Abstract
Introduction
Very few studies have evaluated the efficacy of pentoxifylline and tocopherol (PENT-E) in the management of medication-related osteonecrosis of the jaw (MRONJ), though studies have shown its therapeutic and prophylactic benefit in the management of osteoradionecrosis. We report the outcomes of MRONJ managed with PENT-E in patients with metastatic bone disease/multiple myeloma.
Patients and Methods
Seven patients diagnosed with refractory established cases of MRONJ due to anti-resorptive medications for management of metastatic bone tumors/multiple myeloma were provided PENT-E for a mean period of 16.8 months (range: 3 – 48 months).
Results
At latest follow-up visit, all patients demonstrated relief of symptoms. There was radiographic evidence of new bone fill of prior radiolucent defect in all patients. Two patients had resolution of exposed bone, 2 patients had partial resolution, in 1 patient no change in exposed bone and 1 patient with 3 sites of exposed bone prior to starting PENT-E had resolution of 1 site, partial resolution in another site and no change on the third site. PENT-E was well-tolerated in all patients.
Conclusion
Our case series illustrates that PENT-E could be a safe and effective adjunct in the management of MRONJ.
Introduction
Malignancy such as multiple myeloma and metastases to the bone, a common occurrence in advance-stage disease, may necessitate the use of bone-modifying agents such as anti-resorptive medications, including pamidronate and zolendronate (intravenous bisphosphonates), denosumab (humanized monoclonal antibody) and anti-angiogenics such as sunitinib (tyrosine kinase inhibitor) and bevacizumab (humanized monoclonal antibody).
Medication-related osteonecrosis of the jaw (MRONJ) is a well-known complication of bone-modifying agents used in the prevention of skeletal-related events such as bone fracture, spinal cord compression, radiotherapy or surgery in patients with metastatic disease and patients with osteoporosis or osteopenia1-5. The risk of developing MRONJ in patients treated for metastatic disease is higher compared to patients treated for osteoporosis6. Steroids, tobacco, immunosuppressive therapy use and comorbidities such as a medical history of diabetes mellitus have been associated with an increased risk for MRONJ7-9. In approximately 60% of patients, surgical procedures such as dental extraction, periodontal surgery, or implant placement are considered the major precipitating factors for the development of MRONJ9-11.
Clinical management of MRONJ remains controversial, with no established treatment guidelines. Different therapeutic approaches such as chlorhexidine 0.12% or 2% rinse, antibiotic therapy, hyperbaric oxygen (HBO), low level laser therapy (LLLT), laser surgery, conservative surgery, extensive surgery with or without fluorescence light or plasma rich protein (PRP), and pentoxifylline and tocopherol (PENT-E) have been utilized in the management of MRONJ, with variable success rates1, 2, 4, 12-23.
Patients whose osteoradionecrosis (ORN) is managed by PENT-E have demonstrated significant symptom improvement. A newly proposed theory of pathophysiology of radiation-induced fibrosis accounts for the treatment's effectiveness24, 25. Pentoxifylline was originally approved by the FDA for the management of peripheral artery disease such as ischemic heart disease and intermittent claudication. It improves peripheral blood flow by enhancing vasodilation, reducing blood viscosity and increases erythrocyte flexibility26. It also induces anti-tumor necrosis factor alpha (anti-TNFα) effects, inhibiting inflammation and decreasing fibrosis24, 27. Tocopherol is a potent oxygen radical scavenger that reduces free radical damage generated during oxidative stress and protects cell membranes 28. It also reduces inflammation and tissue fibrosis24, 27.
To date, only two reports have demonstrated the effect of PENT-E on MRONJ21, 22. In one study of 6 patients, all patients with MRONJ experienced improvement after treatment with PENT-E, including a 74% decrease in area of bone exposure, without adverse effects21. The second report detailed a patient treated yearly with zoledronic acid for the management of osteoporosis. This patient demonstrated complete bone remodeling after PENT-E treatment22.
The aim of this observational study is to report the outcomes of MRONJ in patients with metastatic bone disease/multiple myeloma managed with PENT-E.
Patients and Methods
The observational study was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board. Seven patients diagnosed with refractory established MRONJ were referred to Dental Service for evaluation of oral complaints including exposed bone, jaw pain and non-healing extraction sites. They were prescribed PENT-E (pentoxifylline 400mg BID and vitamin E 400 IU BID). All patients had been treated with anti-resorptive medication for management of metastatic bone tumors/multiple myeloma. Clinical and radiographic records were examined to determine if PENT-E provided therapeutic benefit to these patients. Outcomes assessed were symptoms, signs and the area of exposed bone. The outcome of the area of exposed bone was divided into 4 categories: resolution (complete mucosal coverage of prior exposed bone); partial resolution (reduction in size of exposed bone); no change; and progression (increase in size of exposed bone). All patients were also treated with chlorhexidine 0.12% rinse and prescribed antibiotic therapy Augmentin 875mg BID or Clindamycin 300mg QID for seven days or longer as indicated for active infection.
Results
Table 1 summarizes treatment outcomes. There were seven patients (female n = 4, male n = 3; ages 53-68 years). Four patients were being managed for metastatic breast cancer, 2 patients for multiple myeloma and 1 patient metastatic gastrointestinal stromal tumor. Three patients were managed with zoledronic acid only, 1 patient with denosumab only and the remaining 3 patients had a combination of zoledronic acid with either denosumab, sunitinib, or alendronic acid. Anti-resorptive dose ranged from 8 – 100 doses. Three patients were classified as MRONJ stage 3 (cases 1-3), three patients were MRONJ stage 2 (cases 4-6) and one patient was MRONJ stage 0 (case 7).
Table 1. Characteristics of MRONJ Patients managed with PENT-E.
| Age(y) /Sex /Stage | No. of doses prior to ONJ | Clinical presentation prior to PENT-E | Radiographic findings prior to PENT-E | Duration on PENT-E till last follow-up | Clinical presentation at last follow-up | Radiographic findings at last follow-up |
|---|---|---|---|---|---|---|
| 1. 63/M /3 | 40, Z + S | Pain, purulent discharge, mandibular bone exposure (55 mm) | Radiolucent defect reaching the basal bone | 48 months | No pain, discharge or bone exposure | Radiolucent bony defect filling |
| 2. 66 /M /3 | 35Z + 11 A | Pain, purulent discharge, bilateral mandibular bone exposure | Radiolucent defects reaching the basal bone | 13 months | No pain, discharge, bone exposure unchanged | Radiolucent bony defect filling |
| 3. 54 /M /3 | 40, Z | Pain, purulent discharge, mandibular bone exposure | Radiolucent defect reaching the basal bone | 22 months | No pain, discharge or bone exposure | Radiolucent bony defect filling |
| 4. 62/F /2 | 7Z + 2D | Pain, discharge and bone exposure | No radiographic findings | 3 months | No pain or discharge, bone exposure reduced in size | NA |
| 5. 57/F /2 | 8, D | Pain and bone exposure: right maxilla (8 mm), left maxilla (6 mm) and mandible | No radiographic findings | 5 months | No pain or bone exposure on the right maxilla, bone exposure on the left maxilla reduced to 2 mm. The mandibular bone exposure unchanged | NA |
| 6. 68 /F /2 | 100, Z | Pain, bilateral mandibular bone exposure | No radiographic findings | 3 months | No pain, bone exposure reduced in size | NA |
| 7. 53 /F /0 | 63, Z | Pain, swelling, no exposed bone | Radiolucent defect | 24 months | No pain or swelling | Radiolucent bony defect filling |
Z – Zolendronic acid, S – Sunitinib, A – Alendronic acid, D - Denosumab, NA - Not applicable
Prior to commencement of PENT-E, 6 patients had an area of exposed bone (cases 1-6), 4 patients had a radiolucent lesion identified on panoramic radiograph (cases 1-3, 7), and 4 expressed purulent discharge (Cases 1-4). All patients experienced pain at presentation.
Patients were placed on PENT-E for a mean period of 16.8 months (range: 3 – 48 months). At the most recent follow-up visit, all patients demonstrated symptom relief; no patient presented with pain or discharge. On radiographic evaluation there was evidence of new bone fill of radiolucent defect in 4 patients (cases 1-3, 7) (Figures 1 – 3). Sequestrum was removed in 2 patients (Cases 1 and 4), due to outwardly loose necrotic bone. Two patients had resolution of exposed bone (Cases 1 and 3) (Figure 1) and 2 patients had partial resolution (Cases 4 and 6). In one patient there was no change in exposed bone (Case 2), and one patient with 3 sites of exposed bone prior to PENT-E treatment had resolution of 1 site, partial resolution of another site and no change in exposure area of the third site (Case 5). PENT-E was well tolerated with no adverse effects identified in all patients.
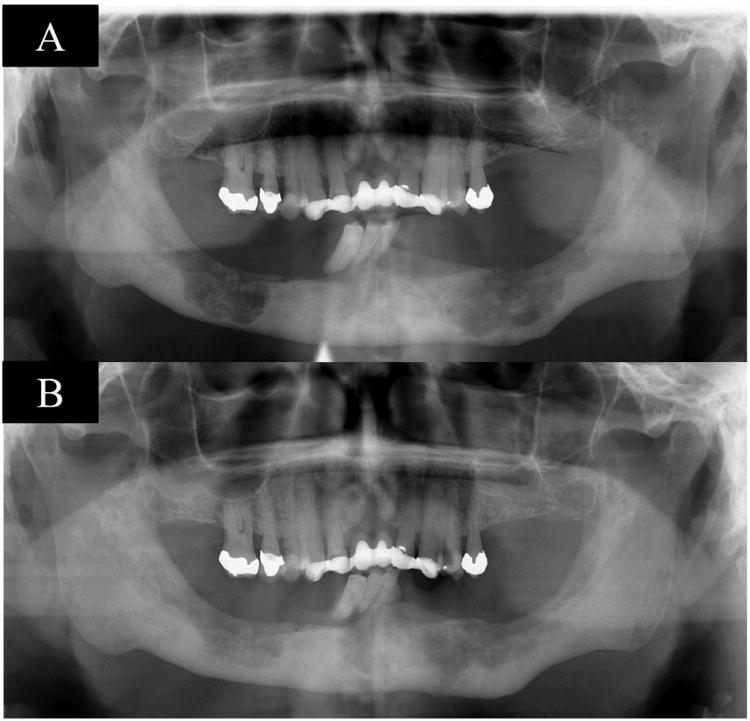
Figure 1. shows pre-therapy panoramic radiograph with a radiolucent defect (A), panoramic radiograph shows a radiographic bone fill after 34 months of therapy (B), case 1.
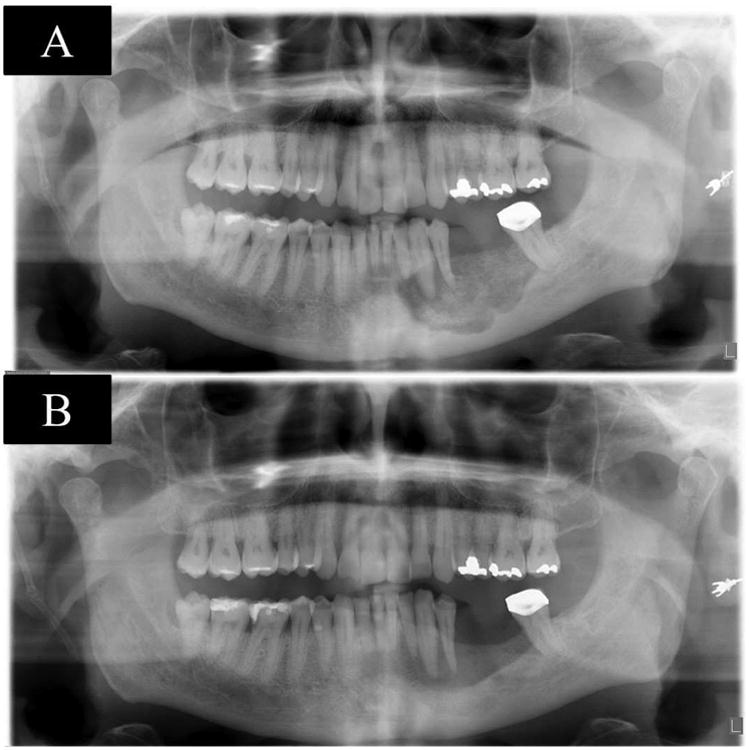
Figure 3. shows pre-therapy panoramic radiograph with a radiolucent defect (A), panoramic radiograph shows a radiographic bone fill after 22 months of therapy (B), case 3.
Discussion
This observational study reports the outcome of MRONJ in patients with metastatic bone disease/multiple myeloma managed with PENT-E. In this present study, all patients demonstrated relief of symptoms and patients who presented with radiolucent defect demonstrated significant new bone formation in the defect at our last follow-up time. PENT-E has demonstrated promising results in the management of radiation-induced fibrosis in soft tissue and established ORN25, 29, 30, with a recent study showing therapeutic benefit in the prophylactic use of PENT-E in patients who required dental extractions after head and neck radiotherapy. In that study, 390 dental extractions were performed in 82 patients who had been irradiated to the head and neck; only one patient developed ORN post-dental extraction31, suggesting a promising application for PENT-E in the management of MRONJ.
In the face of a lack of consensus treatment guidelines, different therapeutic approaches have been employed in the management of MRONJ, all with different outcomes. Nonsurgical options entail the use of antimicrobials such as chlorhexidine 0.12% or 2% rinse or antibiotics. Common antibiotics employed include amoxicillin, amoxicillin with clavulanic acid, clindamycin and/or metronidazole, HBO and low-level laser therapy (LLLT). Surgical options entail conservative surgery (sequestrectomy and/or superficial debridement of sequestrum), extensive surgery (alveoloplasty, resection), and laser surgery. Combination treatments are common, including HBO before and after extensive surgery, HBO or PENT-E with antimicrobial treatment, and others13, 15, 20, 21.
In the 2 previous reports of PENT-E use in the management of MRONJ and in our study 21, 22, the pentoxifylline and tocopherol combination therapy has provided effective relief of patient's symptoms. PENT-E is easily prescribed, less expensive and better tolerated compared to other treatment options such as HBO and extensive surgery. As opposed to surgical procedures which are a well known precipitating factor to the development of MRONJ, it may occur spontaneously secondary to associated endodontic or periodontal infections 16. The use of PENT-E may be considered prophylactically. Limitations to this study are its retrospective nature and an observational study with few patients. Further studies on the therapeutic and prophylactic efficacy of PENT-E in a large cohort of MRONJ patients are needed.
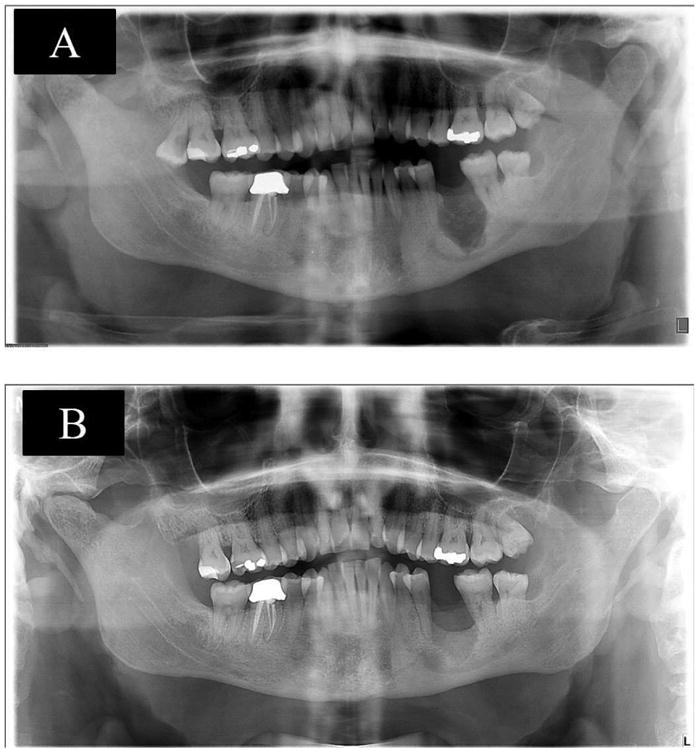
Figure 2. shows pre-therapy panoramic radiograph with bilateral radiolucent defects (A), panoramic radiograph shows bilateral radiographic bone fill after 11 months of therapy (B), case 2.
Statement of Clinical Relevance.
Pentoxifylline and tocopherol were found to be safe and effective in the management of medication-related osteonecrosis of the jaw
Acknowledgments
This study was supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Disclosure: All authors declare that there are no financial conflicts associated with this study.
Abstract of this study was presented at the Annual Meeting of the American Academy of Oral Medicine (AAOM), 2016.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567–1575. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Watters AL, Hansen HJ, Williams T, et al. Intravenous bisphosphonate-related osteonecrosis of the jaw: long-term follow-up of 109 patientsz. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:192–200. doi: 10.1016/j.oooo.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Estilo CL, Van Poznak CH, Wiliams T, et al. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. Oncologist. 2008;13:911–920. doi: 10.1634/theoncologist.2008-0091. [DOI] [PubMed] [Google Scholar]
- 4.Owosho AA, Blanchard A, Levi L, et al. Osteonecrosis of the jaw in patients treated with denosumab for metastatic tumors to the bone: A series of thirteen patients. J Craniomaxillofac Surg. 2016;44:265–270. doi: 10.1016/j.jcms.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercer E, Norton T, Woo S, et al. Ninety-one osteoporosis patients affected with bisphosphonate-related osteonecrosis of the jaw: a case series. Calcif Tissue Int. 2013;93:241–248. doi: 10.1007/s00223-013-9747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodson TB. The Frequency of Medication-related Osteonecrosis of the Jaw and its Associated Risk Factors. Oral Maxillofac Surg Clin North Am. 2015;27:509–516. doi: 10.1016/j.coms.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Wessel JH, Dodson TB, Zavras AI. Zoledronate, smoking, and obesity are strong risk factors for osteonecrosis of the jaw: a case-control study. J Oral Maxillofac Surg. 2008;66:625–631. doi: 10.1016/j.joms.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyrgidis A, Vahtsevanos K, Koloutsos G, et al. Bisphosphonate-related osteonecrosis of the jaws: a case-control study of risk factors in breast cancer patients. J Clin Oncol. 2008;26:4634–4638. doi: 10.1200/JCO.2008.16.2768. [DOI] [PubMed] [Google Scholar]
- 9.Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23:1341–1347. doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]
- 10.Fehm T, Beck V, Banys M, et al. Bisphosphonate-induced osteonecrosis of the jaw (ONJ): Incidence and risk factors in patients with breast cancer and gynecological malignancies. Gynecol Oncol. 2009;112:605–609. doi: 10.1016/j.ygyno.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 11.Vahtsevanos K, Kyrgidis A, Verrou E, et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J Clin Oncol. 2009;27:5356–5362. doi: 10.1200/JCO.2009.21.9584. [DOI] [PubMed] [Google Scholar]
- 12.Lazarovici TS, Yahalom R, Taicher S, et al. Bisphosphonate-related osteonecrosis of the jaws: a single-center study of 101 patients. J Oral Maxillofac Surg. 2009;67:850–855. doi: 10.1016/j.joms.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Freiberger JJ, Padilla-Burgos R, McGraw T, et al. What is the role of hyperbaric oxygen in the management of bisphosphonate-related osteonecrosis of the jaw: a randomized controlled trial of hyperbaric oxygen as an adjunct to surgery and antibiotics. J Oral Maxillofac Surg. 2012;70:1573–1583. doi: 10.1016/j.joms.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Otto S, Baumann S, Ehrenfeld M, Pautke C. Successful surgical management of osteonecrosis of the jaw due to RANK-ligand inhibitor treatment using fluorescence guided bone resection. J Craniomaxillofac Surg. 2013;41:694–698. doi: 10.1016/j.jcms.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 15.Vescovi P, Giovannacci I, Merigo E, et al. Tooth extractions in high-risk patients under bisphosphonate therapy and previously affected with osteonecrosis of the jaws: surgical protocol supported by low-level laser therapy. J Craniofac Surg. 2015;26:696–699. doi: 10.1097/SCS.0000000000001665. [DOI] [PubMed] [Google Scholar]
- 16.Vescovi P, Meleti M, Merigo E, et al. Case series of 589 tooth extractions in patients under bisphosphonates therapy. Proposal of a clinical protocol supported by Nd:YAG low-level laser therapy. Med Oral Patol Oral Cir Bucal. 2013;18:e680–685. doi: 10.4317/medoral.18812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voss PJ, Joshi Oshero J, Kovalova-Muller A, et al. Surgical treatment of bisphosphonate-associated osteonecrosis of the jaw: technical report and follow up of 21 patients. J Craniomaxillofac Surg. 2012;40:719–725. doi: 10.1016/j.jcms.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Stockmann P, Vairaktaris E, Wehrhan F, et al. Osteotomy and primary wound closure in bisphosphonate-associated osteonecrosis of the jaw: a prospective clinical study with 12 months follow-up. Support Care Cancer. 2010;18:449–460. doi: 10.1007/s00520-009-0688-1. [DOI] [PubMed] [Google Scholar]
- 19.Pautke C, Bauer F, Otto S, et al. Fluorescence-guided bone resection in bisphosphonate-related osteonecrosis of the jaws: first clinical results of a prospective pilot study. J Oral Maxillofac Surg. 2011;69:84–91. doi: 10.1016/j.joms.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Curi MM, Cossolin GS, Koga DH, et al. Bisphosphonate-related osteonecrosis of the jaws--an initial case series report of treatment combining partial bone resection and autologous platelet-rich plasma. J Oral Maxillofac Surg. 2011;69:2465–2472. doi: 10.1016/j.joms.2011.02.078. [DOI] [PubMed] [Google Scholar]
- 21.Epstein MS, Wicknick FW, Epstein JB, Berenson JR, Gorsky M. Management of bisphosphonate-associated osteonecrosis: pentoxifylline and tocopherol in addition to antimicrobial therapy. An initial case series. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:593–596. doi: 10.1016/j.tripleo.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 22.Magremanne M, Reychler H. Pentoxifylline and tocopherol in the treatment of yearly zoledronic acid-related osteonecrosis of the jaw in a corticosteroid-induced osteoporosis. J Oral Maxillofac Surg. 2014;72:334–337. doi: 10.1016/j.joms.2013.06.188. [DOI] [PubMed] [Google Scholar]
- 23.Freiberger JJ, Padilla-Burgos R, Chhoeu AH, et al. Hyperbaric oxygen treatment and bisphosphonate-induced osteonecrosis of the jaw: a case series. J Oral Maxillofac Surg. 2007;65:1321–1327. doi: 10.1016/j.joms.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Delanian S, Lefaix JL. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol. 2004;73:119–131. doi: 10.1016/j.radonc.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 25.Delanian S, Chatel C, Porcher R, Depondt J, Lefaix JL. Complete restoration of refractory mandibular osteoradionecrosis by prolonged treatment with a pentoxifylline-tocopherol-clodronate combination (PENTOCLO): a phase II trial. Int J Radiat Oncol Biol Phys. 2011;80:832–839. doi: 10.1016/j.ijrobp.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 26.Delanian S, Porcher R, Rudant J, Lefaix JL. Kinetics of response to long-term treatment combining pentoxifylline and tocopherol in patients with superficial radiation-induced fibrosis. J Clin Oncol. 2005;23:8570–8579. doi: 10.1200/JCO.2005.02.4729. [DOI] [PubMed] [Google Scholar]
- 27.Delanian S, Lefaix JL. Current management for late normal tissue injury: radiation-induced fibrosis and necrosis. Semin Radiat Oncol. 2007;17:99–107. doi: 10.1016/j.semradonc.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Lyons A, Ghazali N. Osteoradionecrosis of the jaws: current understanding of its pathophysiology and treatment. Br J Oral Maxillofac Surg. 2008;46:653–660. doi: 10.1016/j.bjoms.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Delanian S, Porcher R, Balla-Mekias S, Lefaix JL. Randomized, placebo-controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation-induced fibrosis. J Clin Oncol. 2003;21:2545–2550. doi: 10.1200/JCO.2003.06.064. [DOI] [PubMed] [Google Scholar]
- 30.Georges C, Lefaix JL, Delanian S. Case report: resolution of symptomatic epidural fibrosis following treatment with combined pentoxifylline-tocopherol. Br J Radiol. 2004;77:885–887. doi: 10.1259/bjr/62051205. [DOI] [PubMed] [Google Scholar]
- 31.Patel V, Gadiwalla Y, Sassoon I, et al. Prophylactic use of pentoxifylline and tocopherol in patients who require dental extractions after radiotherapy for cancer of the head and neck. Br J Oral Maxillofac Surg. 2016 doi: 10.1016/j.bjoms.2016.02.024. [DOI] [PubMed] [Google Scholar]


