Abstract
During early mouse embryogenesis, each laminin (Lm) chain of the first described Lm, a heterotrimer of α1, β1, and γ1 chains (Lm-1), is essential for basement membrane (BM) assembly, which is required for pregastrulation development. Individual domains may have other functions, not necessarily structural. The cell binding C terminus of Lm α1 chain contains five Lm globular (LG) domains. In vitro, α1LG1–3 domains bind integrins, and α1LG4 binds dystroglycan, heparin, and sulfatides. A prevailing hypothesis is that α1LG4 is crucial as a structural domain for BM assembly, whereas integrin-binding sites conduct signaling. The in vivo role of α1LG4–5 (also called E3) has not been studied. Mice lacking α1LG4–5 were therefore made. Null embryos implanted, but presumptive epiblast cells failed to polarize and did not survive past day 6.5. BM components including truncated Lm α1 were detected in Reichert's membrane. Surprisingly, embryonic BM assembly between visceral endoderm and stem cells was normal in null embryos and in embryoid bodies of α1LG4–5-null embryonic stem cells. Yet, stem cells could not develop into polarized epiblast cells. Thus, α1LG4–5 provides vital signals for the conversion of stem cells to polarized epithelium.
Keywords: epiblast, epithelial polarity, stem cells, mouse development
The three first differentiation events in mammals are conversions of stem cells to epithelial cells. The two first-formed epithelia, the trophectoderm and the primitive endoderm, form no fetal cells. The third, occurring after implantation, leads to formation of epiblast cells from which the entire fetus is derived (1). Similar processes occur throughout development. External factors that initiate stem cell conversion to polarized epithelia are not well known, but growth factors or extracellular matrix may be involved (2).
Epithelial development is accompanied by formation of a basement membrane (BM) (3), an evolutionary ancient extracellular matrix containing laminins (Lm), trimers existing as at least 15 isoforms (4), collagens IV, XV, and XVIII; nidogens; perlecan; agrin; and fibulins. Of these, only seven have been shown to be present during early embryogenesis of mouse, namely Lm-1 (α1β1γ1), Lm-10 (α5β1γ1), nidogen-1 and -2, perlecan, agrin, and collagen IV (α1, α2). These appear either before implantation or shortly after when the first embryonic epithelial sheet forms (4).
Lm-1 is well documented as one of the few essential extracellular matrix proteins in early embryogenesis. First, three different embryonic stem (ES) cell lines that for different reasons are unable to produce Lm-1 cannot form the columnar epiblast epithelia in embryoid body cultures but do so in response to exogenous Lm-1 (5, 6). Second, gene deletions in mouse demonstrated that each of the three Lm-1 chains is essential for early postimplantation embryogenesis (7, 8). Third, the lack of any other single BM component produced at this stage does not affect early embryogenesis and leads to death only at midgestation stages or has no effect on embryogenesis (4).
The structural roles of some Lm domains are well characterized. In vitro, N-terminal domains of each chain are involved in Lm polymerization (4), and one domain in the central region of Lm γ1 binds nidogens with high affinity (9). Cell binding is mainly mediated by the five C-terminal Lm globular (LG) domains of α1, as seen by in vitro studies. The main cell-adhesive site in vitro is composed of α1LG1–3 domains and the C termini of Lm β1 and γ1 chains and binds several integrins (4). The major Lm receptor in vitro, integrin α6β1, is not required for embryogenesis in vivo as shown by gene targeting of integrin α6 (10). Some of the 11 other β1-integrins may compensate, in agreement with data that β1 integrin-null mice die before gastrulation (11, 12). Surprisingly, no Lm-1 is produced by β1 integrin-null ES cells, and β1 integrin-null embryoid bodies can be rescued to epiblast differentiation in embryoid body cultures by the addition of Lm-1 (6). Hence, integrins are probably not the essential receptors for Lm-1-induced epiblast development.
Another cell attachment site for Lm-1 is α1LG4–5, which binds to cell surface receptor dystroglycan, heparin (13, 14), and sulfatides (15). α1LG4–5 is also called E3, because it can be cleaved by elastase (16). Most cell types bind poorly to α1LG4–5 (17), but teratocarcinoma cells, which resemble ES cells, bind well to α1LG4–5 (18). Based on organ culture studies in vitro, it has been suggested that α1LG4–5 has a role in epithelial development (19, 20). Biochemical in vitro assays have led to the major current theory that binding of α1LG4 to dystroglycan, sulfatides, or cell-surface proteoglycans initiates BM assembly (4, 16). However, both the proposals that α1LG4–5 is essential for epithelial development and that this requirement is because of its structural role rely on in vitro data. To study in vivo functions, we therefore made two mouse strains lacking α1LG4–5. This mutation leaves the structural network forming properties and integrin binding sites of Lm-1 intact. The data show that α1LG4–5 is essential for epiblast differentiation, not as a structural component of the embryonic BM but as a previously undescribed differentiation inducer.
Experimental Procedures
Generation of Mutant Mice. A targeting vector was made where an in-frame deletion of the exons coding for Lm α1LG4–5 was accomplished by fusion of a DraIII site with a SmaI site. A phosphoglycerol kinase neomycin (neo) herpes simplex virus thymidine kinase cassette, flanked by loxP sites was added as a selection marker, as detailed in Supporting Methods, which is published as supporting information on the PNAS web site. Then, 45 μg of targeting vector, linearized with XmnI, was electroporated into 3 × 106 R1 ES cells by using a gene pulser (Bio-Rad). Of 384 clones surviving selection in 350 μg/ml G418 (GIBCO/BRL), 1 had undergone homologous recombination in the α1LG4–5 locus. The 0.8-kb probe used was located immediately upstream of the targeting vector and was isolated by EcoRV/NotI digestion. The clone was injected into C57BL/6 blastocysts. A chimeric male founder was crossed with C57BL/6 females to obtain heterozygous F1 offspring. Genotypes were confirmed by Southern blot or PCR of tail biopsies. A second mouse strain with this mutation was generated by following the same procedures, except that selection markers were removed from the heterozygous clone by using procedures for Cre recombinase.
Genotyping by PCR. Mice were subjected to tail biopsies, and DNA was extracted. Primers used for PCR genotyping were as follows: forward, 5′-AGG GGT TCA TAG TTT AGG AT-3′, reverse 1, 5′-CTG AGG AAA ATG GCT TAC-3′, and reverse 2, 5′-TCC GTG TGG CTT TAG TTC-3′. Touchdown PCR with a final annealing temperature of 52°C gave a wild-type (WT) product of 287 base pairs (bp) and a mutant product of 405 bp.
Generation of α1LG4–5-Null (α1LG4–5-/-) ES Cells. ES cells lacking both α1LG4–5 alleles were generated. The frequency of homologous recombination was increased by the addition of diphtheria toxin to the vector, downstream of the short homology arm. The new vector was electroporated into heterozygous ES cells after removal of the selection marker in the targeted allele by Cre recombinase. Of 192 analyzed G418-resistant clones, one had undergone homologous recombination. Embryoid bodies were cultured as described in refs. 5 and 21.
Immunoprecipitation. Tissues were homogenized and sonicated in buffer (500 mM NaCl/50 mM Tris·HCl/10 mM EDTA, pH 7.4) containing EDTA-free complete protease inhibitor mixture (Roche). Lysates were incubated with normal rabbit serum and Sepharose A, then with 1.2 mg/ml antibody against Lm α1 N terminus (22) at +4°C, and were rotated for 24 h after addition of 50% Sepharose A. Reducing loading buffer was added, and samples were heated at 100°C for 5 min and analyzed by SDS/PAGE.
Histology. Sections stained with hematoxylin/eosin or antibodies were analyzed with Zeiss Axioplan 2 and a Leica confocal microscope. All sections were analyzed at ×40 magnification. Primary antibodies were as follows: monoclonal antibody 200 detecting α1LG4 (18), rabbit antisera against Lm α1 N terminus (22), Lm α1LG1–3 (H. Wiedemann and R.T., unpublished data), Lm α3 (23), Lm α5 (24), agrin (T. Sasaki and R.T., unpublished data), perlecan (25);nidogen-1 (26), collagen IV (Chemicon), TROMA-1 against cytokeratin-8 [developed by Rolf Kemler (Max-Planck-Institut for Immunobiology, Freiburg, Germany), obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the National Institute of Child Health and Human Development, and maintained by the University of Iowa (Iowa City)], and GoH3 against integrin α6 (Chemicon). Secondary antibodies were as follows: Alexa Fluor 488, 546, and 633 (Molecular Probes) or Cy3-labeled antibodies (Chemicon). FITC-conjugated phalloidin was from Sigma–Aldrich.
Results
WT and α1LG4–5-/- Mutants on Day 5.5 of Development. Two mouse strains lacking α1LG4–5 were generated, differing only by the presence or absence of the neo cassette (see Fig. 5, which is published as supporting information on the PNAS web site). After implantation, homozygous mutants were identified by monoclonal antibody 200 specific for α1LG4. Whereas 80% of the analyzed embryos resulting from heterozygous matings displayed a strong staining in Reichert's membrane and a weaker staining of the BM separating the visceral endoderm from the epiblast, no staining could be detected in 20% of the embryos (see Table 1, which is published as supporting information on the PNAS web site).
In WT embryos on day 5.5, both Reichert's membrane and the embryonic BM between the visceral endoderm and the columnar epiblast epithelia were stained with antibodies against α1LG1–3 (Fig. 1A), and α1LG4 (Fig. 1C). The truncated Lm-1 molecule was secreted and incorporated into the BMs of the α1LG4–5-/- embryos as shown by staining with an antibody for α1LG1–3 (Fig. 1B). The binding site for integrin α6β1 thus was not abolished by the mutation. Of the 14 α1LG4–5-/- mutants detected at embryonic day 5.5 or 6.5, 13 were analyzed for the presence of BMs. The embryonic BM was present in all of the mutants as determined by staining for α1LG1–3 or the N-terminal of α1. Reichert's membrane was partly present, in either the proximal or the distal part, in eight of the mutants. In two of the mutants, no Reichert's membrane could be detected, and in three mutants, the entire Reichert's membrane was present (see Fig. 3D), although in those cases the structural integrity of Reichert's membrane was poor. No difference according to polarization or cavitation of the epiblast could be detected between the different Reichert's membrane phenotypes. No staining was seen in mutant embryos with the antibody against α1LG4 (Fig. 1D). In WT embryos, endoderm cells surrounded inner polarized epiblast cells that had formed a cavity (Fig. 1E), whereas the endoderm cells in α1LG4–5-/- embryos surrounded undifferentiated stem cells (Fig. 1F). Serial sections of the mutant embryos verified the absence of the proamniotic canal (data not shown). The morphology on day 5.5 of the first set of α1LG4–5-/- embryos with the retained neo cassette and the second set lacking the cassette was indistinguishable.
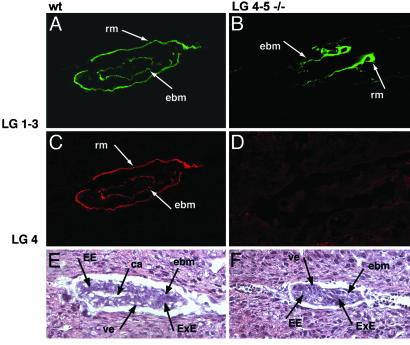
Fig. 1.
Histology of WT (A, C, and E) and α1LG4–5-/- (B, D, and F) embryos on day 5.5. Embryos were stained with polyclonal antibodies against the integrin-binding Lm α1LG-3 domains and monoclonal antibody 200 against Lm α1LG4. Five mutants and 26 WT embryos were analyzed. (A–D) Confocal images of Lm α1LG1–3 domains (green, A and B) and Lmα1LG4–5 domains (red, C and D) of WT (A and C) and α1LG4–5-/- (B and D) embryos. (E and F) Histology of WT (E) and α1LG4–5-/- (F) embryos. rm, Reichert's membrane; ebm, embryonic BM; ca, cavity; ve, visceral endoderm; EE, embryonic ectoderm; ExE, extraembryonic ectoderm.
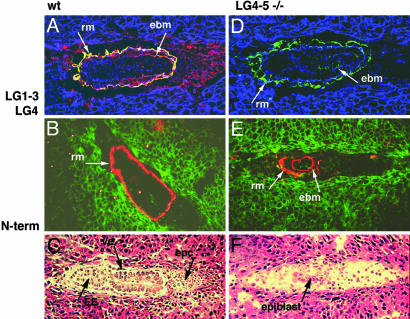
Fig. 3.
WT (A–C) and α1LG4–5-/- (D–F) embryos on day 6.5. (A and D) Confocal images of Lm α1LG1–3 domains (green) and α1LG4 domain (red) in WT (A) and α1LG4–5-/- (D) embryos. Yellow indicates colocalization of α1LG1–3 domains with α1LG4–5 domains in an intact tandem, and blue is F-actin revealed by phalloidin. (B and E) Confocal images of the N terminus of Lm α1 chain (red) and F-actin (green) in WT (B) and α1LG4–5-/- (E) embryos. (C and F) Histology of WT (C) and α1LG4–5-/- (F) embryos. Ten WT and eight mutants were analyzed. rm, Reichert's membrane; ebm, embryonic BM; ve, visceral endoderm; EE, embryonic ectoderm; epc, ectoplacental cone.
In WT embryos, Lm α5 (Fig. 2A), agrin (Fig. 2C), collagen IV (Fig. 2E), and perlecan (Fig. 2G) were found in Reichert's membrane, as well as in the embryonic BM. In all studied α1LG4–5-/- embryos on day 5.5, Lm α5 (Fig. 2B), agrin (Fig. 2D), collagen IV (Fig. 2F), and perlecan (Fig. 2H) were present in the embryonic basement between endoderm and stem cells, but in the particular embryo shown, no Reichert's membrane was detected. Staining for integrin α6 strengthened the view that the mutant epiblast was defective in polarization and cavitation. Distinct staining was detected adjacent to the embryonic BM in both WT and α1LG4–5-/- embryos (Fig. 2 I and J). α1LG4–5-/- embryos displayed a cluster of integrin α6 in the apical part of the cells where there is no integrin α6 present in the WT, suggesting defective polarization. In the WT, integrin α6 could be seen at cell–cell borders in both the ectoderm and endoderm cell layers, but in the mutant integrin α6 was undetectable in the endoderm. The orientation of the embryo and the relationship between parietal endoderm and Reichert's membrane was demonstrated by cytokeratin-8 staining. This finding revealed no apparent differences between the WT and the α1LG4–5-/- embryos (Fig. 2 K and L).
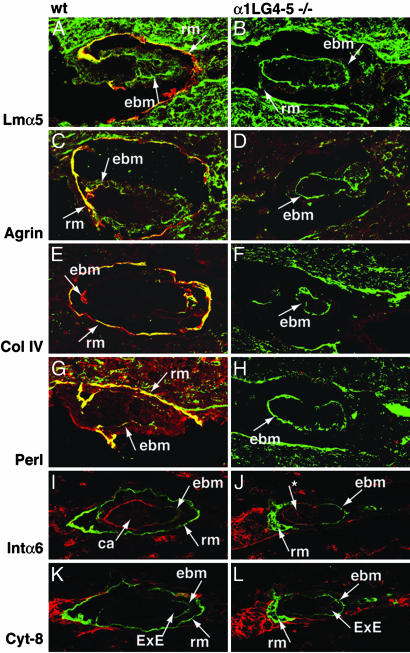
Fig. 2.
Analysis of WT (A, C, E, G, I, and K) and α1LG4–5-/- (B, D, F, H, J, and L) embryos on day 5.5. (A and B) Staining with polyclonal antibodies against Lm α5 chain (green) and monoclonal antibody 200 against α1LG4 (red) in WT (A) and α1LG4–5-/- (B) embryos. Three WT and three mutants were analyzed. (C and D) Staining with polyclonal antibodies against agrin (green) and monoclonal antibody 200 against α1LG4 (red) in WT (C) and α1LG4–5-/- (D) embryos. Three WT and three mutants were analyzed. (E and F) Staining with polyclonal antibodies against collagen IV (Col IV, green) and monoclonal antibody 200 against α1LG4 (red) in WT (E) and α1LG4–5-/- (F) embryos. Three WT and three mutants were analyzed. (G and H) Staining with polyclonal antibodies against perlecan (Perl, green) and monoclonal antibody 200 against α1LG4 (red) in WT (G) and α1LG4–5-/- (H) embryos. Three WT and two mutants were analyzed. (I and J) Staining with polyclonal antibodies against Lmα1LG1–3 (green) and monoclonal antibody GoH3 against integrin α6 (red) in WT (I) and α1LG4–5-/- (J) embryos. The * indicates an apical cluster of intα6. Five WT and one mutant were analyzed. (K and L) Staining with polyclonal antibodies against Lmα1LG1–3 (green) and monoclonal antibody TROMA-1 against cytokeratin-8 (red) in WT (K) and α1LG4–5-/- (L) embryos. Five WT and one mutant were analyzed. rm, Reichert's membrane; ebm, embryonic BM; ca, cavity; ve, visceral endoderm; ExE, extraembryonic ectoderm.
α1LG4–5-/- Mutants Die Before Gastrulation. On day 6.5 of normal embryonic development (Fig. 3 A–C), the α1 chain N terminus was strongly expressed in Reichert's membrane but also in the embryonic BM between the parietal endoderm and the epiblast cells that had converted into columnar ectoderm. α1LG4, but not the N terminus or α1LG1–3, also was detected as bright spots throughout the ectoplacental cone (Fig. 3A), suggesting that α1LG4–5 could exist as a cleaved fragment.
On day 6.5, α1LG4–5-/- embryos expressed truncated α1 chain in Reichert's membrane to a variable degree and invariably in the embryonic BM. Note that the endoderm cells are only partially attached to Reichert's membrane (Fig. 3 D and E). At this stage, the α1LG4–5-/- embryos began to die (Fig. 3F).
Embryoid Body Cultures. Embryoid bodies were made from α1LG4–5-/- and α1LG4–5+/- ES cells. On day 3, about onethird of the heterozygotes, but only a small portion of the null embryoid bodies, had differentiated into an outer endoderm with typical vacuoles and an inner epiblast surrounding a cavity (Fig. 4 A and B). The results were verified quantitatively (Fig. 4C). On day 6 of embryoid body culture, the heterozygotes had formed a BM between the endoderm and the polarized ectoderm as visualized by staining against α1LG4 and Lm α1 N terminus (Fig. 4 D and E). In agreement with in vivo data, embryoid bodies homozygous for the mutation showed no staining for α1LG4 (Fig. 4F), but the presence of a continuous BM was shown by staining against collagen IV (Fig. 4G), Lm α5 (Fig. 4H), Lm α1 N terminus (Fig. 4I), nidogen-1 (Fig. 4J), and perlecan (Fig. 4K). Whereas the null embryoid bodies to some extent formed the visceral endoderm, the epiblast had failed to polarize as revealed by phalloidin staining of F-actin. In heterozygous embryoid bodies, strong staining of the actin filaments was found in the apical part of the columnar epithelial cells lining the cavity, whereas no staining could be detected in the basal part adjacent to the BM (Fig. 4 D, E, and L). In the inner cells of the null embryoid bodies, the actin filaments were distributed evenly along the plasma membrane of the multilayered, octagonal cells (Fig. 4 G–K and M). Expression of Lm α3 seemed up-regulated in null embryoid bodies (Fig. 4L) as compared with heterozygotes (Fig. 4M).
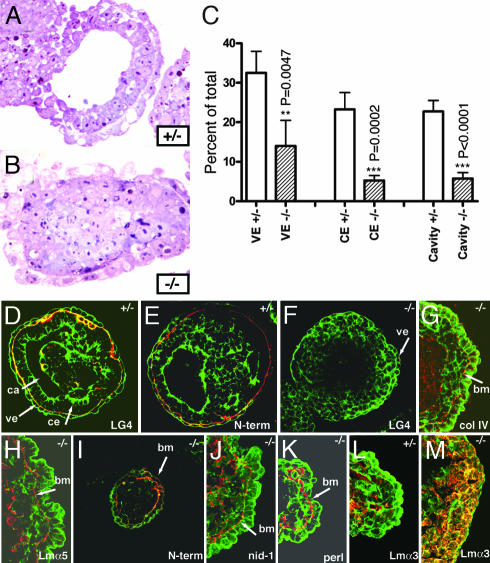
Fig. 4.
Failure of epiblast development in α1LG4–5-/- embryoid bodies in vitro. The α1LG4–5+/- and α1LG4–5-/- ES cells were cultured as embryoid bodies in vitro for 3 days and processed for plastic sections or analyzed as whole mounts or frozen sections by confocal microscopy. (A) In embryoid bodies of the heterozygous ES cells, endodermal cells with typical vacuoles were located as an outer epithelial sheet, surrounding differentiated, pseudostratified epiblast cells with an amniotic cavity in the middle. (B) There were no signs of epiblast development or cavity formation in the embryoid bodies of α1LG4–5-/- ES cells, which formed undifferentiated masses of stem cells. (C) Heterozygous and mutant embryoid bodies were classified according to differentiation of visceral endoderm (VE), columnar ectoderm (CE), and cavity formation. (D, E, and L) Confocal images of heterozygous embryoid bodies from 6-day cultures demonstrating BM components (red) and F-actin (green). The BM contained α1LG4 (D) and the Lm α1 N terminus (E). Lm α3 was expressed diffusely in the inner cells (L). (F–K and M) Confocal images of α1LG4–5-/- embryoid bodies from 6-day cultures demonstrating BM components (red) and F-actin (green). The BM contained collagen IV (G), Lmα5 (H), Lm α1 N terminus (I), nidogen-1 (J), and perlecan (K). No staining was detected with the Lm α1LG4 antibody (F), and Lm α3 was present intracellularly (M).
Discussion
By gene targeting, we showed that the LG4–5 domains of Lm α1 chain are required for stem cell conversion to polarized epiblast epithelial cells, the first fetal cells. Gene targeting of individual chains of the Lm-1 heterotrimer also resulted in early pregastrulation lethal phenotypes (7, 8). In these cruder genetic experiments, the structural and signaling functions could not be distinguished; no BMs form and all BM functions may be lost, including the ability to recruit growth factors. Here, we show that α1LG4–5 has no structural role in the embryonic BM but, rather, could be essential for Lm-1 signaling. Signals that govern self-renewal and differentiation of stem cells often are ascribed to growth factors (27, 28) or to integrins in retention of stem cells (29, 30). Although integrins are major in vitro receptors for Lm, they are not important receptors for signals leading to epiblast differentiation (6, 11). The other known major Lm receptor, dystroglycan, may not be required either because epiblast development can start in vivo and in vitro in the absence of dystroglycan (6, 31). Hence, Lm α1, through its LG4–5 domains, may induce ES cell conversion to epiblast cells by means of yet unknown receptors.
The tandem of five LG domains, present in all five Lm α chains, is the major cell-binding site for all Lms. Physiological cleavage of the tandem occurs almost invariably for α2 (32) and α3 (33), has been shown for α4 (34), and is predicted and to some extent shown for α5 (35). Cleavage of the α1 tandem in cells has not been demonstrated. Our data suggest that cleavage of Lm α1LG4–5 domains might occur in vivo, in the ectoplacental cone.
Embryos lacking α1LG4–5 incorporated a truncated Lm-1 into BMs, but development of epithelial sheets of postimplantation embryos did not occur and the mutant embryos did not survive past day 6.5 of embryogenesis. In the α1LG4–5-/- embryos on day 5.5, we could distinguish endoderm cells and an inner cell mass of stem cells. Hence, the distinct histological abnormality at this stage was the lack of both epiblast polarization and cavity formation. Integrin α6 expression was only partially polarized in epiblast cells in α1LG4–5-/- embryos.
Like the Lm α1-, β1-, and γ1-null phenotypes, the α1LG4–5-/- embryos died after implantation but before gastrulation. There were some differences between these mutations. Embryos lacking any of the β1 or γ1 chains could not form any BMs in the early embryo, and mutants died on embryonic day 5.5 (7, 8). Surprisingly, the Lm α1-/- embryos survived longer than the α1LG4–5 mutants, until embryonic day 7. The epiblast of the Lm α1-/- mutants was polarized and formed a cavity, but no Reichert's membrane was present. The polarization of the epiblast cells may be due to partial compensation by Lm α5 chain in the embryonic BM (8). In the α1LG4–5 mutants, the presence of the truncated Lm α1 chain may prevent the α5 chain from compensating. Hence, the earlier phenotype of the α1LG4–5 mutants might be explained.
A prevailing view is that α1LG4 has a structural role as a nucleation-site for BM assembly, by binding to cell-surface sulfatides, dystroglycan, or cell-surface proteoglycans. Yet, α1LG4–5 is not essential for assembly of Reichert's membrane or embryonic BMs in vivo. In the majority of the pregastrulating embryos examined, parts of Reichert's membrane were detected. A few embryos lacked Reichert's membrane, whereas a few displayed the entire membrane. The variation suggests that Reichert's membrane may require α1LG4–5 for full structural stability. In the embryonic BM, there is more Lm α5 than α1, and here α5 may provide structural stability. It cannot, however, compensate for Lm α1LG4–5-induced epiblast cell polarization and cavitation of the epiblast. The role of α1LG4–5 in BM assembly thus may differ depending on the tissue.
In agreement with in vivo data, α1LG4–5-/- ES cells aggregated to embryoid bodies formed a normal endoderm, but the inner cells remained undifferentiated. Polarized secretion of BM components (36) was detected between outer epithelial cells and the inner cells of both α1LG4–5-/- and α1LG4–5+/- embryoid bodies. Hence, other BM components cannot compensate for absence of α1LG4–5. Lack of robust compensation by overexpression of Lm α5 for the absence of Lm α1 was noted during in vivo development; the embryos died before or during gastrulation (8). This result is remarkable considering that Lm α1 (37) compensates for lack of Lm α2 in mice with muscular dystrophy.
Because studies on extracellular matrix signaling have focused on integrins (38), little is known about α1LG4–5-mediated signal transduction. To our knowledge, the only known signaling by α1LG4–5 is suppression of Lm-1-induced phosphorylation of extracellular signal-regulated kinase 2 (ERK-2) in an epithelial cell line (39), but ERK-2 is required only shortly after gastrulation (40).
It cannot entirely be excluded that lack of α1LG4–5 influences the integrity of integrin binding sites, but integrins do not seem to be required for epiblast polarization (6). We conclude that there may exist in vivo signaling receptors for Lm-1, distinct from dystroglycan and integrins. There is no rationale to believe that such receptors bind LG4 and not LG5, although attention has been focused on LG4 because of previous findings for other receptors. Putative early downstream targets of yet unknown LG4–5 receptors include, despite its name, integrin-linked kinase shown to be required for epiblast polarization (41) and Rho-GTPases (42) known to be important for cell shape in general (43). The suggested role of α1LG4–5 for branching epithelial morphogenesis in general (19, 20) emphasizes the importance of our observations.
Supplementary Material
Acknowledgments
This work was supported by Cancerfonden, Stockholm.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BM, basement membrane; ES, embryonic stem; Lm, laminin.
References
- 1.Beddington, R. S. P. & Robertson, E. J. (1999) Cell 96, 195-209. [DOI] [PubMed] [Google Scholar]
- 2.Eaton, S. & Simons, K. (1995) Cell 82, 5-8. [DOI] [PubMed] [Google Scholar]
- 3.Ekblom, P., Alitalo, K., Vaheri, A., Timpl, R. & Saxén, L. (1980) Proc. Natl. Acad. Sci. USA 77, 485-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miner, J. H. & Yurchenco, P. D. (2004) Ann. Rev. Cell Dev. Biol. 20, 255-284. [DOI] [PubMed] [Google Scholar]
- 5.Li, X., Chen, Y., Schéele, S., Arman, E., Haffner-Krausz, R., Ekblom, P. & Lonai, P. (2001) J. Cell Biol. 153, 811-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li, S., Harrison, D., Carbonetto, S., Fässler, R., Smyth, N., Edgar, D. & Yurchenco, P. D. (2002) J. Cell Biol. 157, 1279-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smyth, N., Vatansever, H. S., Murray, P., Meyer, M., Frie, C., Paulsson, M. & Edgar, D. (1999) J. Cell Biol. 144, 151-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miner, J. H., Li, C., Go, G. & Sutherland, A. E. (2004) Development (Cambridge, U.K.) 131, 2247-2256. [DOI] [PubMed] [Google Scholar]
- 9.Mayer, U., Kohfeldt, E. & Timpl, R. (1998) Ann. N.Y. Acad. Sci. 857, 130-142. [DOI] [PubMed] [Google Scholar]
- 10.Georges-Labouesse, E., Messaddeq, N., Yehia, G., Cadalbert, L., Dierich, A. & LeMeur, M. (1996) Nat. Genet. 13, 370-373. [DOI] [PubMed] [Google Scholar]
- 11.Fässler, R. & Meyer M. (1995) Genes Dev. 9, 405-410. [DOI] [PubMed] [Google Scholar]
- 12.Stephens, L. E., Sutherland, A. E., Klimanskaya, I. V., Andrieux, A., Meneses, J., Pedersen, R. A. & Damsky, C. (1995) Genes Dev. 9, 1883-18895. [DOI] [PubMed] [Google Scholar]
- 13.Gee, S. H., Blacher, R. W., Douville, P. J., Provost, P. R., Yurchenco, P. D. & Carbonetto, S. (1993). J. Biol. Chem. 268, 14972-14980. [PubMed] [Google Scholar]
- 14.Ervasti, J. M. & Campbell, K. P. (1993) J. Cell Biol. 122, 809-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taraboletti, G., Rao, C. N., Krutsch, H. C., Liotta, L. A. & Roberts, D. D. (1990) J. Biol. Chem. 265, 12253-12258. [PubMed] [Google Scholar]
- 16.Timpl, R., Tisi, D. Talts, J. F., Andac, Z., Sasaki, T. & Hohenester, E. (2000) Matrix Biol. 19, 309-317. [DOI] [PubMed] [Google Scholar]
- 17.Ekblom, P. & Timpl, R. (1996). The Laminins (Harwood, Amsterdam).
- 18.Sorokin, L. M., Conzelmann, S., Ekblom, P., Battaglia, C., Aumailley, M. & Timpl, R. (1992) Exp. Cell Res. 201, 137-144. [DOI] [PubMed] [Google Scholar]
- 19.Klein, G., Langegger, M., Timpl, R. & Ekblom, P. (1988) Cell 55, 331-341. [DOI] [PubMed] [Google Scholar]
- 20.Durbeej, M., Talts, J. F., Henry, M. D., Yurchenco, P. D., Campbell, K. P. & Ekblom, P. (2001) Differentiation 69, 121-134. [DOI] [PubMed] [Google Scholar]
- 21.Coucouvanis, E. & Martin, G. R. (1995) Cell 83, 279-287. [DOI] [PubMed] [Google Scholar]
- 22.Ettner, N., Göhring, W., Sasaki, T., Mann, K. & Timpl, R. (1998) FEBS Lett. 430, 217-221. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki, T., Göhring, W., Mann, K., Brakebush, C., Yamada, Y., Fässler, R. & Timpl, R. (2001) J. Mol. Biol. 314, 751-763. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki, T & Timpl, R. (2001) FEBS Lett. 509, 181-185. [DOI] [PubMed] [Google Scholar]
- 25.Costell, M., Mann, K, Yamada, Y. & Timpl, R. (1997) Eur. J. Biochem. 243, 115-121. [DOI] [PubMed] [Google Scholar]
- 26.Fox, J.W, Mayer, U, Nischt, R., Aumailley, M, Reinhart, D., Wiedemann, H., Mann, K., Timpl, R., Krieg, T. & Engel, J. (1991) EMBO J. 10, 3137-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birchmeier, C., Birchmeier, W., Gherardi, E. & Vande Woude, G. F. (2003) Nat. Rev. Mol. Cell Biol. 4, 915-925. [DOI] [PubMed] [Google Scholar]
- 28.Ying, Q., Nichols, J., Chambers, J. & Smith, A. (2003) Cell 115, 281-292. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs, E., Tumbar, T. & Guasch, G. (2004) Cell 116, 109-113. [DOI] [PubMed] [Google Scholar]
- 30.Watt, F. (2002) EMBO J. 21, 3919-3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williamson, R. A., Henry, M. D., Daniels, K. J., Hrstka, R. F., Lee, J. C., Sunada, Y., Ibraghimov-Beskrovnaya, O. & Campbell, K. P. (1997) Hum. Mol. Genet. 6, 831-841. [DOI] [PubMed] [Google Scholar]
- 32.Talts, J. F & Timpl, R. (1999) FEBS Lett. 458, 319-323. [DOI] [PubMed] [Google Scholar]
- 33.Aumailley, M., El Kahl, A., Knoss, N. & Tunggal, L. (2003) Matrix Biol. 22, 49-54. [DOI] [PubMed] [Google Scholar]
- 34.Talts, J.F., Sasaki, T., Miosge, N., Göhring, W., Mann, K., Mayne, R. & Timpl, R. (2000) J. Biol. Chem. 275, 35192-35199. [DOI] [PubMed] [Google Scholar]
- 35.Garbe, H. J., Göhring, W., Mann, K., Timpl, R. & Sasaki, T. (2002) Biochem. J. 362, 213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray, P. & Edgar, D. (2000) J. Cell Biol. 150, 1215-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gawlik, K., Miyagoe-Suzuki, Y., Ekblom, P., Takeda, S. & Durbeej, M. (2004) Hum. Mol. Genet. 13, 1775-1784. [DOI] [PubMed] [Google Scholar]
- 38.Miranti, C. K. & Brugge, J. S. (2002) Nat. Cell Biol. 4, E83-E90. [DOI] [PubMed] [Google Scholar]
- 39.Ferletta, M., Kikkawa, Y., Yu, H., Talts, J. F., Durbeej, M., Sonnenberg, A., Timpl, R. Campbell, K. P., Ekblom, P. & Genersch, E. (2003) Mol. Biol. Cell. 14, 2088-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao, Y., Li, W., Wu, J., Germann, U. A., Su, M., Kuida, K. & Boucher, D. M. (2003) Proc. Natl. Acad. Sci. USA. 100, 12759-12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakai, T., Li, S., Docheva, D., Grashoff, C., Sakai, K., Kostka, G., Braun, A., Pfeifer, A., Yurchenco, P. & Fässler, R. (2003) Genes Dev. 17, 926-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li, L., Arman, E., Ekblom, P., Edgar, D., Murray, P. & Lonai, P. (2004) Development (Cambridge, U.K.) 131, 5277-5286. [DOI] [PubMed] [Google Scholar]
- 43.Burridge, K. & Wennerberg, K. (2004) Cell 116, 167-179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.