitted differences in the neural mechanisms of response inhibition predict individual differences in behavior using both cross-sectional and prospective analyses.
4.9. Conclusions
The present study provides the first evidence for genetic influences on neural correlates of response inhibition during adolescence and suggests that ERPs elicited in the Go/No-Go task can serve as intermediate neurophysiological phenotypes (endophenotypes) for the study of disinhibition and impulse control disorders.
Analysis of a unique longitudinal twin dataset led us to the following specific conclusions: 1) neural correlates of response inhibition show significant developmental changes from age 12 to age 16 that are paralleled by significant improvement of performance; 2) despite systematic group-level changes, individual differences show relative developmental stability; 3) neural correlates of response inhibition are highly heritable, with 50 to 60% of inter-individual variability being attributable to genetic factors; 4) the contribution of shared environmental factors is non-significant; 5) overall heritability remains relatively stable with age; 6) genetic influences included both common genetic factors shared across ages as well as novel genetic influences emerging during development (at age 14 and age 16); 7) the rate of developmental changes in the neural correlates of response inhibition is significantly influenced by genetic factors, although heritability is modest.
These findings provide a foundation for studies aimed at the identification of specific genes influencing the neurophysiological mechanisms of response inhibition, investigation of behavioral correlates of genetically transmitted differences in inhibitory control and, ultimately, elucidation of gene-brain-behavior relationships in normal personality variation and psychopathology.
Supplementary Material
Highlights.
Neural correlates of response inhibition undergo significant developmental changes during adolescence, but Individual differences remain relatively stable
Inhibition-related brain activity is heritable, with evidence for both continued and age-limited genetic influences
Individual differences in developmental trajectories are strongly influenced by genetic factors
Acknowledgments
This work was supported by grants DA018899 and DA027096 to A.A. from the National Institutes of Health (NIH). The authors acknowledge organizational and technical assistance by Tara Tinnin, MSW, Olga Novak, and other project staff. The authors also acknowledge the generous giving of time and effort by the study participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Figures: Captions are provided with figures (see Supplementary Material).
References
- Anokhin AP. Genetic psychophysiology: advances, problems, and future directions. Int J Psychophysiol. 2014;93:173–197. doi: 10.1016/j.ijpsycho.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Heath AC, Myers E. Genetics, prefrontal cortex, and cognitive control: A twin study of event-related potentials in a response inhibition task. Neurosci Lett. 2004;368:314–318. doi: 10.1016/j.neulet.2004.07.036. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barry RJ, De Blasio FM. Performance and ERP components in the equiprobable go/no-go task: Inhibition in children. Psychophysiology. 2015;52:1228–1237. doi: 10.1111/psyp.12447. [DOI] [PubMed] [Google Scholar]
- Bekker EM, Kenemans JL, Verbaten MN. Electrophysiological correlates of attention, inhibition, sensitivity and bias in a continuous performance task. Clin Neurophysiol. 2004;115:2001–2013. doi: 10.1016/j.clinph.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nature reviews. 2002;3:872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- Brandeis D, Banaschewski T, Baving L, Georgiewa P, Blanz B, Warnke A, Steinhausen HC, Rothenberger A, Scheuerpflug P. Multicenter P300 brain mapping of impaired attention to cues in hyperkinetic children. J Am Acad Child Adolesc Psychiatry. 2002;41:990–998. doi: 10.1097/00004583-200208000-00018. [DOI] [PubMed] [Google Scholar]
- Bruin KJ, Wijers AA. Inhibition, response mode, and stimulus probability: a comparative event-related potential study. Clin Neurophysiol. 2002;113:1172–1182. doi: 10.1016/s1388-2457(02)00141-4. [DOI] [PubMed] [Google Scholar]
- Casey BJ. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol. 2015;66:295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33:631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Cheung CH, Rijsdijk F, McLoughlin G, Brandeis D, Banaschewski T, Asherson P, Kuntsi J. Cognitive and neurophysiological markers of ADHD persistence and remission. Br J Psychiatry. 2015 doi: 10.1192/bjp.bp.114.145185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dias EC, Foxe JJ, Javitt DC. Changing plans: a high density electrical mapping study of cortical control. Cereb Cortex. 2003;13:701–715. doi: 10.1093/cercor/13.7.701. [DOI] [PubMed] [Google Scholar]
- Donkers FC, van Boxtel GJ. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain Cogn. 2004;56:165–176. doi: 10.1016/j.bandc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Eimer M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biol Psychol. 1993;35:123–138. doi: 10.1016/0301-0511(93)90009-w. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go Nogo tasks and their relation to inhibition. Acta Psychol (Amst) 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Bartsch AJ, Herrmann MJ. Electrophysiological measurements of anterior cingulate function. J Neural Transm. 2002;109:977–988. doi: 10.1007/s007020200080. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Bartsch AJ, Strik WK, Mueller TJ, Eisenack SS, Neuhauser B, Aranda D, Herrmann MJ. Test-retest reliability of electrophysiological parameters related to cognitive motor control. Clin Neurophysiol. 2001;112:198–204. doi: 10.1016/s1388-2457(00)00505-8. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Brandeis D, Strik WK. A robust assessment of the NoGo-anteriorisation of P300 microstates in a cued Continuous Performance Test. Brain Topogr. 1997;9:295–302. doi: 10.1007/BF01464484. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Ehlis AC, Seifert J, Strik WK, Scheuerpflug P, Zillessen KE, Herrmann MJ, Warnke A. Altered response control and anterior cingulate function in attention-deficit/hyperactivity disorder boys. Clin Neurophysiol. 2004;115:973–981. doi: 10.1016/j.clinph.2003.11.036. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Jatzke S, Bartsch AJ, Hamelbeck B, Lesch KP. Serotonin transporter promoter polymorphism influences topography of inhibitory motor control. Int J Neuropsychopharmcol. 1999;2:115–120. doi: 10.1017/S1461145799001455. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Strik WK. The NoGo-anteriorization as a neurophysiological standard-index for cognitive response control. Int J Psychophysiol. 1999;32:233–238. doi: 10.1016/s0167-8760(99)00018-5. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. NeuroImage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Gomez P, Ratcliff R, Perea M. A model of the go/no-go task. J Exp Psychol Gen. 2007;136:389–413. doi: 10.1037/0096-3445.136.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenstein EE, Newman JP. Disinhibitory psychopathology: a new perspective and a model for research. Psychol Rev. 1980;87:301–315. [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Guan M, Liao Y, Ren H, Wang X, Yang Q, Liu X, Wang W. Impaired response inhibition in juvenile delinquents with antisocial personality characteristics: A preliminary ERP study in a Go/Nogo task. Neurosci Lett. 2015;603:1–5. doi: 10.1016/j.neulet.2015.06.062. [DOI] [PubMed] [Google Scholar]
- Helenius P, Laasonen M, Hokkanen L, Paetau R, Niemivirta M. Impaired engagement of the ventral attentional pathway in ADHD. Neuropsychologia. 2011;49:1889–1896. doi: 10.1016/j.neuropsychologia.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Eichele T, Enriquez-Geppert S, Wollbrink A, Kugel H, Konrad C, Pantev C. Multimodal imaging of functional networks and event-related potentials in performance monitoring. NeuroImage. 2011;56:1588–1597. doi: 10.1016/j.neuroimage.2011.03.039. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Enriquez-Geppert S, Lavallee CF, Falkenstein M, Herrmann CS. Electroencephalography of response inhibition tasks: functional networks and cognitive contributions. Int J Psychophysiol. 2013;87:217–233. doi: 10.1016/j.ijpsycho.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Enriquez-Geppert S, Pantev C, Bruchmann M. Variations in midcingulate morphology are related to ERP indices of cognitive control. Brain structure & function. 2014;219:49–60. doi: 10.1007/s00429-012-0483-5. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Jodo E, Kayama Y. Relation of a negative ERP component to response inhibition in a Go/No-go task. Electroencephalogr Clin Neurophysiol. 1992;82:477–482. doi: 10.1016/0013-4694(92)90054-l. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Pleffer CB, Barry RJ, Clarke AR, Smith JL. Development of Inhibitory Processing During the Go/NoGo Task. J Psychophysiol. 2005;19:11–23. [Google Scholar]
- Jonkman LM. The development of preparation, conflict monitoring and inhibition from early childhood to young adulthood: a Go/Nogo ERP study. Brain Res. 2006;1097:181–193. doi: 10.1016/j.brainres.2006.04.064. [DOI] [PubMed] [Google Scholar]
- Jonkman LM, Lansbergen M, Stauder JE. Developmental differences in behavioral and event-related brain responses associated with response preparation and inhibition in a go/nogo task. Psychophysiology. 2003;40:752–761. doi: 10.1111/1469-8986.00075. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go task. Biol Psychol. 2005a;69:353–373. doi: 10.1016/j.biopsycho.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. Spatial-anatomical mapping of NoGo-P3 in the offspring of alcoholics: evidence of cognitive and neural disinhibition as a risk for alcoholism. Clin Neurophysiol. 2005b;116:1049–1061. doi: 10.1016/j.clinph.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo K, Pontifex MB, Khan NA, Raine LB, Scudder MR, Drollette ES, Evans EM, Castelli DM, Hillman CH. The association of childhood obesity to neuroelectric indices of inhibition. Psychophysiology. 2012;49:1361–1371. doi: 10.1111/j.1469-8986.2012.01459.x. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Hester R, Foxe JJ, Shpaner M, Garavan H. Flexible cognitive control: effects of individual differences and brief practice on a complex cognitive task. NeuroImage. 2006;31:866–886. doi: 10.1016/j.neuroimage.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Kiefer M, Marzinzik F, Weisbrod M, Scherg M, Spitzer M. The time course of brain activations during response inhibition: evidence from event-related potentials in a go/no go task. Neuroreport. 1998;9:765–770. doi: 10.1097/00001756-199803090-00037. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Liddle PF. An event-related potential investigation of response inhibition in schizophrenia and psychopathy. Biol Psychiatry. 2000;48:210–221. doi: 10.1016/s0006-3223(00)00834-9. [DOI] [PubMed] [Google Scholar]
- Kirmizi-Alsan E, Bayraktaroglu Z, Gurvit H, Keskin YH, Emre M, Demiralp T. Comparative analysis of event-related potentials during Go/NoGo and CPT: decomposition of electrophysiological markers of response inhibition and sustained attention. Brain Res. 2006;1104:114–128. doi: 10.1016/j.brainres.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. J Abnorm Psychol. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Walker OL, Degnan KA, Henderson HA, Pine DS, McDermott JM, Fox NA. Cognitive control moderates early childhood temperament in predicting social behavior in 7-year-old children: an ERP study. Developmental science. 2014;17:667–681. doi: 10.1111/desc.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MD, Lamm C, Segalowitz SJ, Stieben J, Zelazo PD. Neurophysiological correlates of emotion regulation in children and adolescents. J Cogn Neurosci. 2006;18:430–443. doi: 10.1162/089892906775990633. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Whitfield SL, Ford JM. Anatomy of an error: ERP and fMRI. Biol Psychol. 2003;64:119–141. doi: 10.1016/s0301-0511(03)00105-4. [DOI] [PubMed] [Google Scholar]
- McLoughlin G, Albrecht B, Banaschewski T, Rothenberger A, Brandeis D, Asherson P, Kuntsi J. Electrophysiological evidence for abnormal preparatory states and inhibitory processing in adult ADHD. Behav Brain Funct. 2010;6:66. doi: 10.1186/1744-9081-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller V, Anokhin AP. Neural synchrony during response production and inhibition. PLoS One. 2012;7:e38931. doi: 10.1371/journal.pone.0038931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx:Statistical Modeling. 6th. Department of Psychiatry; Richmond, VA: 2002. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Kluwer Academic Publishers; Dordrecht: 1992. [Google Scholar]
- Nieuwenhuis S, Yeung N, Cohen JD. Stimulus modality, perceptual overlap, and the go/no-go N2. Psychophysiology. 2004;41:157–160. doi: 10.1046/j.1469-8986.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cognitive, affective & behavioral neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overtoom CC, Verbaten MN, Kemner C, Kenemans JL, van Engeland H, Buitelaar JK, Camfferman G, Koelega HS. Associations between event-related potentials and measures of attention and inhibition in the Continuous Performance Task in children with ADHD and normal controls. J Am Acad Child Adolesc Psychiatry. 1998;37:977–985. doi: 10.1097/00004583-199809000-00018. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Weller BJ, Kopell BS. ERPs to response production and inhibition. Electroencephalogr Clin Neurophysiol. 1985;60:423–434. doi: 10.1016/0013-4694(85)91017-x. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Beem AL, de Geus EJ, van Baal GC, von Hjelmborg JB, Iachine I, Boomsma DI. Theory and practice in quantitative genetics. Twin Res. 2003;6:361–376. doi: 10.1375/136905203770326367. [DOI] [PubMed] [Google Scholar]
- Randall WM, Smith JL. Conflict and inhibition in the cued-Go/NoGo task. Clin Neurophysiol. 2011;122:2400–2407. doi: 10.1016/j.clinph.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Reyes S, Peirano P, Peigneux P, Lozoff B, Algarin C. Inhibitory control in otherwise healthy overweight 10-year-old children. International journal of obesity. 2015;39:1230–1235. doi: 10.1038/ijo.2015.49. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief Bioinform. 2002;3:119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Rau H, Lutzenberger W, Birbaumer N. Mapping P300 waves onto inhibition: Go/No-Go discrimination. Electroencephalogr Clin Neurophysiol. 1994;92:44–55. doi: 10.1016/0168-5597(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Rubia K. “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biol Psychiatry. 2011;69:e69–87. doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Rubia K. Functional brain imaging across development. Eur Child Adolesc Psychiatry. 2013;22:719–731. doi: 10.1007/s00787-012-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Sham P. Statistics in Human genetics. Oxford University Press; New York: 1998. [Google Scholar]
- Smith JL, Johnstone SJ, Barry RJ. Movement-related potentials in the Go/NoGo task: the P3 reflects both cognitive and motor inhibition. Clin Neurophysiol. 2008;119:704–714. doi: 10.1016/j.clinph.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Smith JL, Smith EA, Provost AL, Heathcote A. Sequence effects support the conflict theory of N2 and P3 in the Go/NoGo task. Int J Psychophysiol. 2010;75:217–226. doi: 10.1016/j.ijpsycho.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Swainson R, Cunnington R, Jackson GM, Rorden C, Peters AM, Morris PG, Jackson SR. Cognitive control mechanisms revealed by ERP and fMRI: evidence from repeated task-switching. J Cogn Neurosci. 2003;15:785–799. doi: 10.1162/089892903322370717. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Tye C, Asherson P, Ashwood KL, Azadi B, Bolton P, McLoughlin G. Attention and inhibition in children with ASD, ADHD and co-morbid ASD + ADHD: an event-related potential study. Psychol Med. 2013:1–16. doi: 10.1017/S0033291713001049. [DOI] [PubMed] [Google Scholar]
- van Boxtel GJ, van der Molen MW, Jennings JR, Brunia CH. A psychophysiological analysis of inhibitory motor control in the stop-signal paradigm. Biol Psychol. 2001;58:229–262. doi: 10.1016/s0301-0511(01)00117-x. [DOI] [PubMed] [Google Scholar]
- van Dongen J, Slagboom PE, Draisma HH, Martin NG, Boomsma DI. The continuing value of twin studies in the omics era. Nature reviews. 2012;13:640–653. doi: 10.1038/nrg3243. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002a;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci. 2002b;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Heilman KM. Response preparation and response inhibition after lesions of the medial frontal lobe. Arch Neurol. 1987;44:1265–1271. doi: 10.1001/archneur.1987.00520240045010. [DOI] [PubMed] [Google Scholar]
- Vogel F. Neurobiological approaches in human behavior genetics. Behav Genet. 1981;11:87–102. doi: 10.1007/BF01065620. [DOI] [PubMed] [Google Scholar]
- Vogel F, Schalt E, Kruger J, Propping P, Lehnert KF. Electroencephalogram (Eeg) as a Research Tool in Human-Behavior Genetics - Psychological Examinations in Healthy Males with Various Inherited Eeg Variants .1 Rationale of the Study. Material. Methods. Heritability of Test Parameters. Hum Genet. 1979;47:1–45. doi: 10.1007/BF00295569. [DOI] [PubMed] [Google Scholar]
- Whelan R, Conrod PJ, Poline JB, Lourdusamy A, Banaschewski T, Barker GJ, Bellgrove MA, Buchel C, Byrne M, Cummins TD, Fauth-Buhler M, Flor H, Gallinat J, Heinz A, Ittermann B, Mann K, Martinot JL, Lalor EC, Lathrop M, Loth E, Nees F, Paus T, Rietschel M, Smolka MN, Spanagel R, Stephens DN, Struve M, Thyreau B, Vollstaedt-Klein S, Robbins TW, Schumann G, Garavan H. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat Neurosci. 2012;15:920–925. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- Wild-Wall N, Oades RD, Schmidt-Wessels M, Christiansen H, Falkenstein M. Neural activity associated with executive functions in adolescents with attention-deficit/hyperactivity disorder (ADHD) Int J Psychophysiol. 2009;74:19–27. doi: 10.1016/j.ijpsycho.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Woltering S, Granic I, Lamm C, Lewis MD. Neural changes associated with treatment outcome in children with externalizing problems. Biol Psychiatry. 2011;70:873–879. doi: 10.1016/j.biopsych.2011.05.029. [DOI] [PubMed] [Google Scholar]
- Woltering S, Liu Z, Rokeach A, Tannock R. Neurophysiological differences in inhibitory control between adults with ADHD and their peers. Neuropsychologia. 2013;51:1888–1895. doi: 10.1016/j.neuropsychologia.2013.06.023. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minowski A, editor. Regional Development of the Brain in Early life. 1967. pp. 3–70. [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J Abnorm Psychol. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. Am J Med Genet. 2000;96:684–695. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


