Abstract
Rubber elongation factor (REF) and small rubber particle protein (SRPP) are two key factors for natural rubber biosynthesis. To further understand the roles of these proteins in rubber formation, six different genes for latex abundant REF or SRPP proteins, including REF138,175,258 and SRPP117,204,243, were characterized from Hevea brasiliensis Reyan (RY) 7-33-97. Sequence analysis showed that REFs have a variable and long N-terminal, whereas SRPPs have a variable and long C-terminal beyond the REF domain, and REF258 has a β subunit of ATPase in its N-terminal. Through two-dimensional electrophoresis (2-DE), each REF/SRPP protein was separated into multiple protein spots on 2-DE gels, indicating they have multiple protein species. The abundance of REF/SRPP proteins was compared between ethylene and control treatments or among rubber tree clones with different levels of latex productivity by analyzing 2-DE gels. The total abundance of each REF/SRPP protein decreased or changed a little upon ethylene stimulation, whereas the abundance of multiple protein species of the same REF/SRPP changed diversely. Among the three rubber tree clones, the abundance of the protein species also differed significantly. Especially, two protein species of REF175 or REF258 were ethylene-responsive only in the high latex productivity clone RY 8-79 instead of in RY 7-33-97 and PR 107. Some individual protein species were positively related to ethylene stimulation and latex productivity. These results suggested that the specific protein species could be more important than others for rubber production and post-translational modifications might play important roles in rubber biosynthesis.
Keywords: comparative proteomics, Hevea brasiliensis, ethylene stimulation, latex productivity, rubber elongation factor, small rubber particle protein
1. Introduction
Approximately 2500 plants generate natural rubber latex, but Hevea brasiliensis is the only widely used rubber tree for natural latex production [1]. Natural rubber biosynthesis is synthesized in the specialized cells which have a cytoplasm called latex [2]. In H. brasiliensis, these specialized cells are articulated laticifers, and are located within a thin layer under the tree bark. Natural latex contains ~35% rubber particles, ~60% water, and a small amount of lipids, carbohydrates, and lots of proteins [3,4].
Natural rubber polymer is found in rubber particles enclosed by a contiguous, monolayer biomembrane in the laticifer cells [5,6]. Rubber biosynthesis includes three major processes: the synthesis of the rubber monomer isopentenyl pyrophosphate (IPP); the synthesis of initiator molecules such as geranyl pyrophosphate, farnesyl pyrophosphate, and geranylgeranyl pyrophosphate (APPs); and the elongation of isoprene polymers. The synthesis of IPP and APPs is common to all plants [7]. However, the elongation of these subunits into rubber in latex-producing plants occurs at the surface of the rubber particles and is catalyzed by many protein complexes [8,9]. An experiment incubating the extracted rubber particles with [1-14C]-IPP confirmed the production of rubber polymer from IPP by the particles [5,9,10].
Although several candidates for rubber biosynthetic proteins have been identified through their association with rubber particles and in vitro biosynthesis rubber assay, the process of rubber biosynthesis is far from being understood. Two related classes of proteins have been shown to play a role in natural rubber biosynthesis: one is rubber elongation factor (REF) [11], the other is small rubber particle protein (SRPP) [12]. Hevea laticifers are layers of contiguous cells that are formed parallel to the vascular cambium [13]. Immunogold staining showed that REF is localized in both large and small rubber particles and in all laticifer layers, but SRPP is predominantly localized in small rubber particles and in laticifer layers in conducting phloem [14]. Computational study predicted that REF protein had organized aggregates of β-sheet, whereas SRPP protein formed helical fold structures [15]. Both REF and SRPP are highly hydrophobic proteins, however, they exhibit different affinities for the monolayer of rubber particle, and ellipsometry experiments showed that REF seems to penetrate into the rubber particle membrane while SRPP binds on the membrane surface [15,16].
REF and SRPP share a common REF domain. Studies on REFs and SRPPs in H. brasiliensis have been focused on a 14.6 kDa REF (gi|132270) and a 24 kDa SRPP (gi|14423933); their precise roles in natural rubber biosynthesis are still unknown. To better understand the roles of REF and SRPP in rubber biosynthesis, in this research, we characterized the six genes coding for the most abundant REF/SRPP proteins in latex from H. brasiliensis RY 7-33-97, compared their protein sequence structures and cellular locations, then investigated their expression pattern in response to ethylene at both the mRNA and the proteomics levels, and finally compared their abundance among three rubber tree clones with different levels of latex productivity. The results revealed that all the REF/SRPP proteins all have multiple protein species, but only a few protein species responded positively to ethylene stimulation and related to the latex productivity of rubber tree clones.
2. Results
2.1. REF and SRPP Subfamily Members in H. brasiliensis
Rubber elongation factor (REF) and small rubber particle protein (SRPP) are two key factors for natural rubber biosynthesis. A total of 18 REF/SRPP like sequences were predicted from the genome of H. brasiliensis after genomic sequencing [17]. In addition, in our previous work, we found the 13 of the 18 sequences coding protein can be found in rubber latex by shotgun analysis [18], and only 6 of them were abundant. Based on this information, the sequences of the six REF/SRPP genes were confirmed in H. brasiliensis by cDNA clone, and named as REF138, REF175, REF258, and SRPP117, SRPP204, SRPP243 based on their protein length. REF138, REF175, SRPP117, SRPP204 and SRPP243 are identical to the predicted sequences by genome, but REF258 has a much longer N-terminal than the predicted one.
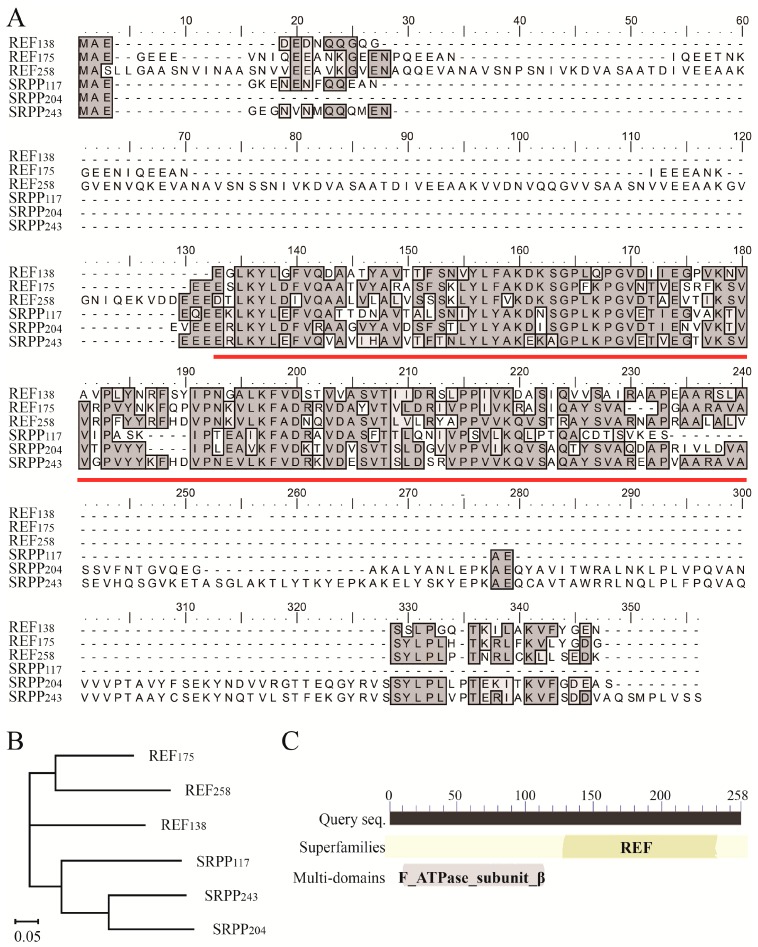
Sequence alignment of the six REF and SRPP proteins from H. brasiliensis RY 7-33-97 showed the relatively conserved REF domain [19]. The conserved REF domain is ~110 amino acid (aa) in all proteins except for SRPP117, which has a small deletion (Figure 1A). Other than the REF domain, the N- and C-terminal of the six REF and SRPP members have clear differences in length and compositions of amino acids (Figure 1A). REF subfamily members have a short C-terminal of constant length but an N-terminal of variable length, while SRPP subfamily members have a short N-terminal but a C-terminal of variable length. REF138 and SRPP117 have only a brief N- and C-terminal sequence flanking the REF domain, while REF258 has the longest N-terminal sequence, consisting of about 120 aa and containing a sequence similar to the β-subunit of ATPase (Figure 1C). Evolutionary analysis of the six protein members of REF and SRPP revealed that REF138, REF175, REF258, and SRPP117, SRPP204, SRPP243 are spread across the three major branches of the evolutionary tree, and REF258 is close to REF175 in the evolutionary relationship (Figure 1B).
Figure 1.
Multiple protein sequence alignment and phylogeny analysis of different REF/SRPP family members in the rubber tree. (A) Sequence alignment of REF and SRPP. Threshold into gray frames is 50% and the approximate location of the REF domain is underlined. Identical and similar amino acids are colored with dark and light gray frames, respectively; (B) Phylogeny relationships of REF and SRPP by complete protein sequence. Branch lengths are proportional to accumulated amino acid substitutions. Accession numbers in GeneBank of the six genes are listed in the Materials and Methods section; (C) The multiple domains of REF258.
2.2. Detection of the Six REF and SRPP Genes in H. brasiliensis Genomic DNA
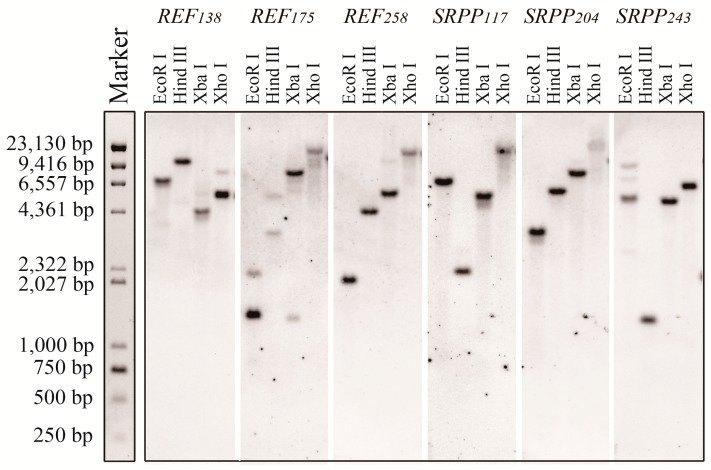
The six REF and SRPP members are quite different from each other (Figure 1A). Southern hybridization was used to identify whether there are additional homologies of the six REF and SRPP genes in the genome of H. brasiliensis. Partial sequences of each REF or SRPP were used as probes, and endonuclease EcoR I, Hind III, Xba I, and Xho I were used for genomic DNA digestion. The results showed that only one band was visible hybridization for most of the endonuclease-digested products of REF138, REF258, SRPP117, SRPP204, and SRPP243 (Figure 2), suggesting that REF138, REF258, SRPP117, SRPP204, and SRPP243 have no other close homologs in the rubber genome. Two bands were visible upon REF175 hybridization for most endonuclease-digested products (Figure 2), suggesting that there is one close homolog to REF175.
Figure 2.
Southern blot of REFs and SRPPs in genomic DNA isolated from rubber tree clone RY 7-33-97. Endonucleases EcoR I, Hind III, Xba I, and Xho I were used for digestion of genomic DNA. DNA molecular mass markers are shown on the left.
2.3. Subcellular Localization of REF and SRPP in Arabidopsis Protoplasts
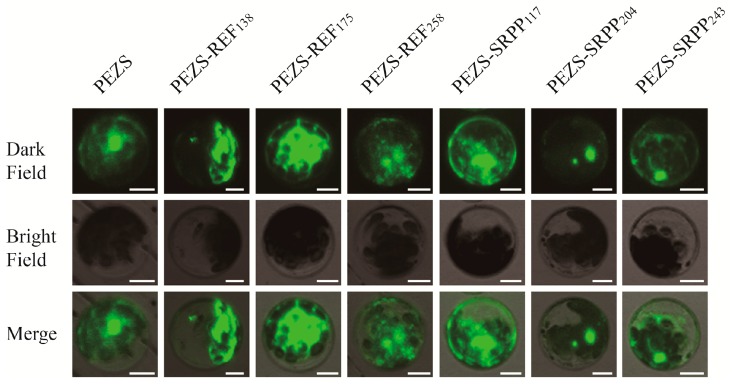
The 14.6 kDa REF138 protein is integral to the rubber particle membrane, and 24 kDa SRPP204 adheres to the membrane of rubber particles [16], and 19.6 kDa REF175 and 27.3 kDa REF258 are more tightly bound to the membrane than REF138 [20]. Transient expression of REF/SRPP-GFP in Arabidopsis protoplasts have been used to investigate if the subcellular localizations of these REF and SRPP proteins are similar to each other. The locations of REF and SRPP proteins in mesophyll cells of Arabidopsis leaves are not exclusive, as judged from the green fluorescence protein (GFP) fluorescence, but present two different tendencies for cellular location. The REF proteins demonstrated concentrate GFP fluorescence under shadow region observed in bright field by confocal which consisted of multiple organelles such as plastids. In contrast, the SRPP proteins showed diffused GFP fluorescence throughout the whole cells in cytoplasm, nuclear, and plasma membrane. Additionally, REF258 and the three SRPPs also demonstrated a kind of dotted pattern of GFP fluorescence in cytoplasm (Figure 3). The results revealed that in mesophyll cells REF175 and REF258 conducted a similar subcellular location as REF138, while SRPP117 and SRPP243 are located in a similar region as SRPP204, suggesting that they might have similar locations in laticifers. In addition, the dotted pattern for REF258, SRPP117 and SRPP243 is similar to the location of AtSRPPs/LDAPs in mesophyll cells in a certain way, which also confined to the cytosolic fraction with dot-like structures, and probably localized in lipid droplets [21,22].
Figure 3.
Subcellular localization of REF and SRPP proteins in Arabidopsis protoplasts using GFP-fluorescence. PEZS plasmids containing REF-GFP or SRPP-GFP fusions were transfected into the protoplasts. As a control a PEZS vector (containing GFP alone) was transfected. REF proteins appear to be gathered in the shadow zone of the cell under the view of Bright Field, and SRPP proteins appear to have diffused GFP fluorescence throughout the cells. GFP was excited with an argon laser at 488 nm and fluorescence images were taken using a confocal laser scanning microscope. Scale bars = 10 μm.
2.4. Gene Expression Patterns of REF and SRPP in Rubber Latex under Ethylene Treatment
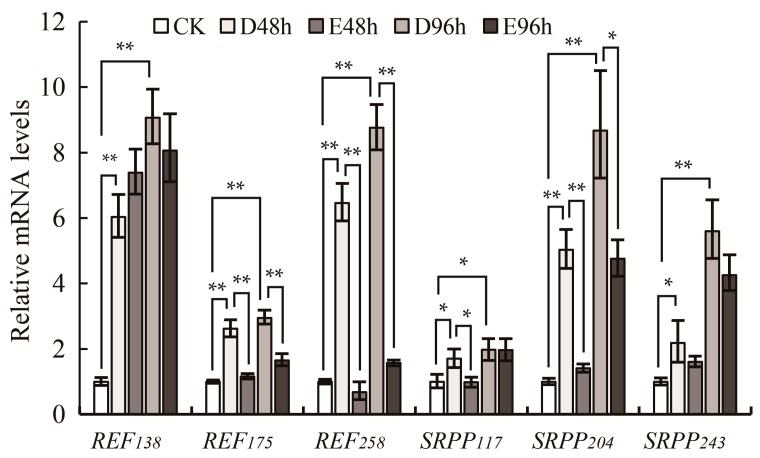
Ethylene stimulation strongly promotes formation of fresh latex and increases latex dry matter yield [23]. Based on studies of REF138 and SRPP204, some researchers suggested that ethylene stimulation did not change the expression levels of REF and SRPP in H. brasiliensis [19], but others suggested that ethylene treatment had a direct and positive effect on induction of REF gene expression [24]. We characterized the response to ethylene of the expression of the six REF and SRPP genes including REF138 and SRPP204. Our data reveals that the six REF/SRPP genes showed a similar expression pattern, that they were increased by tapping and decreased or little influenced by ethylene (Figure 4). Among them, REF258 and SRPP204 displayed the significant changes upon both tapping and ethylene, while REF138, SRPP117 and SRPP243 had little changes upon ethylene stimulation (Figure 4).
Figure 4.
The expression patterns of REF and SRPP in the latex of rubber tree clone RY 7-33-97. The relative expression levels of the six REF and SRPP genes were normalized to HbActin. CK (control check), latex from the first tapping; D48h, D96h, latex harvested 48 and 96 h after tapping, distilled water treatment; E48h, E96h, latex harvested 48 and 96 h after tapping, ethylene treatment. Data from qRT-PCR contains three biological repeats and the error bars indicate the standard deviations. Above an error bar, the symbol * indicates a difference of p < 0.05 (Student’s t-test), and the symbol ** indicates a difference of p < 0.01 (Student’s t-test); D48h and D96h were compared to CK, and E48h and E96h were respectively compared to D48h and D96h.
2.5. Identification of Protein Species of Each REF/SRPP Protein
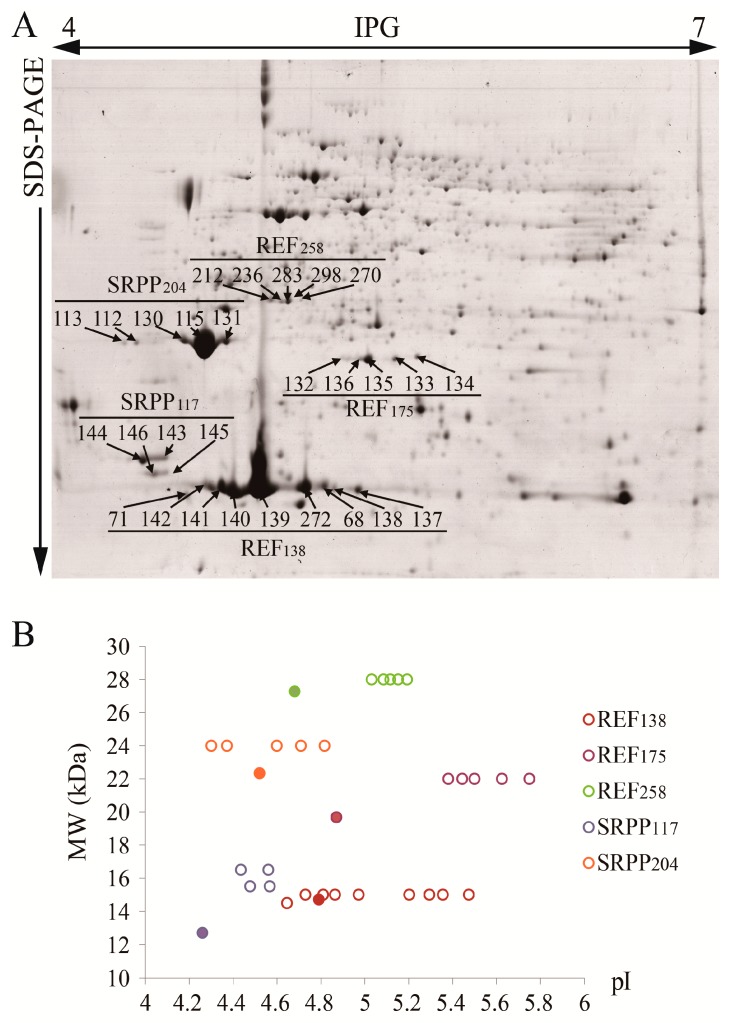
In a previous work on latex proteomics, we identified some protein spots as protein species of REF and SRPP, and noted that some of them were phosphorylated [23]. However, at that time the database used for identification of protein spots only included five REF/SRPP proteins, and the analysis did not distinguish these protein spots of individual REF and SRPP. To more comprehensively delineate the protein species of each REF and SRPP, we fractionated latex protein extracts using 2-DE and identified the protein species of each of the six abundant REF/SRPP proteins (Figure 5 and Table S1).
Figure 5.
The REF and SRPP protein species. (A) The distributions of REF and SRPP protein species on a typical 2-DE gel of latex proteins from rubber tree RY 7-33-97 are presented; (B) The distribution of MW (molecular weight) and pI (isoelectric point) of each REF and SRPP protein species. The filled circles represent the theoretical MW and pI of each REF and SRPP protein, and the hollow circles represent the experimental MW and pI of each REF and SRPP protein species.
By searching the resultant mass spectrum (MS) data with a self-built database of the REF/SRPP proteins, a total of 28 protein spots were positively identified as five REF/SRPP proteins (Figure 5A), and SRPP243 was not matched to any protein spots on the 2-DE gel, probably due to its isoelectric point (pI) is beyond the separation range of the 4–7 immobilized pH gradient (IPG) strip used in the experiments. Multiple protein species identified as a particular REF or SRPP appeared to be much different in pI but not in molecular weight (MW). Among them, REF138 was the most abundant REF/SRPP protein, and had at least 9 protein species. Spots identified as REF138, REF258 or SRPP204 are distributed around their theoretical MW, while spots identified as REF175 and SRPP117 are clearly bigger than their theoretical MW (Figure 5B). The pI of protein species of an individual REF (or SRPP) was quite diverse. For example, the pI of the nine protein species of REF138 were distributed from pI 4.65 to 5.4, compared to the theoretical pI of 4.79 (Figure 5B).
2.6. Characterization of REF/SRPP Protein Species in Different Rubber Tree Clones in Response to Tapping or Ethylene Treatment
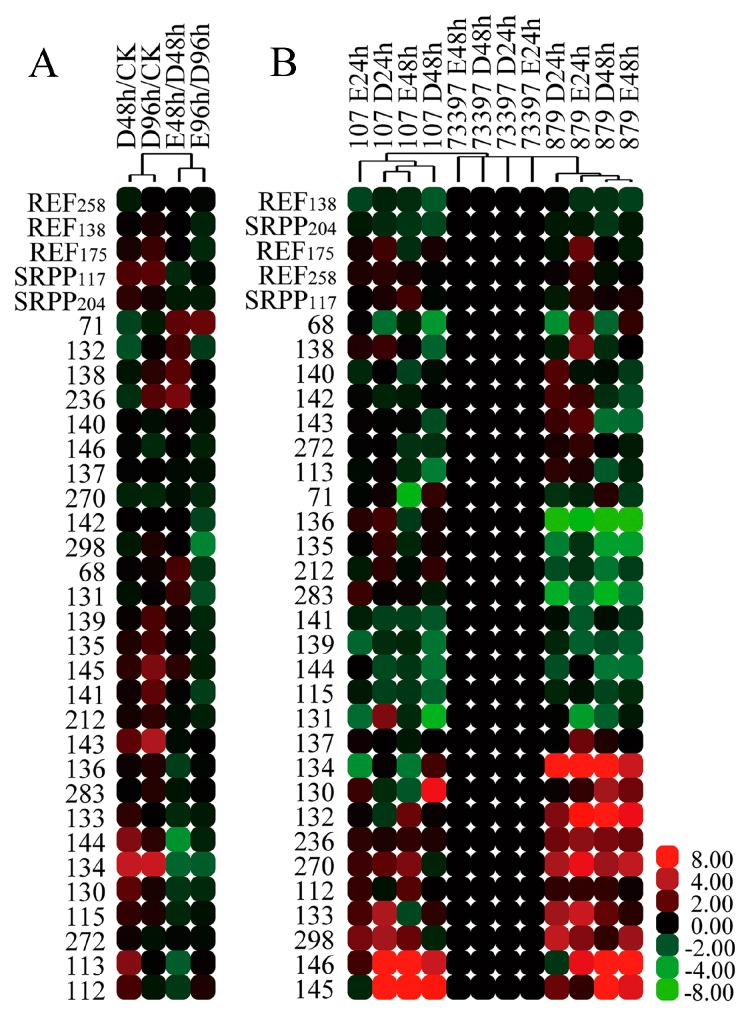
To analyze the influence of tapping and ethylene on the accumulation of these REF/SRPP protein species, total latex was collected from Hevea clone RY 7-33-97 before the treatments, and 48 and 96 h after treating with ddH2O (control) or with 3% ethephon. The total protein abundance of each REF/SRPP was calculated by adding all the abundance of their protein species together. The total abundance of REF/SRPP proteins was up-regulated or little changed after tapping, and down-regulated or little changed after ethylene treatment (Figure 6A), consistent with their gene expression patterns (Figure 4). The abundance of multiple protein species of a same REF/SRPP was diverse from each other. Most of these protein species were up-regulated by tapping, but down-regulated by ethylene. Spot 134 of REF175 is the most representative one, which is significantly up-regulated by tapping and down-regulated by ethylene. Only a few of these protein species were up-regulated by ethylene, such as spot 71 of REF138, spot 236 of REF258 (Figure 6A).
Figure 6.
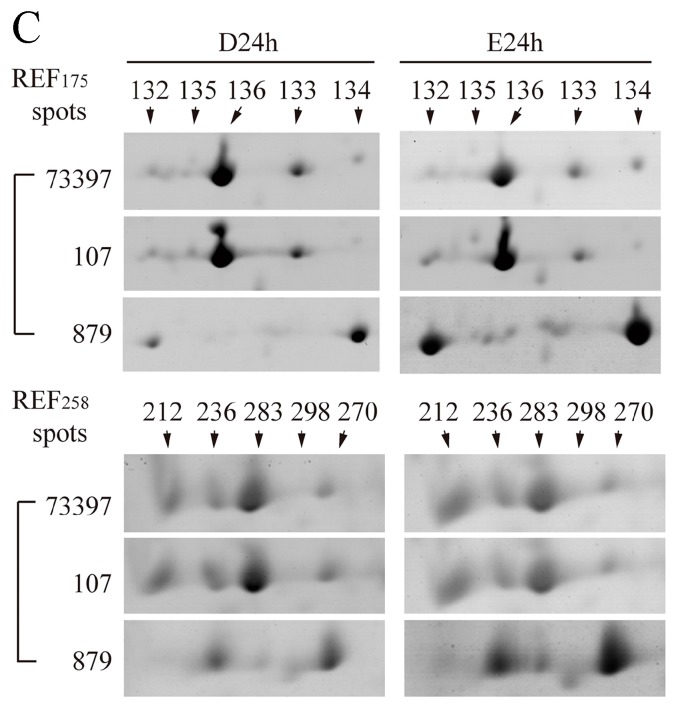
The abundance of the protein species of each REF/SRPP in high yielding and lower yielding clones of rubber tree upon ethylene treatment. (A) Clustering analysis of the relative abundance of the total protein and its protein species of each REF/SRPP after tapping and ethylene treatment. The abundance of each protein species in different treatments was normalized to their corresponding values in the control, CK; (B) Clustering analysis of the relative abundance of the total protein and the protein species of each REF/SRPP in rubber tree clones RY 7-33-97, PR 107 and RY 8-79. The abundance values of each protein or protein species were normalized to their corresponding values in RY 7-33-97; (C) 2-DE gel images of the protein species of REF175 and REF258 in rubber clones RY 7-33-97, PR 107 and RY 8-79. CK, the latex collected from the first tapping. D24h, D48h, D96h, latex from rubber trees at 24, 48, and 96 h under distilled water treatment. E24h, E48h, E96h, latex from rubber trees at 24, 48, and 96 h the under 3% ethephon treatment.
The abundance of different protein species of individual REF and SRPP was also determined in three different rubber tree clones, which have different latex productivity followed the order of RY 8-79 > RY 7-33-97 > PR 107 [25,26,27]. The total protein abundance of these REF/SRPPs showed small difference, but the abundance of individual protein species of them was very different among the three rubber tree clones (Figure 6B). Compared to RY 7-33-97 and PR 107, spots 132, 134, 236 and 270, had an obviously higher abundance, whereas spots 135, 136, 212 and 283 were extremely lower in RY 8-79 (Figure 6B). These eight protein species were all originating from REF175 and REF258 (Figure 6C), suggesting the two proteins are the most diverse REF/SRPP members among the three different rubber tree clones. The protein species 132, 134 of REF175 and 236, 270 of REF258 were not only predominant but also quite promoted by ethylene treatment in the high latex productivity rubber clone RY 8-79 (Figure 6C), suggesting the possible positive connection of these four protein species to latex productivity.
3. Discussion
3.1. The REF/SRPP Family in H. brasiliensis
Plant REF/SRPP family consists of either rubber biosynthesis related REF/SRPP proteins in latex-producing plants or some stress-related proteins in non-latex producing plants. All protein members in this family have a similar ~110 aa region called REF domain, but the differences between REF and SRPP subfamily have not been defined yet. In this work, the difference of REF and SRPP protein subfamily has been defined: REF subfamily members have a changeable N-terminal and a relatively short C-terminal beyond REF domain, while SRPP subfamily members have a short N-terminal and a changeable C-terminal (Figure 1A). In H. brasiliensis, through genome sequencing, eighteen predicted coding sequence (CDS) sequences for REF/SRPP were recently discovered, and these REF/SRPPs were annotated as eight REFs and ten SRPPs [17], most of these REF/SRPP sequences are unconfirmed by mRNA or proteins yet. In our previous work, we found only 13 of the 18 predicted REF/SRPP proteins could be identified in rubber latex by shotgun analysis [18]. In some other lactiferous plants, also are many REF/SRPP like proteins, which have demonstrated function in rubber biosynthesis. As in Taraxacum brevicorniculatum, six REF/SRPPs have been discovered, in which, SRPP 3, 4, and 5 are more close to REF of Hevea, and SRPP 2 is more close to SRPP of Hevea [28].
3.2. REF258 Is a Multiple-Domain Protein in REF/SRPP Family
The six latex abundant REF/SRPP protein genes are corresponding to REF1 (REF138), REF3 (REF175), REF8 (REF258), REF7 (SRPP117), SRPP1 (SRPP204), and SRPP2 (SRPP243) of the genome predicted CDS [28]. The REF8 and REF258 are not completely consistent, that predicted REF8 misses a 108 bp sequence compared to REF258, which causes the protein of REF8 misses a 36 aa peptides in the N-terminal. We blasted REF258 in NCBI protein database, and identified a ~120 aa domain in the N-terminal as a β subunit of ATP synthase (Figure 1C). The N-terminal region has some similarity to Mycobacterial beta/delta fusion protein, and to a subunit from a Methanosarcina barkeri protein, which is with sodium-translocating ATP synthase.
We searched proteins with both REF domain and other extra domain like REF258 in the NCBI database, and noticed that this multiple domain architecture also existed in many other proteins such as Trehalase-REF (gi|596158908|XP_007222867, gi|645230894|XP_008222142, gi|658009949|XP_008340200), TM_PBP1_branched-REF (gi|460403916|XP_004247432, gi|565387203|XP_006359392), and MCP_signal-REF (gi|495337605|WP_008062341) (Figure S1). Unfortunately, there is no functional study of these proteins. In a multiple domain protein, each domain may fulfill a function independently or in concert with its neighbors [29]. REF is important for rubber biosynthesis, and the REF protein can aggregate with itself or with SRPP [15,30]. REF also has been shown to display strong aggregating properties with native neutral lipids extracted from H. brasiliensis latex [31]. In addition, REF175 and REF258 have been demonstrated bind more tightly to rubber particles than REF138 [20]. We speculated that REF258 could form complexes with other REF and SRPP proteins, and even other ATP synthase subunits, providing a new cooperative function of rubber biosynthesis and ATP usage. Such as REF-like proteins in avocado (Persea americana), which have revealed two aspects of functions: the packaging of triacylglycerols as well as oil biosynthesis on the oil body surface in mesocarp cells [32].
3.3. The Multiple Protein Species of REF/SRPP in H. brasiliensis
Ethylene stimulation increases the production of latex [33]. Studies showed ethylene stimulation not only accelerated the glycolytic pathway, which supplies precursors for the biosynthesis of IPP and natural rubber [34], but also activated the general biosynthesis of natural rubber between tappings [23]. The gene expression of most REF/SRPPs decreased (Figure 4), and the total protein abundance of each REF/SRPP was slightly changed after ethylene treatment (Figure 6A). At the same time, the abundance of multiple protein species of each REF/SRPP changed diversely among the three rubber tree clones with different latex productivity (Figure 6B). Multiple protein species of a protein could be created through single-nucleotide polymorphisms (SNPs), alternative splicing (AS), post translational modifications (PTMs) or protein degradation, but more than 90% of them are generated by PTMs [35]. In our previously work, spots 140/141/142, 144/146, 115/130 were found to be phosphorylated modifications on REF138, SRPP117, and SRPP204, respectively [23]. In this experiment, none of these phosphorylated modifications changed much after ethylene treatment (Figure 6A), or showed obvious difference among different rubber clones (Figure 6B). However, we found some protein species of REF175 (132, 134) and REF258 (236, 270) were not only predominant but also ethylene-responsive in high latex productivity clone RY 8-79 (Figure 6C), suggesting their positive relations to latex production. These four protein species of REF175 and REF258 seem not to be dyed in red by Pro-Q diamond [23], suggesting they are not phosphorylated protein species, still their modifications were unknown. Protein species of a same REF/SRPP varied diversely in response to ethylene treatment or among rubber tree clones might have biological significance. We proposed specific protein species which are dominant in high latex productivity rubber tree and positive response to ethylene stimulation might be important for natural rubber biosynthesis.
4. Materials and Methods
4.1. Plant Materials and Treatments
Latex samples were obtained from newly tapped mature rubber plants (~8-year-old, H. brasiliensis Mull. Arg., clones RY 7-33-97, RY 8-79 and PR 107) growing at an experimental farm of the Chinese Academy of Tropical Agricultural Sciences at Danzhou city in Hainan province of China. Ethephon is a type of ethylene release agent which is capable of direct chemical decomposition into ethylene under physiological conditions [36,37]. When ethephon is applied to the bark in the region of the tapping cut, ethylene commences immediately at the site of application, and quickly translocates throughout the plant [38]. To determine the direct effects of ethylene stimulation, rubber trees were tapped one day before treatments, then the tree cuts were brushed with ethephon (3%, v/v) or ddH2O (the control) 24 h after the first tapping as described [39]. Latex samples were collected from each rubber tree one day before treatment (as the control, CK) and the second (D24h, E24h), third (D48h, E48h) and fifth (D96h, E96h) day after ethephon or ddH2O treatments. Treatments and sample collections were carried on with three independent biological replicates and latex from 15 trees were harvested for the 3 replicates (5 trees/replicate). Among these clones, RY 7-33-97 and RY 8-79 are the two elite clones bred by Chinese Academy of Tropical Agricultural Sciences, the former one is a fast-growing and high-yielding clone, and the later one is an early maturing and high-yielding clone. PR 107 is a late-maturing clone, having lower yield in its first few years, and gradually increases in later years. These three clones have remarkable differences in their latex production ability in the first few years of tapping, the latex yield being RY 8-79 > RY 7-33-97 > PR 107 [25,26,27].
4.2. Gene Clone
RNA extraction from the latex was performed using high SDS extraction buffer (100 mM Tris-HCl, 300 mM LiCl, 10 mM EDTA-Na2, and 10% SDS). The first strand cDNA was synthesis using 1 μg total RNA and with SMARTer™ RACE cDNA Amplification Kit (Clontech, Dalian, China). The PCR products of six REF/SRPP genes were amplified and separated through an agarose gel and purified with the AxyPrep™ DNA gel extraction kit (Axygen, Suzhou, China). Purified PCR products were ligated into a clone vector PMD18T (Takara, Dalian, China) and transformed into Escherichia coli strain JM109. Multiple clones from each were sent for sequencing (Life Biological Technology Co., Guangzhou, China).
The complete cDNA sequence of REF and SRPP were submitted to the GenBank database under accession numbers of REF138 (KR076812), REF175 (KR076813), REF258 (KR076814), SRPP117 (KR076815), SRPP204 (KR076816), and SRPP243 (KR076817). The MW and pI of the deducted proteins from these genes were calculated using DNAMAN 5.0 software (Lynnon Biosoft, San Ramon, CA, USA). Multiple sequence alignments of REF and SRPP were assembled using the EBI ClustalW [40] and visualized by BioEdit 7.0 software (Ibis Biosciences, Carlsbad, CA, USA). Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 5 software as described [41].
4.3. Southern Blot Analysis
Southern blotting was conducted by using Dig Application Manual for Filter Hybridization (Roche, Basel, Switzerland). H. brasiliensis genomic DNA was extracted from the leaves of rubber tree and digested with BamH I, EcoR I, Hind III, and Xba I (Thermo Scientific, Guangzhou, China). Ten μg of each digestion product was electrophoresed on a 0.8% agarose gel at 1 V/cm overnight, and then transferred onto a positively charged nylon membrane (Roche, Basel, Switzerland) through capillary transfer by 20× SSC (saline sodium citrate) for about 12 h. UV (ultraviolet) crosslink of the DNA to the filter was at a strength of 90,000 μJ/m2. The cross-linked filters were hybridized with ~350 bp Dig PCR labeled probes of each REF and SRPP genes, respectively. The primers for the probes are listed in Table S2. Hybridization was performed at 42 °C and the probes were washed at a low stringent condition. DNA blots were visualized by LAS4000mini (GE Healthcare, Uppsala, Sweden).
4.4. REF/SRPP-GFP Fusion Transient Expression
The sequences of the REF/SRPP genes were amplified by PCR and cloned into the vector, pEZS-NL, which has a GFP tag positioned at the C-terminal of the insert. The purified plasmids were then sequenced to confirm the fusion construct. The plasmids were extracted by an endotoxin free plasmid extraction kit (CWBIO, Shanghai, China) to a final concentration of 2 μg/μL, and stored in −20 °C.
Transient expression assay was performed according to the method [42]. Mesophyll protoplasts were isolated from the leaves of 3-week old Arabidopsis Col-0 plants. Ten μg of each REF/SRPP-GFP fusion plasmids were transfected into 4 × 104 protoplasts using polyethylene glycol (PEG) solution (0.4 g/mL PEG 4000, 0.2 M mannitol, 100 mM CaCl2), incubated at room temperature for 20 min, washed and re-suspended with 1 mL W5 solution (154 mM NaCl, 125 mM CaCl2, 5 mM KCl, 2 mM MES, 5 mM glucose at pH 5.7), placed under a dark condition at 22 °C for 16–24 h, then imaged by confocal laser scanning microscope (Olympus fv1000, Tokyo, Japan).
4.5. Quantitative PCR Analysis of REF and SRPP
The first strand cDNA synthesis was performed using 1 μg of total RNA and a Revert Aid First Strand cDNA Synthesis Kit in 25 μL volume (Thermo Scientific, Guangzhou, China). One μL of each cDNA synthesis was used to amplify the sequence for each REF or SRPP. The primers used for PCR analysis were designed according to the sequence dissimilarity regions among the six REF/SRPP genes. The HbActin gene (JF775488.1) from H. brasiliensis was used as an internal control. All primers are listed in Table S2. For quantitative PCR (qPCR), the amplifications were performed using 2× Maxima SYBR Green/ROX, qPCR Master Mix (Thermo Scientific, Guangzhou, China) and the Mx3005P QPCR System (Agilent Technologies, Santa Clara, CA, USA). The relative mRNA levels were calculated by MxPro, the system included software based on 2−ΔΔCt method [43]. Three biological replicates were performed in qPCR with samples from the same batch of latex of RY 7-33-97 as was used for protein extraction.
4.6. Protein Extraction and 2-DE
Latex proteins were extracted as described [39]. Protein concentration was determined by the Bradford assay with BSA as a standard. For 2-DE, 1300 μg proteins were loaded onto the 24 cm, pH 4–7 linear IPG strips (GE Healthcare, Uppsala, Sweden), then performed isoelectric focusing and SDS-PAGE as described [31]. The MW, pI, and volume% of each protein spot was calculated by ImageMaster 5.0 software (GE Healthcare, Uppsala, Sweden). Spots were eluted from gel for protein enzymolysis and identification through mass spectrometry on an AB 5800 MALDI-TOF/TOF-MS instrument (AB SCIEX, Foster City, CA, USA) as described [44]. The sequences of REF/SRPP were formatted into text files that can be used by ProteinPilot software to provide identification of the 2-DE spots. The values of the vol.% were used to represent the level of each REF/SRPP protein. The total abundance for each REF/SRPP protein was calculated by adding the abundance of its spots (protein species) together. The clustering of REF/SRPP protein species that result from different treatments or different rubber tree clones were performed by Cluster 3.0 software and visualized by TreeView1.1.6 software (Stanford University, Stanford, CA, USA).
5. Conclusions
In this study, we systematically characterized the sequences, locations, expressions, and protein species of the latex-abundant REF/SRPP family members in H. brasiliensis. Based on the obtained results, we further defined the characteristics of REF and SRPP subfamily, clarified the expression patterns of REF/SRPP members upon ethylene treatment, and disclosed the multiple protein species of each REF/SRPP protein. The finding of REF domain and ATPase β subunit united in REF258 implies a coordinating role of REF258 in latex production. More importantly, we noticed that the protein species of each REF/SRPP protein behaved differently among the three rubber tree clones RY 7-33-97, RY 8-79 and PR 107, and REF175 and REF258 each has two protein species predominant and ethylene-responsive in high productivity rubber tree clone RY 8-79. The results suggested that only individual protein species of REF/SRPP protein could positively relate to rubber biosynthesis and latex production. We presented some tips for further study of the not-yet explicit process of rubber biosynthesis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant No. 31500557, 31370681, 31270712, 31570301), the Major Technology Project of Hainan (ZDZX2013023-1) and Central Public-interest Scientific Institution Basal Research Fund for Innovative Research Team Program of CATAS (No. 17CXTD-29).
Supplementary Materials
Supplementary materials can be found at www.mdpi.com/1422-0067/18/5/958/s1.
Author Contributions
Xuchu Wang and Zheng Tong conceived and designed research; Eve Syrkin Wurtele and Ling Li guide the experiments; Zheng Tong conducted experiments of gene clone, Southern hybridization, subcellular location, gene expression analysis; Yong Sun conducted the protein extraction; Dan Wang conducted the 2-DE experiment and analyzed the proteomics data; Qian Yang conducted the MS identification; Limin Wang, Xueru Meng, and Weiqiang Feng assisted the experiments or data analysis; Zheng Tong, Eve Syrkin Wurtele, Ling Li and Xuchu Wang wrote the manuscript; All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bonner J. The history of rubber. In: Whitworth J.W., Whitehead E.E., editors. Guayule Natural Rubber: A Technical Publication with Emphasis on Recent Findings. Guayule Administrative Management Committee and US Department of Agriculture Cooperative State Research Service, Office of Arid Lands Studies, University of Arizona; Tucson, AZ, USA: 1991. pp. 1–16. [Google Scholar]
- 2.Hagel J.M., Yeung E.C., Facchini P.J. Got milk? The secret life of laticifers. Trends Plant Sci. 2008;13:631–639. doi: 10.1016/j.tplants.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Nor H.M., Ebdon J.R. Telechelic liquid natural rubber: A review. Prog. Polym. Sci. 1998;23:143–177. doi: 10.1016/S0079-6700(97)00028-2. [DOI] [Google Scholar]
- 4.Sakdapipanich J.T. Structural characterization of natural rubber based on recent evidence from selective enzymatic treatments. J. Biosci. Bioeng. 2007;103:287–292. doi: 10.1263/jbb.103.287. [DOI] [PubMed] [Google Scholar]
- 5.Cornish K., Backhaus R. Rubber transferase activity in rubber particles of guayule. Phytochemistry. 1990;29:3809–3813. doi: 10.1016/0031-9422(90)85337-F. [DOI] [Google Scholar]
- 6.Cornish K., Wood D.F., Windle J.J. Rubber particles from four different species, examined by transmission electron microscopy and electron-paramagnetic-resonance spin labeling, are found to consist of a homogeneous rubber core enclosed by a contiguous, monolayer biomembrane. Planta. 1999;210:85–96. doi: 10.1007/s004250050657. [DOI] [PubMed] [Google Scholar]
- 7.Chen F., Tholl D., Bohlmann J., Pichersky E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011;66:212–229. doi: 10.1111/j.1365-313X.2011.04520.x. [DOI] [PubMed] [Google Scholar]
- 8.Archer B.L., Audley B.G., Cockbain E.G., McSweeney G.P. The biosynthesis of rubber. Incorporation of mevalonate and isopentenyl pyrophosphate into rubber by Hevea brasiliensis-latex fractions. Biochem. J. 1963;89:565–574. doi: 10.1042/bj0890565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benedict C.R., Madhavan S., Greenblatt G.A., Venkatachalam K.V., Foster M.A. The enzymatic synthesis of rubber polymer in Parthenium argentatum Gray. Plant Physiol. 1990;92:816–821. doi: 10.1104/pp.92.3.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamashita S., Yamaguchi H., Waki T., Aoki Y., Mizuno M., Yanbe F., Ishii T., Funaki A., Tozawa Y., Miyagi-Inoue Y., et al. Identification and reconstitution of the rubber biosynthetic machinery on rubber particles from Hevea brasiliensis. eLife. 2016;5:e19022. doi: 10.7554/eLife.19022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennis M.S., Light D.R. Rubber elongation factor from Hevea brasiliensis. Identification, characterization, and role in rubber biosynthesis. J. Biol. Chem. 1989;264:18608–18613. [PubMed] [Google Scholar]
- 12.Oh S.K., Kang H., Shin D.H., Yang J., Chow K.S., Yeang H.Y., Wagner B., Breiteneder H., Han K.H. Isolation, characterization and functional analysis of a novel cDNA clone encoding a small rubber particle protein from Hevea brasiliensis. J. Biol. Chem. 1999;274:17132–17138. doi: 10.1074/jbc.274.24.17132. [DOI] [PubMed] [Google Scholar]
- 13.Hao B.Z., Wu J.L. Laticifer Differentiation in Hevea brasiliensis: Induction by exogenous jasmonic acid and linolenic acid. Ann. Bot. 2000;85:37–43. doi: 10.1006/anbo.1999.0995. [DOI] [Google Scholar]
- 14.Sando T., Hayashi T., Takeda T., Akiyama Y., Nakazawa Y., Fukusaki E., Kobayashi A. Histochemical study of detailed laticifer structure and rubber biosynthesis-related protein localization in Hevea brasiliensis using spectral confocal laser scanning microscopy. Planta. 2009;230:215–225. doi: 10.1007/s00425-009-0936-0. [DOI] [PubMed] [Google Scholar]
- 15.Berthelot K., Lecomte S., Estevez Y., Coulary-Salin B., Bentaleb A., Cullin C., Deffieux A., Peruch F. Rubber elongation factor (REF), a major allergen component in Hevea brasiliensis latex has amyloid properties. PLoS ONE. 2012;7:e48065. doi: 10.1371/journal.pone.0048065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berthelot K., Lecomte S., Estevez Y., Zhendre V., Henry S., Thevenot J., Dufourc E.J., Alves I.D., Peruch F. Rubber particle proteins, HbREF and HbSRPP, show different interactions with model membranes. Biochim. Biophys. Acta. 2014;1838:287–299. doi: 10.1016/j.bbamem.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Tang C.R., Yang M., Fang Y.J., Luo Y.F., Gao S.H., Xiao X.H., An Z., Zhou B., Zhang B., Tan X., et al. The rubber tree genome reveals new insights into rubber production and species adaptation. Nat. Plants. 2016;2:16073. doi: 10.1038/nplants.2016.73. [DOI] [PubMed] [Google Scholar]
- 18.Feng W.Q., Tong Z., Jin X., Wang D., Sun Y., Meng X.R., Wang X.C. Cloning and Expression of REF/SRPP Gene Family in Rubber Tree (Hevea brasiliensis) Mol. Plant Breed. 2016;14:3024–3032. [Google Scholar]
- 19.Sookmark U., Pujade-Renaud V., Chrestin H., Lacote R., Naiyanetr C., Seguin M., Romruensukharom P., Narangajavana J. Characterization of polypeptides accumulated in the latex cytosol of rubber trees affected by the tapping panel dryness syndrome. Plant Cell Physiol. 2002;43:1323–1333. doi: 10.1093/pcp/pcf161. [DOI] [PubMed] [Google Scholar]
- 20.Dai L., Nie Z., Kang G., Li Y., Zeng R. Identification and subcellular localization analysis of two rubber elongation factor isoforms on Hevea brasiliensis rubber particles. Plant Physiol. Biochem. 2017;111:97–106. doi: 10.1016/j.plaphy.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Kim E.Y., Park K.Y., Seo Y.S., Kim W.T. Arabidopsis small rubber particle protein homolog SRPs play dual roles as positive factors for tissue growth and development and in drought stress responses. Plant Physiol. 2016;170:2494–2510. doi: 10.1104/pp.16.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gidda S.K., Park S.J., Pyc M., Yurchenko O., Cai Y.Q., Wu P., Andrews D.W., Chapman K.D., Dyer J.M., Mullen R.T. Lipid droplet-associated proteins (LDAPs) are required for the 19 he dynamic regulation 20 of neutral lipid compartmentation in plant cells. Plant Physiol. 2016;170:2052–2071. doi: 10.1104/pp.15.01977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X.C., Wang D., Sun Y., Yang Q., Chang L.L., Wang L.M., Meng X., Huang Q., Jin X., Tong Z. Comprehensive proteomics analysis of laticifer latex reveals new insights into ethylene stimulation of natural rubber production. Sci. Rep. 2015;5:13778. doi: 10.1038/srep13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Priya P., Venkatachalam P., Thulaseedharan A. Differential expression pattern of rubber elongation factor (REF) mRNA transcripts from high and low yielding clones of rubber tree (Hevea brasiliensis Muell. Arg.) Plant Cell Rep. 2007;26:1833–1838. doi: 10.1007/s00299-007-0402-z. [DOI] [PubMed] [Google Scholar]
- 25.Huang D.B., Qin Y.X., Tang C.R. Physiological characters of latex from three Hevea clones. Reyan: 8-79, Reyan 7-33-97 and PR107. J. Trop. Subtrop. Bot. 2010;18:170–175. [Google Scholar]
- 26.An F., Zou Z., Cai X., Wang J., Rookes J., Lin W., Cahill D., Kong L. Regulation of HbPIP2;3, a latex-abundant water transporter, is associated with latex dilution and yield in the rubber tree (Hevea brasiliensis Muell. Arg.) PLoS ONE. 2015;10:e0125595. doi: 10.1371/journal.pone.0125595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chao J.Q., Chen Y.Y., Wu S.H., Tian W.M. Comparative transcriptome analysis of latex from rubber tree clone CATAS8–79 and PR107 reveals new cues for the regulation of latex regeneration and duration of latex flow. BMC Plant Biol. 2015;15:1–12. doi: 10.1186/s12870-015-0488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laibach N., Hillebrand A., Twyman R.M., Prüfer D., Gronover C.S. Identification of a Taraxacum brevicorniculatum rubber elongation factor protein that is localized on rubber particles and promotes rubber biosynthesis. Plant J. 2015;82:609–620. doi: 10.1111/tpj.12836. [DOI] [PubMed] [Google Scholar]
- 29.Abdin M.Z., Kiran U., Alam A. Analysis of osmotin, a PR protein as metabolic modulator in plants. Bioinformation. 2011;5:336–340. doi: 10.6026/97320630005336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berthelot K., Lecomte S., Estevez Y., Peruch F. Hevea brasiliensis REF (Hev b 1) and SRPP (Hev b 3): An overview on rubber particle proteins. Biochimie. 2014;106:1–9. doi: 10.1016/j.biochi.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Wadeesirisak K., Castano S., Berthelot K., Vaysse L., Bonfils F., Peruch F., Rattanaporn K., Liengprayoon S., Lecomte S., Bottier C. Rubber particle proteins REF1 and SRPP1 interact differently with native lipids extracted from Hevea brasiliensis latex. Biochim. Biophys. Acta. 2017;1859:201–210. doi: 10.1016/j.bbamem.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Horn P.J., James C.N., Gidda S.K., Kilaru A., Dyer J.M., Mullen R.T., Ohlrogge J.B., Chapman K.D. Identification of a new class of lipid droplet-associated proteins in plants. Plant Physiol. 2013;162:1926–1936. doi: 10.1104/pp.113.222455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D’Auzac J., Ribaillier D. Ethylene, a new agent stimulating latex production in Hevea brasiliensis. CR Hebd. Seances Acad. Sci. D. 1969;268:3046–30499. [Google Scholar]
- 34.Liu J.P., Zhuang Y.F., Guo X.L., Li Y.J. Molecular mechanism of ethylene stimulation of latex yield in rubber tree (Hevea brasiliensis) revealed by de novo sequencing and transcriptome analysis. BMC Genom. 2016;17:257. doi: 10.1186/s12864-016-2587-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang F., Chen J.Y. A method for identifying discriminative isoform-specific peptides for clinical proteomics application. BMC Genom. 2016;17:522. doi: 10.1186/s12864-016-2907-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooke A.R., Randall D.I. 2-Haloethanephosphonic acids as ethylene releasing agents for the induction of flowering in pineapples. Nature. 1968;218:974–975. doi: 10.1038/218974a0. [DOI] [PubMed] [Google Scholar]
- 37.Abraham P.D., Wycherley P.R., Pakianathan S.W. Stimulation of latex flow in Hevea brasiliensis by 4-Amino—3,5,6-Trichloropicolinic acid and 2-chloroethanephosphonic acid. Rubber Chem. Technol. 1972;45:883–899. doi: 10.5254/1.3542895. [DOI] [Google Scholar]
- 38.Audley B.G., Archer B.L., Carruthers I.B. Metabolism of ethephon (2-chloroethylphosphonic acid) and related compounds in Hevea brasiliensis. Arch. Environ. Contam. Toxicol. 1976;4:183–200. doi: 10.1007/BF02221023. [DOI] [PubMed] [Google Scholar]
- 39.Wang X.C., Shi M.J., Wang D., Chen Y.Y., Cai F.G., Zhang S.X., Wang L., Tong Z., Tian W.M. Comparative proteomics of primary and secondary lutoids reveals that chitinase and glucanase play a crucial combined role in rubber particle aggregation in Hevea brasiliensis. J. Proteome Res. 2013;12:5146–5159. doi: 10.1021/pr400378c. [DOI] [PubMed] [Google Scholar]
- 40.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 41.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoo S.D., Cho Y.H., Sheen J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007;2:1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
- 43.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(t) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 44.Wang X.C., Shi M.J., Lu X.L., Ma R.F., Wu C.G., Guo A.P., Peng M., Tian W. A method for protein extraction from different subcellular fractions of laticifer latex in Hevea brasiliensis compatible with 2-DE and MS. Proteome Sci. 2010;8:1–10. doi: 10.1186/1477-5956-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.