Abstract
In this review, the sugar portions of glycoproteins, glycolipids, and glycosaminoglycans constitute the glycome, and the genes involved in their biosynthesis, degradation, transport and recognition are referred to as “glycogenes“. The extreme complexity of the glycome requires the regulatory layer to be provided by the epigenetic mechanisms. Almost all types of cancers present glycosylation aberrations, giving rise to phenotypic changes and to the expression of tumor markers. In this review, we discuss how cancer-associated alterations of promoter methylation, histone methylation/acetylation, and miRNAs determine glycomic changes associated with the malignant phenotype. Usually, increased promoter methylation and miRNA expression induce glycogene silencing. However, treatment with demethylating agents sometimes results in silencing, rather than in a reactivation of glycogenes, suggesting the involvement of distant methylation-dependent regulatory elements. From a therapeutic perspective aimed at the normalization of the malignant glycome, it appears that miRNA targeting of cancer-deranged glycogenes can be a more specific and promising approach than the use of drugs, which broad target methylation/acetylation. A very specific type of glycosylation, the addition of GlcNAc to serine or threonine (O-GlcNAc), is not only regulated by epigenetic mechanisms, but is an epigenetic modifier of histones and transcription factors. Thus, glycosylation is both under the control of epigenetic mechanisms and is an integral part of the epigenetic code.
Keywords: glycome, glycosyltransferases, DNA methylation, miRNA targeting
1. Introduction
As a part of the genome, genes involved in the biosynthesis, degradation, transport, and recognition of the sugar portions of glycoconjugates (collectively referred to as glycogenes) undergo epigenetic regulation. While the simple gene expression/protein expression model could at best account for primary gene products, the existence of epigenetic mechanisms adds another layer of complexity and opportunity for regulating biological functions. Since glycans are not primary gene products, but secondary products of a complex interplay among glycogenes, the epigenetic regulation of the glycome moves a step forward to understanding the complexity and opportunity of biological regulation. It has been hypothesized that the epigenetic regulation of glycogenes provides mammalians with a quick and heritable mechanism generating glycome plasticity to cope with microorganisms [1,2]. As a consequence of epigenetic regulation of glycogenes in solid tissues, the whole glycome of plasma proteins can be modulated. This leads to important functional changes, as well as to the appearance of disease-related glycomic markers [3,4,5]. In light of the enormous impact of epigenetics on human cancers, which are very frequently associated with dramatic deregulation of glycan expression, a growing number of reports have focused on the epigenetic mechanisms affecting glycosylation in cancer.
In this review, we discuss the glycogenes that were found to be regulated by promoter methylation [6,7,8], histone methylation/acetylation [9,10,11], and microRNAs [12,13], in cancer. We see how different epigenetic mechanisms converge to regulate specific pathways of cancer-associated carbohydrate structures. We also summarize the case of O-linked GlcNAc, in which glycosylation is not only the target of epigenetic mechanisms, but contributes to the generation of the epigenetic code. We expect that the relatively small number of glycogenes epigenetically modulated in cancer so far identified are only a minority of those actually undergoing epigenetic regulation.
2. Epigenetic-Regulation of Cancer Associated Structures
2.1. Core Glycosylation of N-Linked Chains
In this section, we will discuss the epigenetic regulation of three well-known cancer associated carbohydrate structures present in the internal core of N-glycans.
2.1.1. Core Fucosylation
Core fucosylation consists in the addition of a Fuc residue α1,6-linked to the innermost GlcNAc residue of N-linked chain (Figure 1A). The biosynthesis of this structure is due to a single enzyme: fucosyltransferase 8, encoded by the FUT8 gene [14]. In mice, the genetic ablation of FUT8 dysregulates TGF-β signaling, leading to abnormal lung development and emphysema-like phenotype [15]. Increased core fucosylation has been reported during hepatocarcinogenesis, in both cell-associated and secreted proteins, including α-fetoprotein and α1-antitrypsin, which are plasma markers of the disease [16]. However, the expression of FUT8 in cancer biology is double-hedged—it can be associated with both increased [17] or decreased [18] malignancy in different tissues. FUT8 is the target of different miRNA. In colorectal cancer, miR-198 up-regulates FUT8 expression at both the mRNA and protein level, leading to an invasive phenotype [19]. In liver cancer cells, FUT8 appears to be down-regulated by miR-122, miR-34a [20], miR-26a, miR-34a, and miR-146a [21]. In some cases, miR-34a modifies protein but not mRNA levels [20], showing that some miRNAs can exert their effects only at a translational level.
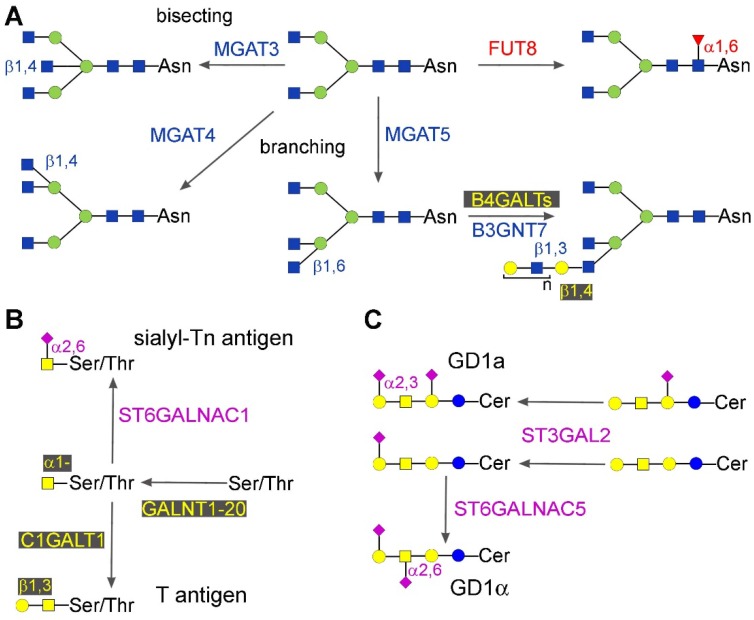
Figure 1.
Structure of some core glycans. Monosaccharides are depicted according to this representation: blue square—GlcNAc, N-acetylglucosamine; yellow square—GalNAc, N-acetylgalactosamine; yellow circle—Gal, galactose; blue circle—Glc, glucose; green circle—Man, mannose; red triangle—Fuc, fucose; pink diamond—sialic acid, Sia. Anomers, linkage positions, and enzymes involved in relevant reactions are indicated. (A) N-glycans. As an example, the reactions are indicated using only the simple bi-antennary core structure as the substrate. Note that they are not alternative and can occur in various orders, because many of the indicated products can act as the substrate for several of the reported enzymes. An exception is represented by MGAT3 (bisecting enzyme) and MGAT5 (branching enzyme), whose reactions are mutually exclusive; (B) O-glycans; (C) Gangliosides. In both panels, the enzymes are indicated in the order in which they act.
2.1.2. Bisecting GlcNAc, β1,6 and β1,4 Branching
Bisecting GlcNAc consists of a GlcNAc residue β1,4-linked to the innermost Man residue of the N-glycan core (Figure 1A). Its biosynthesis is due to β-N-acetylglucosaminyltransferase 3, product of the gene MGAT3. The addition of GlcNAc residues either with a β1,4- or a β1,6-linkage to the two external Man residues of the core is mediated by β-N-acetylglucosaminyltransferases 4 and -5, product of the genes MGAT4 and MGAT5, respectively (Figure 1A). The epigenetically regulated alternative addition of the bisecting GlcNAc, or of the β1,6-linked GlcNAc, to the N-glycans of E-cadherin [22] provides a very good example of the modulation of a basic cancer property by glycans. When bisecting GlcNAc is replaced with β1,6-linked GlcNAc on the N-glycans of E-cadherin because of decreased MGAT3 expression β-catenin (which is usually leashed by binding to the cytoplasmic side of E-cadherin), it is released in the cytoplasm, leading to epithelial to mesenchymal transition (EMT) [23,24,25]. The methylation of the MGAT3 promoter is responsible for MGAT3 down-regulation and the consequent EMT [25]. In general, the presence of bisecting GlcNAc is associated with reduced malignancy, mainly because it prevents the addition of the metastasis-associated β1,6-branching. However, in some cases, an association with increased malignancy has also been reported [26]. Among epigenetic mechanisms, promoter methylation appears to play a pivotal role in inhibiting MGAT3 gene expression. Bisecting GlcNAc is up-regulated in ovarian cancers, while treatment with 5-AZA of ovarian cancer cell lines induces MGAT3, bisecting GlcNAc expression [27], and changes in the pattern of glycosylation of secreted glycoproteins [28]. Among ovarian and breast basal-like cancer patients, high MGAT3 promoter methylation correlates with longer survival [26]. 5-AZA treatment of epatocarcinoma HepG2 cells resulted in modulation of about 20% of the glycogenes, although the consequent glycomic changes where mainly consistent with increased expression of MGAT3 [29].
The link between β1,6 branching and metastasis is supported by a large body of evidence [30], but the underlying mechanisms are complex and only partially understood. A key role is certainly played by the preferential binding of galectin-3 to terminal structures carried by β1,6-linked branches [31]. It is not clear whether MGAT5 is directly regulated by promoter methylation. In fact, changes in glycans of glycoproteins secreted by 5-AZA-treated ovarian cancer cells have been attributed to changes in MGAT5 expression [28]. On the other hand, in macrophage-melanoma fusion hybrids, treatment with 5-AZA does not result in the activation of MGAT5 transcription, but results in its down-regulation. This was explained with the demethylation-induced up-regulation of the negative regulator nm23 encoded by the NME1 gene [32].
The addition of β1,4-linked GlcNAc, catalyzed by MGAT4, can be inhibited in mammary epithelial cells by miR-424, leading to cell cycle arrest through CCND1 down-regulation [33].
2.2. Mucin-Type O-Glycosylation
The addition of the first GalNAc O-linked to serine or threonine of mucin-type glycans is mediated by a family of 20 peptides: GalNAc transferases (Figure 1B), which are the products of genes GALNT1-GALNT20. Members of the GALNT family are aberrantly expressed in various cancer types, mainly as a consequence of deregulated miRNA expression.
GALNT1 is negatively regulated by miR-129 in bladder cancer [34]. In hepatocellular carcinoma [35] and in cervical cancer cells [36], down-regulation of GALNT4 by miR-9 promotes invasive growth. Depending on the cancer type, GALNT7 displays opposite tumor-promoting or tumor-suppressing activities, and is regulated by different miRNAs. In laryngeal squamous cell carcinoma, it is down-regulated by miR-34a and miR-34c, with consequent reduced cell proliferation and invasion [37]. GALNT7 promotes tumor growth also in esophageal squamous cell carcinoma, where it is overexpressed because of reduced miR-214 [38]. On the other hand, miR-30b/30d upregulation in melanoma correlates with stage, metastatic potential, shorter time to recurrence, and reduced overall survival, because of reduced expression of GALNT7. This leads to increased synthesis of the immunosuppressive cytokine IL-10, and to reduced immune cell activation [39]. GALNT7 displays a tumor-suppressor effect also in hepatocellular carcinoma cells. In fact, targeting GALNT7 by the passenger miR-17-3p enhances proliferation and migration [40]. Interestingly, the mature miR-17-5p also enhances tumor growth by targeting the tumor suppressor PTEN [40]. Decreased expression of miR-122, which is under the control of hepatocyte nuclear factor 4α in hepatitis B virus hepatocarcinoma, leads to overexpression of GALNT10 and consequently to increased O-glycosylation and signaling through the EGF pathway [41]. In mammary epithelial cells, miR-424 down-regulates GALNT13 [33]. miR-125a regulates ovarian cancer proliferation and invasion by repressing GALNT14 [42].
Without further sugar additions, the O-linked GalNAc forms the Tn antigen, which is a well-known cancer-associated structure. The second step of the biosynthesis of the O-linked chains is often represented by the addition of a β1,3-linked galactose residue to GalNAc, forming the Core 1 or Thomsen-Friedenreich (or T) antigen (Figure 1B), mediated by core 1 β1,3-galactosyltransferase (C1GALT1 or T-synthase). This enzyme requires the molecular chaperone Cosmc for its activity. In the presence of a dysfunctional Cosmc, the Tn antigen accumulates. Cosmc expression is down-regulated by promoter methylation [43], and together with T-synthase, provides a good example of coordinated, but differential, expression. In fact, both genes are regulated by transcription factors of the same SP/Kruppel-like transcription factors, but in a B cell line only the Cosmc promoter undergoes methylation, despite the presence of CpG islands in the promoter region of both genes [44].
The addition of α2,6-linked sialic acid to the Tn antigen, mainly mediated by ST6GALNAC1, leads to the formation of the cancer-associated sialyl-Tn antigen and blocks further chain elongation (Figure 1B). The ST6GALNAC1 gene undergoes hypermethylation out of the promoter region in breast cancer patients positive for both estrogen and progesterone receptors, but not in those negative [45]. The effect of such differential DNA methylation on ST6GALNAC1 expression is not reported yet. Conversely, ST6GALNAC1 down-regulation was reported in esophageal squamous cell carcinoma patients, and was found to be associated with promoter methylation in cell lines [46].
2.3. Gangliosides
Gangliosides are sialylated glycolipids whose expression is frequently deranged in cancer [47]. The final sialylation of gangliosides is mediated by several sialyltransferases, some of which are regulated by promoter methylation or miRNAs. In prostate cancer cell lines, the biosynthesis of ganglioside GD1a (Figure 1C) is regulated by sialyltransferase ST3GAL2, whose promoter methylation blocks the transcriptional stimulation operated by testosterone [48]. Sialyltransferase ST6GALNAC5, responsible for GD1α biosynthesis, displays CpG hypermethylation in both adenomas and carcinomas of the colon, but without effect on gene expression [49]. In one study, it was shown that three glycosyltransferases, ST3GAL5, ST6GALNAC5, and B3GLCT (the first two involved in ganglioside biosynthesis, the third in elongation of O-linked fucosylglycans), affect EMT, and are regulated by miRNAs of the miR-200 family. Silencing of these glycogenes induces EMT, like the expression of miR-200f, indicating a causal role of these gangliosides in EMT [50]. A very recent study confirmed that targeting the 3′UTR of ST3GAL5 by miR-26a, miR-548l and miR-34a downregulates enzyme expression and the growth of hepatocarcinoma cells [51].
2.4. Type 1 and Type 2 Chain Elongation
Both N- and O-glycans can be elongated by repeating units of galactose linked to GlcNAc either through a β1,3 or a β1,4 linkage, giving rise to type 1 or type 2 (lactosamininc) chains, respectively (Figure 2A). Several β1,3-galactosyltransferases (B3GALTs) or β1,4-galactosyltransferases (B4GALTs) mediate the elongation of type 1 or type 2 chains, respectively, and are regulated by epigenetic mechanisms. B3GALT5, which is responsible for switching oligosaccharide elongation to the type 1 chain in many epithelial cells, provides quite a complex example of epigenetic regulation. Two main transcripts were described with different 3′ UTR, but with an identical coding sequence. One, named the native transcript since it is conserved through the evolution, is driven by a promoter affected by the ubiquitous NF-Y transcription factor, and is placed in the context of two CpG islands [52]. In colon cancer, hypermethylation of the native promoter results in gene silencing [53]. The same promoter appears to be hypomethylated in both normal and cancer pancreatic tissues, allowing relevant expression of the transcript [54]. The other B3GALT5 transcript, named the LTR transcript due to its retroviral origin, appeared late in the evolution [55], and is driven by a promoter affected by HNF1 transcription factor [56]. This promoter is very strong in normal colon mucosa giving rise to a very high expression of B3GALT5, but is silenced in colon cancer due the demethylation of a distant DNA sequence [56], probably located about 1 kb far from the transcription initiation site [54]. Treating cell lines that express the LTR transcript with 5-AZA dramatically impairs B3GALT5 expression. In the pancreas, the LTR transcript is expressed at low levels and is not apparently regulated in cancer, where the same DNA region is not differentially methylated [54]. Among other galactosyltransferases regulated in cancer by epigenetic mechanisms, B4GALT1 is down-regulated because of promoter methylation in a significant percentage of colon cancer cases [57]. An unconventional example of stimulation of gene expression by miRNA is provided by miR-27a, which binds to the 3’UTR of B4GALT3, resulting in its overexpression, and contributing to its oncogenic activity in cervical cancer cells [58].
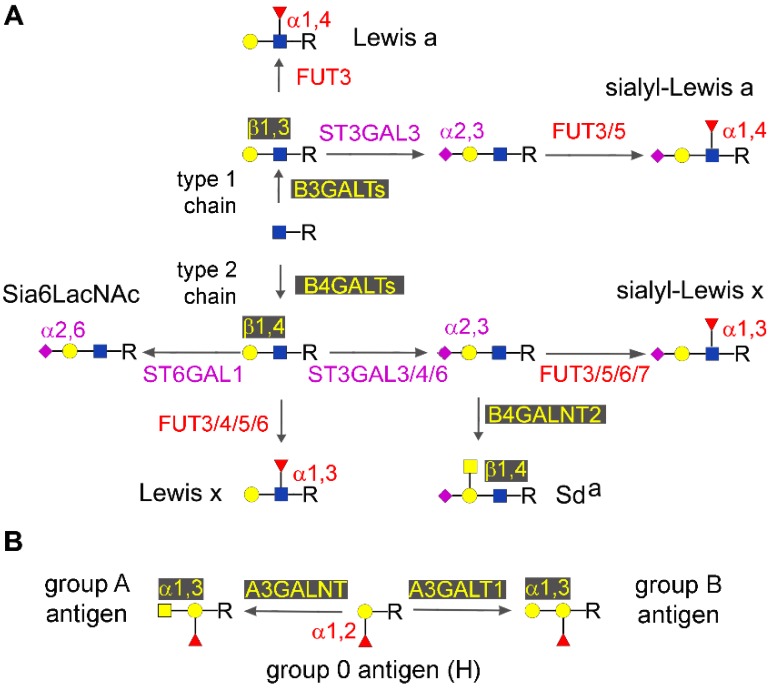
Figure 2.
Structure of some oligosaccharide chain terminations. Monosaccharides are depicted as in Figure 1. Anomers, linkage positions, and enzymes involved in relevant reactions are indicated. (A) Origin of type 1 and 2 chains, and termination by bioactive ends; (B) AB0 antigens.
The B3GNT7 gene encodes β1,3-N-acetylglucosaminyltransferase 7, that mainly acts on extending sulfated polylactosaminic chains (Figure 1A) on which sLex and sLea antigens are mounted. The down-regulation of B3GNT7 observed in colon cancer is partially due to promoter methylation [59].
2.5. Type 1 and type 2 Chain Termination
2.5.1. Major Glycosyltransferases Involved in Chain Termination
The termination of type 1 or type 2 chains frequently occurs by the addition of a few types of monosaccharides, arranged in well-defined structures recognized by antibodies or lectins. The addition of these monosaccharides is mediated by specific glycosyltransferases, many of which display epigenetic regulation. Sialic acids are a group of sugars carrying a negative electric charge at physiological pH values always mounted in terminal position, except in the case of polysialic acids, where sialic acid residues form linear polymers. Sialyltransferases, the enzymes which transfer sialic acids, are a family of 20 enzymes which can be grouped according to the sugar acceptor (Gal, GalNAc, GlcNAc, Sia) and the position (α2,3-α2,6-α2,8-) of the linkage they form [60,61,62].
Members of the fucosyltransferase family [63] mostly involved in the elaboration of terminal epitopes are FUT1 and FUT2, which mediate the α1,2-fucosylation of terminal galactose, and FUT3-10, which catalyze the α1,3/4-fucosylation of the subterminal GlcNAc residue, giving rise to the group of Lewis antigens [63,64,65].
Relevant examples of chain termination are provided by the α2,3-sialylated/α1,3/4 fucosylated sialyl-Lewis type antigens, the Sda antigen, the Sia6LacNAc, and the well-known AB0 antigens.
2.5.2. Lewis and Sda Antigens
The biological relevance of Lewis type, and in particular of sialyl-Lewis (sLe) type antigens, in cancer biology is vast [64] and is mainly related to the ability of these structures to serve as ligands for cell adhesion molecules of the selectin family, favoring the metastatic process and angiogenesis [66]. The terminal steps of the biosynthesis of sLe structures are represented by the α2,3-sialylation of type 1 or type 2 chains, and by the successive addition of fucose to GlcNAc in α1,4- or α1,3 linkage, yielding sLea and sLex, respectively. Both the sialylation and fucosylation steps can be mediated by multiple enzymes (Figure 2A).
Among the reasons for the low expression of the sLea antigen in normal colonic tissues, an alternative biosynthesis of the disialyl-Lewis structure has been proposed, in which an additional α2,6-linked sialic acid is mounted on the GlcNAc residue by the action of sialyltransferase ST6GALNAC6 [67]. Treatment of the colon cancer cell line DLD-1 with 5-AZA and/or butyrate up-regulates the transcript, suggesting that promoter methylation and/or histone deacetylation are involved in its regulation [67]. In a gastric cancer cell line, Lea is regulated by the levels of FUT3 that appear to be reduced by methylation of the promoter region [68]. The differential methylation of the FUT3 promoter was recently reported to occur in tongue cancer, and to be associated with some clinical features [69]. ST3GAL3, coding a sialyltransferase involved in the biosynthesis of type 1 chain active ends including sLea in several tissues, was recently reported to be differentially methylated during childhood [70], but data specifically related to cancer are not yet available. In a colon cancer cell line, 5-AZA treatment induced sLex expression on MUC1 by stimulating ST3GAL6 transcription, which is impaired by promoter methylation [71]. Among the mechanisms claimed to be responsible for low sLex expression in normal colon, there is the biosynthesis of two alternative structures. One is sialyl 6-sulfo sLex, which critically depends on the activity of the sulfate transporter DTDST, whose down-regulated in colon cancer tissues is restored by histone deacetylase inhibitors [72].
The other structure is the Sda antigen (Figure 2A), which is formed by a α2,3-sialylated type 1, 2 or 3 chain in which a GalNAc residue is β1,4-linked to subterminal galactose (Figure 2A) [73]. The terminal step of the biosynthesis of the Sda antigen, is mediated by a single enzyme, a product of the B4GALNT2 locus [74,75]. This enzyme and its cognate Sda antigen mediate multiple biological and pathological functions [76]. The putative promoter regions of the human B4GALNT2 gene are embedded in CpG islands, suggesting that DNA methylation could play a role in regulating B4GALNT2 gene expression, particularly in the down-regulation observed in gastrointestinal cancers [77,78,79]. It has been reported that in colon cancer cells lines, B4GALNT2 promoter methylation is associated with a very low level of mRNA expression. Further, demethylating treatments can induce a partial activation of the gene, reaching an expression level which remains far below that of the normal colonic mucosa [80]. The B4GALNT2 gene was found to be methylated in about half of the gastric cancer cases examined, and in the majority of gastric and colon cancer cell lines [81]. Treatment of cell lines with anti DNA-methylation agents induced a weak expression of the B4GALNT2 transcript, and of the Sda antigen [81]. Altogether, these results are consistent with the view that methylation of the B4GALNT2 genomic regulatory region plays a role in switching off gene expression during carcinogenesis of gastrointestinal tissues, but that the removal of epigenetic marks is not sufficient to drive the level of enzyme activation close to that of normal colonic mucosa. Taken together, the down regulation of sLea [82] and Sda antigens, the up regulation of sLex [83] in colon cancer, and the persistent expression of some Lewis antigens in pancreatic cancer [54], appear as the consequence of a complex tissue- and cancer-specific regulation of several glycogenes operated by various epigenetic mechanisms. At present, they cannot be recapitulated in a simplified minimal cancer-associated signature. Consequently, a single oriented approach, i.e general DNA demethylation obtained through methyltransferase inhibitors, could be not useful in these types of cancers.
Of the fucosyltransferases involved in the biosynthesis of Lewis type antigens, the methylation of FUT4 promoter has been reported to be inversely correlated with gene overexpression and tumor invasion [84]. In breast cancer cells, FUT4 overexpression, due to down-regulation of miR-224-3p, is associated with chemoresistance [85], while its inhibition by miR-493-5p attenuates invasiveness and tumorigenicity [86]. Although FUT6 is involved in the biosynthesis of the cancer associated sLex antigen, it has also been reported that FUT6 down-regulation induced by miR-106b targeting leads to increased invasion of breast cancer cells [87].
2.5.3. Sia6LAcNAc and ST6GAL1
The termination of type 2 chains with α2,6-linked sialic acid (Figure 2A), giving rise to α2,6 sialyllactosamine (Sia6LacNAc), is mainly mediated by sialyltransferase ST6GAL1 [88]. Sia6LacNAc decorates a variety of soluble and cell membrane glycoproteins, and plays roles in cell adhesion [89] and immune and inflammatory processes [90,91,92] (reviewed in [93]).
Sialyltransferase ST6GAL1 is frequently up-regulated in colon cancer [94] and other malignancies, but its impact on cancer biology is multifaceted [60,61,93,95,96]. ST6GAL1 down-regulation by promoter methylation has been documented in bladder cancer [97], in breast cancer patients ER/PR positive, with TP53 mutations and high grade [98], and in gliomas [99]. In all these cases, ST6GAL1 down-regulation is associated with increased invasion.
An example of how miRNA can indirectly modulate a biological function by targeting glycosylation is provided by miR-199a, which reduced both the sialylation and the protein level of Nectin-like Molecule 2/Cell Adhesion Molecule 1 by targeting ST6GAL1 [100].
2.5.4. Other Sialyltransferases
In breast cancer cells, the overexpression of sialyltransferase ST8SIA4, involved in polysialic acid biosynthesis, is associated with invasive growth. A main regulatory mechanism involves miR26a-26b, which targets the 3′UTR region of the transcript determining its down-regulation [101]. Interestingly, ST3GAL6, whose upregulation in hepatocarcinoma is associated with increased invasion, is also targeted and negatively regulated by miR26a [102]. It has been reported that miR-4701-5p is responsible for multi drug resistance of chronic myeloid leukemia cells, at least in part, by targeting and down-regulating ST3GAL1 [103]. The invasive properties and tumorigenicity of human follicular thyroid carcinoma is mediated by miR-4299, through targeting and silencing ST6GALNAC4 [104].
2.5.5. AB0
Histo-blood group AB0 antigens (Figure 2B) derive from either the addition of α1,3GalNAc residue (group A), an α1,3Gal residue (group B), or nothing (group 0), to an α1,2-fucosylated terminal galactose (Figure 2B). Three allelic variants of the ABO genetic locus encoding either an α1,3GalNAc transferase (A3GALNT), an α1,3Gal transferase (A3GALT), or an inactive molecule, are responsible for the A, B and 0 phenotypes, respectively [105,106].
The expression of AB0 antigens in bladder cancer is frequently lost either because of allelic lost or because promoter methylation of the AB0 gene [107]. The fact that the AB0 gene is silenced in about two-thirds of the oral cancer cases [108] suggests that its down-regulation provides a growth advantage [109]. However, in only one-third of the cases, silencing is due to promoter hypermethylation, being the remaining due to allelic lost or other mechanisms [108]. AB0 promoter hypermethylation was also found in hyperplastic or dysplastic tissues adjacent to the tumors, suggesting that it is an early event in tumorigenesis [108]. Downregulation of microRNA-15b by hepatitis B virus X enhances hepatocellular carcinoma proliferation, via FUT2-induced Globo H expression [110].
2.6. Glycosaminoglycans
Glycosaminoglycans are important regulators of the growth of connective tissue cells. In chondrosarcoma, heparan sulfate biosynthesis is inhibited because of the methylation of the 3-O-sulfotransferase [111]. The exostoses like-3 (EXTL3) gene encodes a putative tumor suppressor glycosyltransferase involved in the biosynthesis of glycosaminoglycans. In a high percentage of the colon cancers of the mucinous type, EXTL3 is down-regulated by promoter methylation. This results in the loss of heparan sulfate with a probable impact on the mechanisms of growth control [112]. On the contrary, hypermethylation of EXTL3 promoter has never been reported in non-mucinous colon cancers.
3. Epigenetic Regulation of Sugar binding Molecules: The Galectins
Galectins are a family of 15 galactose binding proteins that regulate many aspects of the cell life through sugar-dependent, but also sugar independent, interactions. The different members of the galectin family can play opposite effects by promoting cell growth or inducing apoptosis. The role of galectins in cancer and immune regulation is well-known [113].
3.1. Galectin 1
The gene LGALS1, encoding galectin-1, is usually hypermethylated in colorectal cancer cells—its induction by demethylating treatments induces apoptosis because of down-regulation of Wnt signaling [114]. Hepatic stellate cells play a role in hepatocarcinoma development by locally suppressing the immune response. In hepatic stellate cells of hepatocarcinoma patients, galectin-1 is much higher than in those from normal liver. Meanwhile, in MiR-22, which targets galectin-1, was lower [115], indicating its role in the regulation of the anti-cancer immune response.
3.2. Galectin 3
The LGALS3 gene, encoding galectin-3, shows promoter methylation-dependent regulation in different cancers. It is unmethylated in normal and benign prostate tumors, but it is silenced by methylation in prostate cancer [116], albeit in a stage-specific manner. In fact, it is heavily methylated in stage I and II adenocarcinoma, and lightly methylated in stage III and IV, allowing discrimination of the stages [117]. Promoter methylation is a major mechanism of LGALS3 gene silencing also in mucinous colorectal cancers [118], as well as in pituitary tumors, and breast and thyroid cancer cell lines [119]. Conversely, the average hypomethylation of five CpG sites in the promoter sequence is associated with high LGALS3 expression in thyroid cancer, and allows to distinguish it from normal thyroid tissues [120]. One of the reasons for galectin-3 overexpression in colorectal cancer, which is associated with disease progression and shorter survival, is the decreased levels of the inhibitory miR-128 [121]. Mucin MUC-1 and Galectin-3 provide an example of a self-fueling loop involving a miRNA. MUC1 is transmembrane glycoprotein whose Asn36 residue of the C-terminal subunit can be decorated by an N-linked chain. Owing to its dimeric nature, galectin-3 can bound in both Asn35 of MUC1 and EGFR, activating a signal transduction pathway leading to the suppression of miR-322. Down-regulation of miR-322 stabilizes galectin-3 transcript, leading to increased galectin-3 levels with a positive feedback on MUC1 signaling [122].
3.3. Galectin 7
A few reports document regulation of the LGALS7 gene, encoding galectin-7, in cancer. Promoter hypomethylation is at the basis of the up-regulation of galectin-7 during lymphoma progression [123], while promoter hypermethylation is responsible for galectin-7 downregulation in gastric cancer, a tumor in which it has a tumor suppressive function [124].
3.4. Galectin 9
Galectin-9 has been reported to exert anticancer activity in gallbladder cancer by modulation of the miRNA expression profile [125]. On the other hand, in liver cancer, galectin-9 is overexpressed because of miR-22 downregulation. As a consequence, lymphocyte apoptosis is increased and tumor growth is promoted [126].
4. Glycosylation as a Part of the Epigenetic Code: O-GlcNAc
In the previous sections, we reviewed how epigenetic mechanisms can regulate the expression of a variety of glycogenes. In this last section, we describe how a very peculiar type of glycosylation, the O-linked GlcNAc (O-GlcNAc), is itself part of the epigenetic code. The addition of O-GlcNAc is basically different from the “conventional“ N- and O-glycosylation for the following reasons. GlcNAc is added to cytoplasmic and nuclear (rather than membrane or secreted) proteins by the action of a single enzyme: O-GlcNAc transferase (OGT), and is removed by the action of O-GlcNAc ase (OGA). The addition/removal of O-GlcNAc is a dynamic and reversible process which prevents phosphorylation of the Ser/Thr residues, to which O-GlcNAc is attached. The OGT activity is crucially dependent on the availability of its donor substrate UDP-GlcNAc, whose concentration is directly linked to the nutritional state of the cell. O-GlcNAc can be added to transcription factors involved in embryonic development [127] and histones [128], with a large impact on gene expression. A very recent paper reports that the inhibition of OGT results in a reduction of colon cancer stem cells population. At least in part, this phenomenon is mediated through the O-GlcNAc-mediated methylation of the CpG islands in the promoter region of the MYBL1 gene, encoding a transcription factor which inhibits colon cancer stem cells growth [129]. On the other hand, OGT itself is epigenetically regulated, being a target of miR-424 [33].
5. Concluding Remarks
The review of recently published data showed that many cancer-associated alterations of the glycan profile of cells and tissues depend on the epigenetic deregulation of glycogenes. They include the methylation of promoter sequence, and the aberrant expression of miRNAs, as the most frequent mechanisms, both acting in many cases as inhibitors of glycogene expression (Figure 3). As originally shown for tumor suppressor genes, in cancer there is a general hypermethylation of the promoter of many genes, determining their transcriptional inhibition, and favoring the malignant phenotype.
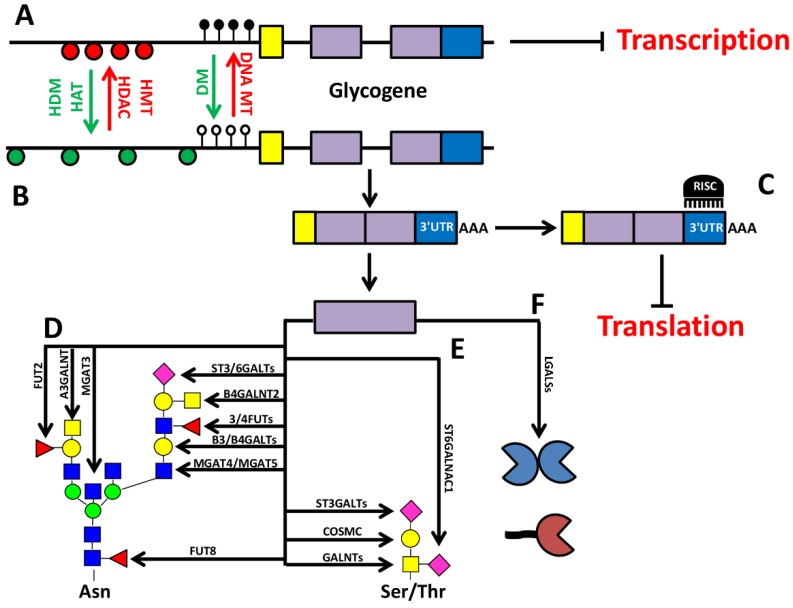
Figure 3.
Summary of the common epigenetic mechanisms of glycosylation control. A hypothetical glycogene is represented in which the activity of histone methyltransferase (HMT), histone deacetylase (HDAC), and DNA methyltransferase (DNA MT) results in chromatin condensation determining a transcriptionally inactive state; closely spaced red circles represent nucleosomes, black lollipops represent methylated cytosine residues (A). The gene can be turned into a transcriptionally active state by the activity of histone demethylase (HDM) and histone acetyltransferase (HAT), which reduce chromatin condensation (loosely spaced green circles), and by demethylation of the promoter region (white lollipops) (B). Transcription of the gene results in a mRNA comprised of a 5′UTR (yellow), a protein coding region (violet), and a 3′UTR (blue), which can be targeted by miRNA, resulting in translation inhibition; RISC, RNA-induced silencing complex (C). This hypothetical glycogene is a paradigm of many others controlled by epigenetic mechanisms. They are involved in a variety of biosynthetic steps that are grouped in the hypothetic N- and O-linked structures depicted in (D) and (E). The epitopes shown in (D) and (E) are often mutually exclusive, and are presented here as a single structure only for didactic purposes. Moreover, glycogenes encode molecules like galectins (F) involved in the biological roles of glycans.
Gene reactivation can be obtained by DNA demethylating agents, restoring the normal levels of gene expression. However, relevant exceptions exist, and can be useful to shed light on the general mechanisms of epigenetic control. In fact, promoter hypermethylation can be tissue specific and evident in cancer cell lines, but not in the corresponding native tumors [46]. Treatment with demethylating agents, which is usually the first approach in determining whether a gene is subjected to promoter methylation, sometimes appear unable to counteract hypermethylation and/or to restore transcription levels [49,56,116,120]. Moreover, treatment with demethylating agents inhibits, instead of stimulating, several glycogenes [28,54,99]. The opposite role of methylation of specific CpG pairs in promoters, or in distinct DNA regions far from the promoter sequences, should be kept in mind in this regard [45,56,120]. Furthermore, some of the glycogenes reactivated through demethylating agents, and some of those inhibited by the same treatment do not attenuate, but instead sustain the malignant phenotype [26,32,71], an observation relevant in view of the clinical use of such agents. The complexity of the picture is recapitulated by the expanding role of miRNAs that also appear to be able to determine double-edged effects on glycogenes (Table 1). Being more specific and straightforward, they are thus promising tools for therapies aimed at the inhibition of such genes.
Table 1.
List of glycogenes regulated by epigenetic mechanisms.
| Target | Epigenetic Mechanism | Effect | Tissues/Cells Involved | Reference |
|---|---|---|---|---|
| Galactosyltransferases | ||||
| A3GALT1/A3GALNT | Promoter methylation | Down-regulation | Bladder and oral cancer | [107,108] |
| B3GALT5 native | Promoter methylation | Down-regulation | Colon cancer | [53,54] |
| B3GALT5 LTR | Distant sequence methylation | Up-regulation | Colon cancer | [54,56] |
| B4GALT1 | Promoter methylation | Down-regulation | Colon cancer | [57] |
| B4GALT3 | miR-27a | Up-regulation | Cervical cancer | [58] |
| C1GALT1 chaperone Cosmc | Promoter methylation | Down-regulation | B lymphocyte and other model cell lines | [43,44] |
| N-acetyl-galactosaminyl transferases | ||||
| GALNT1 | miR-129 | Down-regulation | Bladder cancer | [34] |
| GALNT4 | miR-9 | Down-regulation | Liver and cervical cancer | [35,36] |
| GALNT7 | miR-34° -34c | Down-regulation | Laryngeal cancer | [37] |
| miR-214 | Down-regulation | Esophageal cancer | [38] | |
| miR-17-3p | Down-regulation | Liver cell lines and mouse model | [40] | |
| GALNT10 | miR-122 | Down-regulation | Liver cancer | [41] |
| GALNT13 | miR-424 | Down-regulation | Breast and HEK293 cell lines | [33] |
| GALNT14 | miR-125a | Down-regulation | Ovarian cancer | [42] |
| A3GALNT/A3GALT1 | Promoter methylation | Down-regulation | Bladder and oral cancer | [107,108] |
| B4GALNT2 | Promoter methylation | Down-regulation | Gastrointestinal cancer | [80,81] |
| N-acetyl-glucosaminyl transferases | ||||
| MGAT3 | Promoter methylation | Down-regulation | Mammary model and ovarian and liver cell lines | [25,26,27,28,29] |
| MGAT4 | miR-424 | Down-regulation | Breast and HEK293 cell lines | [33] |
| MGAT5 | Distant methylation | Down-regulation | Ovarian cell line | [28] |
| MGAT5 | Distant methylation | Up-regulation | Melanoma hybrid cell line | [32] |
| B3GLCT | miR-200 | Down-regulation | Breast cell line | [50] |
| B3GNT7 | Promoter methylation | Down-regulation | Colon cancer | [59] |
| OGT | miR-424 | unclear | Breast and HEK293 cell lines | [33] |
| Sialyltransferases | ||||
| ST3GAL1 | miR-4701-5p | Down-regulation | Chronic myeloid leukemia | [103] |
| ST3GAL2 | Promoter methylation | Down-regulation | Prostate cell lines | [48] |
| ST3GAL3 | Promoter methylation | unknown | Whole methylome | [70] |
| ST3GAL5 | miR-200 | Down-regulation | Breast cell line | [50] |
| miR-26a, miR-548l, miR-34a | Down-regulation | Liver cancer | [51] | |
| ST3GAL6 | Promoter methylation | Down-regulation | Colon cancer cell line | [71] |
| miR-26a | Down-regulation | Liver cancer | [102] | |
| ST6GAL1 | Promoter methylation | Down-regulation | Glioma, bladder and breast cancer | [97,98,99] |
| miR-199a | Down-regulation | Lung and HEK293 cell lines | [100] | |
| ST6GALNAC1 | Promoter methylation | Down-regulation | Esophageal cancer | [46] |
| ST6GALNAC4 | miR-4299 | Down-regulation | Thyroid cancer | [104] |
| ST6GALNAC5 | Promoter methylation | No regulation | Colon cancer | [49] |
| miR-200 | Down-regulation | Breast cell line | [50] | |
| ST6GALNAC6 | Putative promoter methylation/ histone deacetylation | Down-regulation | Colon cancer | [67] |
| ST8SIA4 | miR26a-26b | Down-regulation | Breast cancer | [101] |
| Fucosyltransferases | ||||
| FUT2 | miR-15b | Down-regulation | Liver cancer | [110] |
| FUT3 | Promoter methylation | Down-regulation | Gastric and tongue cell lines | [68,69] |
| FUT4 | Promoter methylation | Down-regulation | Skin cell lines | [84] |
| miR-224-3p | Down-regulation | Breast cancer | [85] | |
| miR-493-5p | Down-regulation | Breast cancer | [86] | |
| FUT6 | miR-106b | Down-regulation | Breast cancer | [87] |
| FUT8 | miR-198 | Up-regulation | Colon cancer | [19] |
| miR-122 -34a 26a -146a | Down-regulation | Liver cancer | [20,21] | |
| Sulfotransferases | ||||
| HS3STs | Promoter methylation | Down-regulation | Chondrosarcoma cell line | [111] |
| Nucleotide donor transporters | ||||
| DTDST | Histone deacetylation | Down-regulation | Colon cancer | [72] |
| Galectins | ||||
| LGALS3 | Promoter methylation | Down-regulation | Colon, prostate and pituitary cancer | [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119] |
| Promoter hypomethylation | Up-regulation | Thyroid cancer | [120] | |
| miR-322 | Down-regulation | Breast, lung, prostate and HEK293 cell lines | [122] | |
| miR-128 | Down-regulation | Colon cancer | [121] | |
| LGALS7 | Promoter methylation | Down-regulation | Gastric cancer and lymphomas | [123,124] |
| LGALS9 | miR-22 | Down-regulation | Liver cancer | [126] |
Acknowledgments
This work was supported by grants from the University of Insubria to Marco Trinchera and from the University of Bologna and from the Pallotti Legacy to Fabio Dall’Olio.
Abbreviations
| 5-AZA | 5-Aza-2′-deoxycytidine |
| EMT | Epithelial to mesenchymal transition |
Author Contributions
Fabio Dall’Olio and Marco Trinchera contributed equally to the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lauc G., Zoldos V. Epigenetic regulation of glycosylation could be a mechanism used by complex organisms to compete with microbes on an evolutionary scale. Med. Hypotheses. 2009;73:510–512. doi: 10.1016/j.mehy.2009.03.059. [DOI] [PubMed] [Google Scholar]
- 2.Lauc G., Vojta A., Zoldos V. Epigenetic regulation of glycosylation is the quantum mechanics of biology. Biochim. Biophys. Acta. 2014;1840:65–70. doi: 10.1016/j.bbagen.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Zoldos V., Novokmet M., Beceheli I., Lauc G. Genomics and epigenomics of the human glycome. Glycoconj. J. 2013;30:41–50. doi: 10.1007/s10719-012-9397-y. [DOI] [PubMed] [Google Scholar]
- 4.Zoldos V., Horvat T., Novokmet M., Cuenin C., Muzinic A., Pucic M., Huffman J.E., Gornik O., Polasek O., Campbell H., et al. Epigenetic silencing of HNF1A associates with changes in the composition of the human plasma N-glycome. Epigenetics. 2012;7:164–172. doi: 10.4161/epi.7.2.18918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoldos V., Horvat T., Lauc G. Glycomics meets genomics, epigenomics and other high throughput omics for system biology studies. Curr. Opin. Chem. Biol. 2013;17:34–40. doi: 10.1016/j.cbpa.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Vojta A., Samarzija I., Bockor L., Zoldos V. Glyco-genes change expression in cancer through aberrant methylation. Biochim. Biophys. Acta. 2016;1860:1776–1785. doi: 10.1016/j.bbagen.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Moriwaki K., Narisada M., Imai T., Shinzaki S., Miyoshi E. The effect of epigenetic regulation of fucosylation on TRAIL-induced apoptosis. Glycoconj. J. 2010;27:649–659. doi: 10.1007/s10719-010-9310-5. [DOI] [PubMed] [Google Scholar]
- 8.Kizuka Y., Nakano M., Miura Y., Taniguchi N. Epigenetic regulation of neural N-glycomics. Proteomics. 2016;16:2854–2863. doi: 10.1002/pmic.201600053. [DOI] [PubMed] [Google Scholar]
- 9.Horvat T., Muzinic A., Barisic D., Bosnar M.H., Zoldos V. Epigenetic modulation of the HeLa cell membrane N-glycome. Biochim. Biophys. Acta. 2012;1820:1412–1419. doi: 10.1016/j.bbagen.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Horvat T., Dezeljin M., Redzic I., Barisic D., Herak B.M., Lauc G., Zoldos V. Reversibility of Membrane N-Glycome of HeLa Cells upon Treatment with Epigenetic Inhibitors. PLoS ONE. 2013;8:e54672. doi: 10.1371/journal.pone.0054672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki Y., Yanagisawa M., Ariga T., Yu R.K. Histone acetylation-mediated glycosyltransferase gene regulation in mouse brain during development. J. Neurochem. 2011;116:874–880. doi: 10.1111/j.1471-4159.2010.07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agrawal P., Kurcon T., Pilobello K.T., Rakus J.F., Koppolu S., Liu Z., Batista B.S., Eng W.S., Hsu K.L., Liang Y., et al. Mapping posttranscriptional regulation of the human glycome uncovers microRNA defining the glycocode. Proc. Natl. Acad. Sci. USA. 2014;111:4338–4343. doi: 10.1073/pnas.1321524111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasper B.T., Koppolu S., Mahal L.K. Insights into miRNA regulation of the human glycome. Biochem. Biophys. Res. Commun. 2014;445:774–779. doi: 10.1016/j.bbrc.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uozumi N., Yanagidani S., Miyoshi E., Ihara Y., Sakuma T., Gao C.X., Teshima T., Fujii S., Shiba T., Taniguchi N. Purification and cDNA cloning of porcine brain GDP-L-Fuc: N-acetyl-β-D-glucosaminide a1-->6fucosyltransferase. J. Biol. Chem. 1996;271:27810–27817. doi: 10.1074/jbc.271.44.27810. [DOI] [PubMed] [Google Scholar]
- 15.Wang X., Inoue S., Gu J., Miyoshi E., Noda K., Li W., Mizuno-Horikawa Y., Nakano M., Asahi M., Takahashi M., et al. Dysregulation of TGF-β1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc. Natl. Acad. Sci. USA. 2005;102:15791–15796. doi: 10.1073/pnas.0507375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comunale M.A., Lowman M., Long R.E., Krakover J., Philip R., Seeholzer S., Evans A.A., Hann H.W., Block T.M., Mehta A.S. Proteomic Analysis of Serum Associated Fucosylated Glycoproteins in the Development of Primary Hepatocellular Carcinoma. J. Proteome Res. 2006;5:308–315. doi: 10.1021/pr050328x. [DOI] [PubMed] [Google Scholar]
- 17.Wang X., Chen J., Li Q.K., Peskoe S.B., Zhang B., Choi C., Platz E.A., Zhang H. Overexpression of α (1,6) fucosyltransferase associated with aggressive prostate cancer. Glycobiology. 2014;24:935–944. doi: 10.1093/glycob/cwu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y.P., Xu X.Y., Fang M., Wang H., You Q., Yi C.H., Ji J., Gu X., Zhou P.T., Cheng C., et al. Decreased core-fucosylation contributes to malignancy in gastric cancer. PLoS ONE. 2014;9:e94536. doi: 10.1371/journal.pone.0094536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M., Wang J., Kong X., Chen H., Wang Y., Qin M., Lin Y., Chen H., Xu J., Hong J., et al. MiR-198 represses tumor growth and metastasis in colorectal cancer by targeting fucosyl transferase 8. Sci. Rep. 2014;4:6145. doi: 10.1038/srep06145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernardi C., Soffientini U., Piacente F., Tonetti M.G. Effects of microRNAs on fucosyltransferase 8 (FUT8) expression in hepatocarcinoma cells. PLoS ONE. 2013;8:e76540. doi: 10.1371/journal.pone.0076540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng L., Gao S., Song X., Dong W., Zhou H., Zhao L., Jia L. Comprehensive N-glycan profiles of hepatocellular carcinoma reveal association of fucosylation with tumor progression and regulation of FUT8 by microRNAs. Oncotarget. 2016;7:61199–61214. doi: 10.18632/oncotarget.11284. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Gu J., Sato Y., Kariya Y., Isaji T., Taniguchi N., Fukuda T. A mutual regulation between cell-cell adhesion and N-glycosylation: Implication of the bisecting GlcNAc for biological functions. J. Proteome Res. 2009;8:431–435. doi: 10.1021/pr800674g. [DOI] [PubMed] [Google Scholar]
- 23.Pinho S.S., Reis C.A., Paredes J., Magalhaes A.M., Ferreira A.C., Figueiredo J., Xiaogang W., Carneiro F., Gartner F., Seruca R. The role of N-acetylglucosaminyltransferase III and V in the post-transcriptional modifications of E-cadherin. Hum. Mol. Genet. 2009;18:2599–2608. doi: 10.1093/hmg/ddp194. [DOI] [PubMed] [Google Scholar]
- 24.Pinho S.S., Seruca R., Gartner F., Yamaguchi Y., Gu J., Taniguchi N., Reis C.A. Modulation of E-cadherin function and dysfunction by N-glycosylation. Cell Mol. Life Sci. 2011;68:1011–1020. doi: 10.1007/s00018-010-0595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinho S.S., Oliveira P., Cabral J., Carvalho S., Huntsman D., Gartner F., Seruca R., Reis C.A., Oliveira C. Loss and recovery of MGAT3 and GnT-III Mediated E-cadherin N-glycosylation is a mechanism involved in epithelial-mesenchymal-epithelial transitions. PLoS ONE. 2012;7:e33191. doi: 10.1371/journal.pone.0033191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohler R.S., Anugraham M., Lopez M.N., Xiao C., Schoetzau A., Hettich T., Schlotterbeck G., Fedier A., Jacob F., Heinzelmann-Schwarz V. Epigenetic activation of MGAT3 and corresponding bisecting GlcNAc shortens the survival of cancer patients. Oncotarget. 2016;7:51674–51686. doi: 10.18632/oncotarget.10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anugraham M., Jacob F., Nixdorf S., Everest-Dass A.V., Heinzelmann-Schwarz V., Packer N.H. Specific glycosylation of membrane proteins in epithelial ovarian cancer cell lines: Glycan structures reflect gene expression and DNA methylation status. Mol. Cell Proteom. 2014;13:2213–2232. doi: 10.1074/mcp.M113.037085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saldova R., Dempsey E., Perez-Garay M., Marino K., Watson J.A., Blanco-Fernandez A., Struwe W.B., Harvey D.J., Madden S.F., Peracaula R., et al. 5-AZA-2′-deoxycytidine induced demethylation influences N-glycosylation of secreted glycoproteins in ovarian cancer. Epigenetics. 2011;6:1362–1372. doi: 10.4161/epi.6.11.17977. [DOI] [PubMed] [Google Scholar]
- 29.Klasic M., Kristic J., Korac P., Horvat T., Markulin D., Vojta A., Reiding K.R., Wuhrer M., Lauc G., Zoldos V. DNA hypomethylation upregulates expression of the MGAT3 gene in HepG2 cells and leads to changes in N-glycosylation of secreted glycoproteins. Sci. Rep. 2016;6:24363. doi: 10.1038/srep24363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granovsky M., Fata J., Pawling J., Muller W.J., Khokha R., Dennis J.W. Suppression of tumor growth and metastasis in MGAT5-deficient mice. Nat. Med. 2000;6:306–312. doi: 10.1038/73163. [DOI] [PubMed] [Google Scholar]
- 31.Lau K.S., Partridge E.A., Grigorian A., Silvescu C.I., Reinhold V.N., Demetriou M., Dennis J.W. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell. 2007;129:123–134. doi: 10.1016/j.cell.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 32.Chakraborty A.K., Sousa J.F., Chakraborty D., Funasaka Y., Bhattacharya M., Chatterjee A., Pawelek J. GnT-V expression and metastatic phenotypes in macrophage-melanoma fusion hybrids is down-regulated by 5-Aza-dC: Evidence for methylation sensitive, extragenic regulation of GnT-V transcription. Gene. 2006;374:166–173. doi: 10.1016/j.gene.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 33.Vaiana C.A., Kurcon T., Mahal L.K. MicroRNA-424 Predicts a Role for b1,4 Branched Glycosylation in Cell Cycle Progression. J. Biol. Chem. 2016;291:1529–1537. doi: 10.1074/jbc.M115.672220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dyrskjot L., Ostenfeld M.S., Bramsen J.B., Silahtaroglu A.N., Lamy P., Ramanathan R., Fristrup N., Jensen J.L., Andersen C.L., Zieger K., et al. Genomic profiling of microRNAs in bladder cancer: miR-129 is associated with poor outcome and promotes cell death in vitro. Cancer Res. 2009;69:4851–4860. doi: 10.1158/0008-5472.CAN-08-4043. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y., Liu H., Yang L., Wu Q., Liu W., Fu Q., Zhang W., Zhang H., Xu J., Gu J. Loss of N-acetylgalactosaminyltransferase-4 orchestrate oncogenic microRNA-9 in hepatocellular carcinoma. J. Biol. Chem. 2017 doi: 10.1074/jbc.M116.751685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng R.Q., Wan H.Y., Li H.F., Liu M., Li X., Tang H. MicroRNA-214 Suppresses Growth and Invasiveness of Cervical Cancer Cells by Targeting UDP-N-acetyl-α-D-galactosamine:Polypeptide N-Acetylgalactosaminyltransferase 7. J. Biol. Chem. 2012;287:14301–14309. doi: 10.1074/jbc.M111.337642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W., Ma H., Sun J. MicroRNA34a/c function as tumor suppressors in Hep2 laryngeal carcinoma cells and may reduce GALNT7 expression. Mol. Med. Rep. 2014;9:1293–1298. doi: 10.3892/mmr.2014.1929. [DOI] [PubMed] [Google Scholar]
- 38.Lu Q., Xu L., Li C., Yuan Y., Huang S., Chen H. miR-214 inhibits invasion and migration via downregulating GALNT7 in esophageal squamous cell cancer. Tumour Biol. 2016;37:14605–14614. doi: 10.1007/s13277-016-5320-7. [DOI] [PubMed] [Google Scholar]
- 39.Gaziel-Sovran A., Segura M.F., Di Micco R., Collins M.K., Hanniford D., Vega-Saenz de Miera E., Rakus J.F., Dankert J.F., Shang S., Kerbel R.S., et al. miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell. 2011;20:104–118. doi: 10.1016/j.ccr.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shan S.W., Fang L., Shatseva T., Rutnam Z.J., Yang X., Du W., Lu W.Y., Xuan J.W., Deng Z., Yang B.B. Mature miR-17-5p and passenger miR-17-3p induce hepatocellular carcinoma by targeting PTEN, GalNT7 and vimentin in different signal pathways. J. Cell Sci. 2013;126:1517–1530. doi: 10.1242/jcs.122895. [DOI] [PubMed] [Google Scholar]
- 41.Wu Q., Liu H.O., Liu Y.D., Liu W.S., Pan D., Zhang W.J., Yang L., Fu Q., Xu J.J., Gu J.X. Decreased Expression of Hepatocyte Nuclear Factor 4α (Hnf4α)/MicroRNA-122 (miR-122) Axis in Hepatitis B Virus-associated Hepatocellular Carcinoma Enhances Potential Oncogenic GALNT10 Protein Activity. J. Biol. Chem. 2015;290:1170–1185. doi: 10.1074/jbc.M114.601203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang J., Li G., Zhang K. MiR-125a regulates ovarian cancer proliferation and invasion by repressing GALNT14 expression. Biomed. Pharmacother. 2016;80:381–387. doi: 10.1016/j.biopha.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 43.Mi R., Song L., Wang Y., Ding X., Zeng J., Lehoux S., Aryal R.P., Wang J., Crew V.K., van Die I., et al. Epigenetic silencing of the chaperone cosmc in human leukocytes expressing tn antigen. J. Biol. Chem. 2012;287:41523–41533. doi: 10.1074/jbc.M112.371989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng J., Mi R., Wang Y., Li Y., Lin L., Yao B., Song L., van Die I., Chapman A.B., Cummings R.D., et al. Promoters of human Cosmc and T-synthase are similar in structure, yet different in epigenetic regulation. J. Biol. Chem. 2015;290:19018–19033. doi: 10.1074/jbc.M115.654244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L., Lee K.M., Han W., Choi J.Y., Lee J.Y., Kang G.H., Park S.K., Noh D.Y., Yoo K.Y., Kang D. Estrogen and progesterone receptor status affect genome-wide DNA methylation profile in breast cancer. Hum. Mol. Genet. 2010;19:4273–4277. doi: 10.1093/hmg/ddq351. [DOI] [PubMed] [Google Scholar]
- 46.Iwaya T., Sawada G., Amano S., Kume K., Ito C., Endo F., Konosu M., Shioi Y., Akiyama Y., Takahara T., et al. Downregulation of ST6GALNAC1 is associated with esophageal squamous cell carcinoma development. Int. J. Oncol. 2017;50:441–447. doi: 10.3892/ijo.2016.3817. [DOI] [PubMed] [Google Scholar]
- 47.Daniotti J.L., Lardone R.D., Vilcaes A.A. Dysregulated Expression of Glycolipids in Tumor Cells: From Negative Modulator of Anti-tumor Immunity to Promising Targets for Developing Therapeutic Agents. Front Oncol. 2015;5:300. doi: 10.3389/fonc.2015.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hatano K., Miyamoto Y., Mori M., Nimura K., Nakai Y., Nonomura N., Kaneda Y. Androgen-regulated transcriptional control of sialyltransferases in prostate cancer cells. PLoS ONE. 2012;7:e31234. doi: 10.1371/journal.pone.0031234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oster B., Thorsen K., Lamy P., Wojdacz T.K., Hansen L.L., Birkenkamp-Demtroder K., Sorensen K.D., Laurberg S., Orntoft T.F., Andersen C.L. Identification and validation of highly frequent CpG island hypermethylation in colorectal adenomas and carcinomas. Int. J. Cancer. 2011;129:2855–2866. doi: 10.1002/ijc.25951. [DOI] [PubMed] [Google Scholar]
- 50.Kurcon T., Liu Z., Paradkar A.V., Vaiana C.A., Koppolu S., Agrawal P., Mahal L.K. miRNA proxy approach reveals hidden functions of glycosylation. Proc. Natl. Acad. Sci. USA. 2015;112:7327–7332. doi: 10.1073/pnas.1502076112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai H., Zhou H., Miao Y., Li N., Zhao L., Jia L. MiRNA expression profiles reveal the involvement of miR-26a, miR-548l and miR-34a in hepatocellular carcinoma progression through regulation of ST3GAL5. Lab. Investig. 2017 doi: 10.1038/labinvest.2017.12. [DOI] [PubMed] [Google Scholar]
- 52.Mare L., Trinchera M. Comparative Analysis of Retroviral and Native Promoters Driving Expression of b1,3-Galactosyltransferase b3Gal-T5 in Human and Mouse Tissues. J. Biol. Chem. 2007;282:49–57. doi: 10.1074/jbc.M606666200. [DOI] [PubMed] [Google Scholar]
- 53.Caretti A., Sirchia S.M., Tabano S., Zulueta A., Dall’Olio F., Trinchera M. DNA methylation and histone modifications modulate the β1,3 galactosyltransferase β3Gal-T5 native promoter in cancer cells. Int. J. Biochem. Cell Biol. 2012;44:84–90. doi: 10.1016/j.biocel.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 54.Aronica A., Avagliano L., Caretti A., Tosi D., Bulfamante G.P., Trinchera M. Unexpected distribution of CA19.9 and other type 1 chain Lewis antigens in normal and cancer tissues of colon and pancreas: Importance of the detection method and role of glycosyltransferase regulation. Biochim. Biophys. Acta. 2016;1861:3210–3220. doi: 10.1016/j.bbagen.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Dunn C.A., van de Lagemaat L.N., Baillie G.J., Mager D.L. Endogenous retrovirus long terminal repeats as ready-to-use mobile promoters: The case of primate β3GAL-T5. Gene. 2005;364:2–12. doi: 10.1016/j.gene.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 56.Zulueta A., Caretti A., Signorelli P., Dall’Olio F., Trinchera M. Transcriptional control of the B3GALT5 gene by a retroviral promoter and methylation of distant regulatory elements. FASEB J. 2014;28:946–955. doi: 10.1096/fj.13-236273. [DOI] [PubMed] [Google Scholar]
- 57.Poeta M.L., Massi E., Parrella P., Pellegrini P., de Robertis M., Copetti M., Rabitti C., Perrone G., Muda A.O., Molinari F., et al. Aberrant promoter methylation of β-1,4 galactosyltransferase 1 as potential cancer-specific biomarker of colorectal tumors. Genes Chromosomes Cancer. 2012;51:1133–1143. doi: 10.1002/gcc.21998. [DOI] [PubMed] [Google Scholar]
- 58.Sun Y., Yang X., Liu M., Tang H. B4GALT3 up-regulation by miR-27a contributes to the oncogenic activity in human cervical cancer cells. Cancer Lett. 2016;375:284–292. doi: 10.1016/j.canlet.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 59.Lu C.H., Wu W.Y., Lai Y.J., Yang C.M., Yu L.C. Suppression of B3GNT7 gene expression in colon adenocarcinoma and its potential effect in the metastasis of colon cancer cells. Glycobiology. 2014;24:359–367. doi: 10.1093/glycob/cwu002. [DOI] [PubMed] [Google Scholar]
- 60.Dall’Olio F., Chiricolo M. Sialyltransferases in cancer. Glycoconj. J. 2001;18:841–850. doi: 10.1023/A:1022288022969. [DOI] [PubMed] [Google Scholar]
- 61.Dall’Olio F., Malagolini N., Trinchera M., Chiricolo M. Sialosignaling: Sialyltransferases as engines of self-fueling loops in cancer progression. Biochim. Biophys. Acta. 2014;1840:2752–2764. doi: 10.1016/j.bbagen.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 62.Harduin-Lepers A., Vallejo-Ruiz V., Krzewinski-Recchi M., Samyn-Petit B., Julien S., Delannoy P. The human sialyltransferase family. Biochimie. 2001;83:727–737. doi: 10.1016/S0300-9084(01)01301-3. [DOI] [PubMed] [Google Scholar]
- 63.De Vries T., Knegtel R.M., Holmes E.H., Macher B.A. Fucosyltransferases: Structure/function studies. Glycobiology. 2001;11:119R–128R. doi: 10.1093/glycob/11.10.119R. [DOI] [PubMed] [Google Scholar]
- 64.Trinchera M., Aronica A., Dall’Olio F. Selectin Ligands Sialyl-Lewis a and Sialyl-Lewis x in Gastrointestinal Cancers. Biology (Basel) 2017;6 doi: 10.3390/biology6010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mollicone R., Candelier J.J., Reguigne I., Couillin P., Fletcher A., Oriol R. Molecular genetics of a-L-fucosyltransferase genes (H, Se, Le, FUT4, FUT5 and FUT6) Transfus. Clin. Biol. 1994;1:91–97. doi: 10.1016/S1246-7820(94)80002-2. [DOI] [PubMed] [Google Scholar]
- 66.Terraneo L., Avagliano L., Caretti A., Bianciardi P., Tosi D., Bulfamante G.P., Samaja M., Trinchera M. Expression of carbohydrate-antigen sialyl-Lewis a on colon cancer cells promotes xenograft growth and angiogenesis in nude mice. Int. J. Biochem. Cell Biol. 2013;45:2796–2800. doi: 10.1016/j.biocel.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 67.Miyazaki K., Ohmori K., Izawa M., Koike T., Kumamoto K., Furukawa K., Ando T., Kiso M., Yamaji T., Hashimoto Y., et al. Loss of disialyl Lewisa the ligand for lymphocyte inhibitory receptor sialic acid-binding immunoglobulin-like lectin-7 (Siglec-7) associated with increased sialyl Lewis a expression on human colon cancers. Cancer Res. 2004;64:4498–4505. doi: 10.1158/0008-5472.CAN-03-3614. [DOI] [PubMed] [Google Scholar]
- 68.Serpa J., Mesquita P., Mendes N., Oliveira C., Almeida R., Santos-Silva F., Reis C.A., Lependu J., David L. Expression of Lea in gastric cancer cell lines depends on FUT3 expression regulated by promoter methylation. Cancer Lett. 2006;242:191–197. doi: 10.1016/j.canlet.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 69.Krishnan N.M., Dhas K., Nair J., Palve V., Bagwan J., Siddappa G., Suresh A., Kekatpure V.D., Kuriakose M.A., Panda B. A Minimal DNA Methylation Signature in Oral Tongue Squamous Cell Carcinoma Links Altered Methylation with Tumor Attributes. Mol. Cancer Res. 2016;14:805–819. doi: 10.1158/1541-7786.MCR-15-0395. [DOI] [PubMed] [Google Scholar]
- 70.Walton E., Pingault J.B., Cecil C.A., Gaunt T.R., Relton C.L., Mill J., Barker E.D. Epigenetic profiling of ADHD symptoms trajectories: A prospective, methylome-wide study. Mol. Psychiatry. 2017;22:250–256. doi: 10.1038/mp.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chachadi V.B., Cheng H., Klinkebiel D., Christman J.K., Cheng P.W. 5-Aza-2′-deoxycytidine increases sialyl Lewis X on MUC1 by stimulating b-galactoside:a2,3-sialyltransferase 6 gene. Int. J. Biochem. Cell Biol. 2011;43:586–593. doi: 10.1016/j.biocel.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yusa A., Miyazaki K., Kimura N., Izawa M., Kannagi R. Epigenetic silencing of the sulfate transporter gene DTDST induces sialyl Lewisx expression and accelerates proliferation of colon cancer cells. Cancer Res. 2010;70:4064–4073. doi: 10.1158/0008-5472.CAN-09-2383. [DOI] [PubMed] [Google Scholar]
- 73.Donald A.S., Yates A.D., Soh C.P., Morgan W.T., Watkins W.M. A blood group Sda-active pentasaccharide isolated from Tamm-Horsfall urinary glycoprotein. Biochem. Biophys. Res. Commun. 1983;115:625–631. doi: 10.1016/S0006-291X(83)80190-9. [DOI] [PubMed] [Google Scholar]
- 74.Presti L., Cabuy E., Chiricolo M., Dall’Olio F. Molecular Cloning of the Human b1,4 N-Acetylgalactosaminyltransferase Responsible for the Biosynthesis of the Sda Histo-Blood Group Antigen: The Sequence Predicts a Very Long Cytoplasmic Domain. J. Biochem. (Tokyo) 2003;134:675–682. doi: 10.1093/jb/mvg192. [DOI] [PubMed] [Google Scholar]
- 75.Montiel M.D., Krzewinski-Recchi M.A., Delannoy P., Harduin-Lepers A. Molecular cloning, gene organization and expression of the human UDP-GalNAc:Neu5Aca2-3Galb-R b1,4-N-acetylgalactosaminyltransferase responsible for the biosynthesis of the blood group Sda/Cad antigen: Evidence for an unusual extended cytoplasmic domain. Biochem. J. 2003;373:369–379. doi: 10.1042/bj20021892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dall’Olio F., Malagolini N., Chiricolo M., Trinchera M., Harduin-Lepers A. The expanding roles of the Sda/Cad carbohydrate antigen and its cognate glycosyltransferase B4GALNT2. Biochim. Biophys. Acta. 2014;1840:443–453. doi: 10.1016/j.bbagen.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 77.Groux-Degroote S., Wavelet C., Krzewinski-Recchi M.A., Portier L., Mortuaire M., Mihalache A., Trinchera M., Delannoy P., Malagolini N., Chiricolo M., et al. B4GALNT2 gene expression controls the biosynthesis of Sda and sialyl Lewis X antigens in healthy and cancer human gastrointestinal tract. Int. J. Biochem. Cell Biol. 2014;53:442–449. doi: 10.1016/j.biocel.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 78.Malagolini N., Dall’Olio F., Di Stefano G., Minni F., Marrano D., Serafini-Cessi F. Expression of UDP-GalNAc:NeuAc α2,3Gal β-R β 1,4(GalNAc to Gal) N-acetylgalactosaminyltransferase involved in the synthesis of Sda antigen in human large intestine and colorectal carcinomas. Cancer Res. 1989;49:6466–6470. [PubMed] [Google Scholar]
- 79.Malagolini N., Santini D., Chiricolo M., Dall’Olio F. Biosynthesis and expression of the Sda and sialyl Lewis x antigens in normal and cancer colon. Glycobiology. 2007;17:688–697. doi: 10.1093/glycob/cwm040. [DOI] [PubMed] [Google Scholar]
- 80.Wang H.R., Hsieh C.Y., Twu Y.C., Yu L.C. Expression of the human Sda β-1,4-N-acetylgalactosaminyltransferase II gene is dependent on the promoter methylation status. Glycobiology. 2008;18:104–113. doi: 10.1093/glycob/cwm120. [DOI] [PubMed] [Google Scholar]
- 81.Kawamura Y.I., Toyota M., Kawashima R., Hagiwara T., Suzuki H., Imai K., Shinomura Y., Tokino T., Kannagi R., Dohi T. DNA hypermethylation contributes to incomplete synthesis of carbohydrate determinants in gastrointestinal cancer. Gastroenterology. 2008;135:142–151. doi: 10.1053/j.gastro.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 82.Mare L., Caretti A., Albertini R., Trinchera M. CA19.9 antigen circulating in the serum of colon cancer patients: Where is it from? Int. J. Biochem. Cell Biol. 2013;45:792–797. doi: 10.1016/j.biocel.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 83.Trinchera M., Malagolini N., Chiricolo M., Santini D., Minni F., Caretti A., Dall’Olio F. The biosynthesis of the selectin-ligand sialyl Lewis x in colorectal cancer tissues is regulated by fucosyltransferase VI and can be inhibited by an RNA interference-based approach. Int. J. Biochem. Cell Biol. 2011;43:130–139. doi: 10.1016/j.biocel.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 84.Li H., Tong S., Liu J., Han L., Yang X., Hou H., Yan Q., Wang X.Q. Differential fucosyltransferase IV expression in squamous carcinoma cells is regulated by promoter methylation. Cell Mol. Biol. Lett. 2012;17:206–216. doi: 10.2478/s11658-012-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feng X., Zhao L., Gao S., Song X., Dong W., Zhao Y., Zhou H., Cheng L., Miao X., Jia L. Increased fucosylation has a pivotal role in multidrug resistance of breast cancer cells through miR-224-3p targeting FUT4. Gene. 2016;578:232–241. doi: 10.1016/j.gene.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 86.Zhao L., Feng X., Song X., Zhou H., Zhao Y., Cheng L., Jia L. miR-493-5p attenuates the invasiveness and tumorigenicity in human breast cancer by targeting FUT4. Oncol. Rep. 2016;36:1007–1015. doi: 10.3892/or.2016.4882. [DOI] [PubMed] [Google Scholar]
- 87.Li N., Liu Y., Miao Y., Zhao L., Zhou H., Jia L. MicroRNA-106b targets FUT6 to promote cell migration, invasion, and proliferation in human breast cancer. IUBMB Life. 2016;68:764–775. doi: 10.1002/iub.1541. [DOI] [PubMed] [Google Scholar]
- 88.Weinstein J., Lee E.U., McEntee K., Lai P.H., Paulson J.C. Primary structure of β-galactoside α 2,6-sialyltransferase. Conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J. Biol. Chem. 1987;262:17735–17743. [PubMed] [Google Scholar]
- 89.Seales E.C., Jurado G.A., Brunson B.A., Wakefield J.K., Frost A.R., Bellis S.L. Hypersialylation of b1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 2005;65:4645–4652. doi: 10.1158/0008-5472.CAN-04-3117. [DOI] [PubMed] [Google Scholar]
- 90.Jones M.B., Nasirikenari M., Feng L., Migliore M.T., Choi K.S., Kazim L., Lau J.T. Role for hepatic and circulatory ST6Gal-1 sialyltransferase in regulating myelopoiesis. J. Biol. Chem. 2010;285:25009–25017. doi: 10.1074/jbc.M110.104406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nasirikenari M., Chandrasekaran E.V., Matta K.L., Segal B.H., Bogner P.N., Lugade A.A., Thanavala Y., Lee J.J., Lau J.T. Altered eosinophil profile in mice with ST6Gal-1 deficiency: An additional role for ST6Gal-1 generated by the P1 promoter in regulating allergic inflammation. J. Leukoc. Biol. 2010;87:457–466. doi: 10.1189/jlb.1108704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nasirikenari M., Veillon L., Collins C.C., Azadi P., Lau J.T. Remodeling of Marrow Hematopoietic Stem and Progenitor Cells by Non-self ST6Gal-1 Sialyltransferase. J. Biol. Chem. 2014;289:7178–7189. doi: 10.1074/jbc.M113.508457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dall’Olio F. The sialyl-a2,6-lactosaminyl-structure: Biosynthesis and functional role. Glycoconj. J. 2000;17:669–676. doi: 10.1023/A:1011077000164. [DOI] [PubMed] [Google Scholar]
- 94.Dall’Olio F., Malagolini N., Di Stefano G., Minni F., Marrano D., Serafini-Cessi F. Increased CMP-NeuAc:Galb1,4GlcNAc-R a 2,6 sialyltransferase activity in human colorectal cancer tissues. Int. J. Cancer. 1989;44:434–439. doi: 10.1002/ijc.2910440309. [DOI] [PubMed] [Google Scholar]
- 95.Schultz M.J., Holdbrooks A.T., Chakraborty A., Grizzle W.E., Landen C.N., Buchsbaum D.J., Conner M.G., Arend R.C., Yoon K.J., Klug C.A., et al. The Tumor-Associated Glycosyltransferase ST6Gal-I Regulates Stem Cell Transcription Factors and Confers a Cancer Stem Cell Phenotype. Cancer Res. 2016;76:3978–3988. doi: 10.1158/0008-5472.CAN-15-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhuo Y., Chammas R., Bellis S.L. Sialylation of b1 integrins blocks cell adhesion to galectin-3 and protects cells against galectin-3-induced apoptosis. J. Biol. Chem. 2008;283:22177–22185. doi: 10.1074/jbc.M800015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Antony P., Rose M., Heidenreich A., Knuchel R., Gaisa N.T., Dahl E. Epigenetic inactivation of ST6GAL1 in human bladder cancer. BMC Cancer. 2014;14:901. doi: 10.1186/1471-2407-14-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fleischer T., Edvardsen H., Solvang H.K., Daviaud C., Naume B., Borresen-Dale A.L., Kristensen V.N., Tost J. Integrated analysis of high-resolution DNA methylation profiles, gene expression, germline genotypes and clinical end points in breast cancer patients. Int. J. Cancer. 2014;134:2615–2625. doi: 10.1002/ijc.28606. [DOI] [PubMed] [Google Scholar]
- 99.Kroes R.A., Moskal J.R. The Role of DNA Methylation in ST6Gal1 Expression in Gliomas. Glycobiology. 2016;26:1271–1283. doi: 10.1093/glycob/cww058. [DOI] [PubMed] [Google Scholar]
- 100.Minami A., Shimono Y., Mizutani K., Nobutani K., Momose K., Azuma T., Takai Y. Reduction of the ST6 β-Galactosamide α-2,6-Sialyltransferase 1 (ST6GAL1)-catalyzed Sialylation of Nectin-like Molecule 2/Cell Adhesion Molecule 1 and Enhancement of ErbB2/ErbB3 Signaling by MicroRNA-199a. J. Biol. Chem. 2013;288:11845–11853. doi: 10.1074/jbc.M112.405993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma X., Dong W., Su Z., Zhao L., Miao Y., Li N., Zhou H., Jia L. Functional roles of sialylation in breast cancer progression through miR-26a/26b targeting ST8SIA4. Cell Death Dis. 2016;7:e2561. doi: 10.1038/cddis.2016.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun M., Zhao X., Liang L., Pan X., Lv H., Zhao Y. Sialyltransferase ST3GAL6 mediates the effect of microRNA-26a on cell growth, migration, and invasion in hepatocellular carcinoma through the protein kinase B/mammalian target of rapamycin pathway. Cancer Sci. 2017;108:267–276. doi: 10.1111/cas.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y., Luo S., Dong W., Song X., Zhou H., Zhao L., Jia L. Α-2, 3-sialyltransferases regulate the multidrug resistance of chronic myeloid leukemia through miR-4701-5p targeting ST3GAL1. Lab. Investig. 2016;96:731–740. doi: 10.1038/labinvest.2016.50. [DOI] [PubMed] [Google Scholar]
- 104.Miao X., Jia L., Zhou H., Song X., Zhou M., Xu J., Zhao L., Feng X., Zhao Y. miR-4299 mediates the invasive properties and tumorigenicity of human follicular thyroid carcinoma by targeting ST6GALNAC4. IUBMB Life. 2016;68:136–144. doi: 10.1002/iub.1467. [DOI] [PubMed] [Google Scholar]
- 105.Denomme G.A. Molecular basis of blood group expression. Transfus. Apher. Sci. 2011;44:53–63. doi: 10.1016/j.transci.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 106.Dotz V., Wuhrer M. Histo-blood group glycans in the context of personalized medicine. Biochim. Biophys. Acta. 2016;1860:1596–1607. doi: 10.1016/j.bbagen.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chihara Y., Sugano K., Kobayashi A., Kanai Y., Yamamoto H., Nakazono M., Fujimoto H., Kakizoe T., Fujimoto K., Hirohashi S., et al. Loss of blood group A antigen expression in bladder cancer caused by allelic loss and/or methylation of the ABO gene. Lab. Investig. 2005;85:895–907. doi: 10.1038/labinvest.3700268. [DOI] [PubMed] [Google Scholar]
- 108.Gao S., Worm J., Guldberg P., Eiberg H., Krogdahl A., Liu C.J., Reibel J., Dabelsteen E. Genetic and epigenetic alterations of the blood group ABO gene in oral squamous cell carcinoma. Int. J. Cancer. 2004;109:230–237. doi: 10.1002/ijc.11592. [DOI] [PubMed] [Google Scholar]
- 109.Dabelsteen E., Gao S. ABO blood-group antigens in oral cancer. J. Dent. Res. 2005;84:21–28. doi: 10.1177/154405910508400103. [DOI] [PubMed] [Google Scholar]
- 110.Wu C.S., Yen C.J., Chou R.H., Chen J.N., Huang W.C., Wu C.Y., Yu Y.L. Downregulation of microRNA-15b by hepatitis B virus X enhances hepatocellular carcinoma proliferation via fucosyltransferase 2-induced Globo H expression. Int. J. Cancer. 2014;134:1638–1647. doi: 10.1002/ijc.28501. [DOI] [PubMed] [Google Scholar]
- 111.Bui C., Ouzzine M., Talhaoui I., Sharp S., Prydz K., Coughtrie M.W., Fournel-Gigleux S. Epigenetics: Methylation-associated repression of heparan sulfate 3-O-sulfotransferase gene expression contributes to the invasive phenotype of H-EMC-SS chondrosarcoma cells. FASEB J. 2010;24:436–450. doi: 10.1096/fj.09-136291. [DOI] [PubMed] [Google Scholar]
- 112.Karibe T., Fukui H., Sekikawa A., Shiratori K., Fujimori T. EXTL3 promoter methylation down-regulates EXTL3 and heparan sulphate expression in mucinous colorectal cancers. J. Pathol. 2008;216:32–42. doi: 10.1002/path.2377. [DOI] [PubMed] [Google Scholar]
- 113.Liu F.T., Rabinovich G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 114.Satelli A., Rao U.S. Galectin-1 is silenced by promoter hypermethylation and its re-expression induces apoptosis in human colorectal cancer cells. Cancer Lett. 2011;301:38–46. doi: 10.1016/j.canlet.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.You Y., Tan J.X., Dai H.S., Chen H.W., Xu X.J., Yang A.G., Zhang Y.J., Bai L.H., Bie P. MiRNA-22 inhibits oncogene galectin-1 in hepatocellular carcinoma. Oncotarget. 2016;7:57099–57116. doi: 10.18632/oncotarget.10981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ahmed H., Banerjee P.P., Vasta G.R. Differential expression of galectins in normal, benign and malignant prostate epithelial cells: Silencing of galectin-3 expression in prostate cancer by its promoter methylation. Biochem. Biophys. Res. Commun. 2007;358:241–246. doi: 10.1016/j.bbrc.2007.04.114. [DOI] [PubMed] [Google Scholar]
- 117.Ahmed H., Cappello F., Rodolico V., Vasta G.R. Evidence of heavy methylation in the galectin 3 promoter in early stages of prostate adenocarcinoma: Development and validation of a methylated marker for early diagnosis of prostate cancer. Transl. Oncol. 2009;2:146–156. doi: 10.1593/tlo.09118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ben Mahmoud L.K., Arfaoui A., Khiari M., Chaar I., el Amine O., Ben Hmida A.M., Gharbi L., Mzabi S.R., Bouraoui S. Loss of Galectin-3 Expression in Mucinous Colorectal Carcinomas is Associated With 5′CpG Island Methylation in Tunisian Patients. Appl. Immunohistochem. Mol. Morphol. 2011;19:258–265. doi: 10.1097/PAI.0b013e3181f869bb. [DOI] [PubMed] [Google Scholar]
- 119.Ruebel K.H., Jin L., Qian X., Scheithauer B.W., Kovacs K., Nakamura N., Zhang H., Raz A., Lloyd R.V. Effects of DNA methylation on galectin-3 expression in pituitary tumors. Cancer Res. 2005;65:1136–1140. doi: 10.1158/0008-5472.CAN-04-3578. [DOI] [PubMed] [Google Scholar]
- 120.Keller S., Angrisano T., Florio E., Pero R., Decaussin-Petrucci M., Troncone G., Capasso M., Lembo F., Fusco A., Chiariotti L. DNA methylation state of the galectin-3 gene represents a potential new marker of thyroid malignancy. Oncol. Lett. 2013;6:86–90. doi: 10.3892/ol.2013.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu W., Wang J., Yang G., Yu N., Huang Z., Xu H., Li J., Qiu J., Zeng X., Chen S., et al. Posttranscriptional regulation of Galectin-3 by miR-128 contributes to colorectal cancer progression. Oncotarget. 2017;8:15242. doi: 10.18632/oncotarget.14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ramasamy S., Duraisamy S., Barbashov S., Kawano T., Kharbanda S., Kufe D. The MUC1 and galectin-3 oncoproteins function in a microRNA-dependent regulatory loop. Mol. Cell. 2007;27:992–1004. doi: 10.1016/j.molcel.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Demers M., Couillard J., Giglia-Mari G., Magnaldo T., St Pierre Y. Increased galectin-7 gene expression in lymphoma cells is under the control of DNA methylation. Biochem. Biophys. Res. Commun. 2009;387:425–429. doi: 10.1016/j.bbrc.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 124.Kim S.J., Hwang J.A., Ro J.Y., Lee Y.S., Chun K.H. Galectin-7 is epigenetically-regulated tumor suppressor in gastric cancer. Oncotarget. 2013;4:1461–1471. doi: 10.18632/oncotarget.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tadokoro T., Morishita A., Fujihara S., Iwama H., Niki T., Fujita K., Akashi E., Mimura S., Oura K., Sakamoto T., et al. Galectin-9: An anticancer molecule for gallbladder carcinoma. Int. J. Oncol. 2016;48:1165–1174. doi: 10.3892/ijo.2016.3347. [DOI] [PubMed] [Google Scholar]
- 126.Yang Q., Jiang W., Zhuang C., Geng Z., Hou C., Huang D., Hu L., Wang X. microRNA-22 downregulation of galectin-9 influences lymphocyte apoptosis and tumor cell proliferation in liver cancer. Oncol. Rep. 2015;34:1771–1778. doi: 10.3892/or.2015.4167. [DOI] [PubMed] [Google Scholar]
- 127.Love D.C., Krause M.W., Hanover J.A. O-GlcNAc cycling: Emerging roles in development and epigenetics. Semin. Cell Dev. Biol. 2010;21:646–654. doi: 10.1016/j.semcdb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sakabe K., Wang Z., Hart G.W. Beta-N-acetylglucosamine (O-GlcNAc) is part of the histone code. Proc. Natl. Acad. Sci. USA. 2010;107:19915–19920. doi: 10.1073/pnas.1009023107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guo H., Zhang B., Nairn A.V., Nagy T., Moremen K.W., Buckhaults P., Pierce M. O-Linked N-Acetylglucosamine (O-GlcNAc) Expression Levels Epigenetically Regulate Colon Cancer Tumorigenesis by Affecting the Cancer Stem Cell Compartment via Modulating Expression of Transcriptional Factor MYBL1. J. Biol. Chem. 2017;292:4123–4137. doi: 10.1074/jbc.M116.763201. [DOI] [PMC free article] [PubMed] [Google Scholar]