Abstract
Ocotea species present economic importance and biological activities attributed to their essential oils (EOs) and extracts. For this reason, various strategies have been developed for their conservation. The chemical compositions of the essential oils and matK DNA sequences of O. caudata, O. cujumary, and O. caniculata were subjected to comparison with data from O. floribunda, O. veraguensis, and O. whitei, previously reported. The multivariate analysis of chemical composition classified the EOs into two main clusters. Group I was characterized by the presence of α-pinene (9.8–22.5%) and β-pinene (9.7–21.3%) and it includes O. caudata, O. whitei, and O. floribunda. In group II, the oils of O. cujumary and O. caniculata showed high similarity due amounts of β-caryophyllene (22.2% and 18.9%, respectively). The EO of O. veraguensis, rich in p-cymene (19.8%), showed minor similarity among all samples. The oils displayed promising antimicrobial and cytotoxic activities against Escherichia coli (minimum inhibitory concentration (MIC) < 19.5 µg·mL−1) and MCF-7 cells (median inhibitory concentration (IC50) ≅ 65.0 µg·mL−1), respectively. The analysis of matK gene displayed a good correlation with the main class of chemical compounds present in the EOs. However, the matK gene data did not show correlation with specific compounds.
Keywords: Lauraceae, volatile compounds, terpenes, matK gene, phylogenetic analysis
1. Introduction
The genus Ocotea is not a monophyletic group of the Lauraceae, which includes about 400 species occurring mostly in tropical and subtropical regions (Central and South America, the West Indies, and Africa) [1,2]. These species present alternate penninerved leaves; inflorescenses thyrsopaniculate to botryoid. Flowers are trimerous, bisexual, polygamous, or unisexual; tepals equal, rarely persistent on the rim; nine fertile stamens, the third whorl with glands; anthers with four loculos; receptacle very small and deeply tubular; in male flowers, rudimentary ovary to absent; fruit and cupule extremely variable in size and shape [1]. It is a very variable genus morphologically, being the largest genus in the Neotropics, with 170 species occurring in Brazil [3,4].
The economic importance of Ocotea species in the Amazon region has been related to numerous applications such as the use of their wood in lightweight construction and luxury furniture [5]. Phytochemical studies reported the occurrence of benzylisoquinoline alkaloids, neolignans, catechins from leaves and bark of O. porosa [6,7]; aporphine alkaloids from leaves of O. macrophylla; flavonols from O. vellosiana; and eudesmane sesquiterpenoids from O. corymbosa [8,9,10]. The volatile chemical profiles of Ocotea species are characterized by high concentrations of phenylpropanoids and terpenoids (hydrocarbons or oxygenated) [11,12].
Many studies have been reported on the biological activities of Ocotea metabolites: the alkaloid reticuline isolated from extract of O. duckei showed potent central nervous system depressant action [13]. (−)-Caaverine, a noraporphine alkaloid isolated from O. lancifolia, has shown high antiprotozoal activity against Leishmania and Trypanosoma cruzi parasites [14]. The chloroform fraction obtained from an extract of fruits of O. puberula and the alkaloid dicentrine displayed antinociceptive effects [15]. The butanolides isolated from roots of O. macrocarpa showed good cytotoxic activities against the A2780 ovarian cell line [16]. The flavonoids of O. notata showed antimycobacterial activity and ability to inhibit NO production by macrophages [17]. The essential oil of O. quixos, rich in trans-cinnamaldehyde and methyl cinnamate, showed anti-inflammatory properties in vitro and in vivo models [18]. Studies on the chemical characteristics of the species O. caudata, O. cujumary, and O. caniculata are rare and important because Ocotea species are classified as threatened to extinction by the Brazilian List and the risk of extinction is increased due the reduction of genetic variability [19,20] so knowledge of the genetic diversity is necessary for our understanding of the factors that determine essential oil quantity and quality in these economically important species [21].
2. Results and Discussion
2.1. Essential Oil Chemical Composition
The essential oil (EO) of Ocotea species provided different yields and the higher yields were found in the leaf EO for all samples (0.7–0.8%) (Table 1).
Table 1.
Collection data and essential oil yield of the samples of Ocotea occurring in Caxiuanã National Forest, Amazon, Brazil.
| Species | Geographic Coordinate | Voucher | Plant Material | Sample | Oil Yield (%) |
|---|---|---|---|---|---|
| O. caudata | S 01.0° 44.0′ 18.8′′ | MG 216263 | Leaves | Cau-L | 0.7 |
| W 51.0° 27.0′ 27.4′′ | Branches | Cau-B | 0.1 | ||
| O. cujumary | S 01.0° 44.0′ 14.1′′ | MG 216269 | Leaves | Cuj-L | 0.8 |
| W 51.0° 27.0′ 20.4′′ | Branches | Cuj-B | 0.5 | ||
| O. caniculata | S 01.0° 44.0′ 14.1′′ | MG 216262 | Leaves | Can-L | 0.7 |
| W 51.0° 27.0′ 20.4′′ | Branches | Can-B | 0.2 |
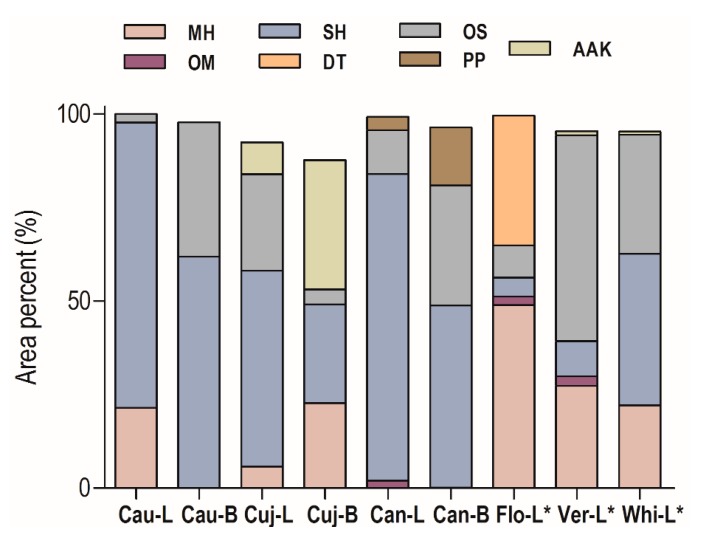
The most representative compounds class was sesquiterpene hydrocarbons (56.3–82.0%) in all samples from Caxianuã Forest (Figure 1). The O. caudata oils showed different chemical profiles in the tissues; in the EO from leaves (Cau-L) the monoterpene hydrocarbons (21.5%) were present, while in the branches (Cau-B) there was a high accumulation of oxygenated sesquiterpenoids (35.9%). The EO of O. cujumary leaves (Cuj-L) showed higher concentrations of oxygenated sesquiterpenoids (25.8%) than branches (Cuj-B, 4.0%), which showed significant amounts of monoterpene hydrocarbons (22.7%) and fatty acid derivatives (34.6%). The O. caniculata oils showed amounts of phenylpropanoids, especially in the branches (Can-B, 15.5%). Seventy volatile components were identified, comprising approximately 95.1% of the total composition of the oils (Table 2).
Figure 1.
Distribution of compound classes in essential oils (Eos) of Ocotea species. Monoterpene hydrocarbons (MH), Oxygenated monoterpenoids (OM), Sesquiterpene hydrocarbons (SH), Oxygenated sesquiterpenoids (OS), Diterpenes (DT), Phenylpropanoids (PP), Alkanes, aldehydes, and ketones (AAK). O. floribunda leaves (Flo-L), O. veraguensis leaves (Ver-L), O. whitei leaves (Whi-L); * Literature data [22]. Cau-L (O. caudata leaves); Cau-B (O. caudata bark); Cuj-L (O. cujimary leaves); Cuj-B (O. cujimary bark); Can-L (O. caniculata leaves); Can-B (O. caniculata bark).
Table 2.
Chemical composition of essential oils of Ocotea species. RICalc, calculated retention index; RILit, literature retention index.
| Constituents | RICalc | RILit | Cau-L | Cau-B | Cuj-L | Cuj-B | Can-L | Can-B | Flo-L * | Ver-L * | Whi-L * |
|---|---|---|---|---|---|---|---|---|---|---|---|
| E-2-Hexenal | 856 | 854 | 1.1 | 0.8 | |||||||
| α-Thujene | 933 | 931 | 0.2 | ||||||||
| α-Pinene | 936 | 932 | 9.8 | 2.1 | 22.5 | 0.7 | 12.7 | ||||
| Camphene | 956 | 953 | 1.7 | 0.1 | 0.3 | ||||||
| Sabinene | 979 | 976 | 0.1 | ||||||||
| β-Pinene | 980 | 974 | 9.7 | 1.8 | 2.2 | 21.3 | 0.3 | 7.3 | |||
| Myrcene | 995 | 991 | 0.7 | 1.1 | 0.5 | ||||||
| α-Phellandrene | 1009 | 1005 | 1 | ||||||||
| p-Cymene | 1028 | 1026 | 19.8 | ||||||||
| Limonene | 1030 | 1024 | 2.1 | 1.8 | 20.5 | 2.7 | 1.1 | ||||
| β-Phellandrene | 1032 | 1031 | 4.0 | ||||||||
| 1,8-Cineole | 1033 | 1033 | 1.3 | ||||||||
| γ-Terpinene | 1064 | 1062 | 0.1 | ||||||||
| α-Terpinolene | 1089 | 1088 | 0.1 | ||||||||
| α-Pinene oxide | 1097 | 1095 | 0.1 | ||||||||
| Linalool | 1104 | 1098 | 1.7 | ||||||||
| Borneol | 1168 | 1165 | 0.1 | ||||||||
| Terpinen-4-ol | 1178 | 1177 | 2.0 | 0.2 | |||||||
| α-Terpineol | 1191 | 1189 | 0.2 | 1.3 | |||||||
| Cuminal | 1238 | 1239 | 0.1 | ||||||||
| 2-Undecanol | 1301 | 1301 | 1.2 | 4.6 | |||||||
| δ-Elemene | 1340 | 1335 | 2.2 | ||||||||
| α-Cubebene | 1351 | 1345 | 2.0 | 2.0 | 0.8 | 2.2 | |||||
| α-Ylangene | 1373 | 1373 | 0.8 | 5.1 | |||||||
| α-Copaene | 1377 | 1374 | 1.0 | 0.1 | 0.1 | 0.8 | |||||
| β-Cubebene | 1392 | 1387 | 1.6 | 0.1 | |||||||
| β-Bourbonene | 1384 | 1387 | 0.7 | 0.1 | 0.1 | ||||||
| δ-Elemene | 1392 | 1389 | 1.5 | 0.4 | 1.1 | 0.8 | 0.3 | 0.7 | 0.3 | ||
| Z-Caryophyllene | 1416 | 1408 | 0.3 | ||||||||
| β-Caryophyllene | 1421 | 1417 | 9.6 | 2.5 | 22.2 | 8.1 | 18.9 | 7.1 | 2.5 | 2.3 | 15.2 |
| 2,5-Dimethoxy-p-cymene | 1424 | 1424 | 0.9 | ||||||||
| β-Copaene | 1428 | 1430 | 0.4 | ||||||||
| β-Gurjunene | 1432 | 1432 | 0.2 | ||||||||
| α-trans-Bergamotene | 1436 | 1432 | 0.9 | 1.9 | |||||||
| γ-Elemene | 1435 | 1434 | 0.8 | 0.1 | |||||||
| α-Guaiene | 1439 | 1439 | 0.1 | ||||||||
| Aromadendrene | 1440 | 1439 | 0.1 | ||||||||
| Z-β-Farnesene | 1443 | 1440 | 0.9 | ||||||||
| Spirolepechinene | 1451 | 1449 | 0.7 | 1.4 | 0.6 | ||||||
| α-Humulene | 1455 | 1452 | 1.8 | 2.4 | 3.8 | 2.5 | 2.5 | 1.7 | 0.3 | 1.7 | 1.7 |
| Sesquisabinene | 1458 | 1457 | 0.9 | ||||||||
| Dehydroaromadendrane | 1460 | 1460 | 0.6 | ||||||||
| E-β-Farnesene | 1462 | 1458 | 0.9 | ||||||||
| allo-Aromadendrene | 1463 | 1461 | 0.2 | ||||||||
| trans-Cadina-1(6),4-diene | 1473 | 1475 | 0.5 | ||||||||
| γ-Selinene | 1477 | 1470 | 0.2 | ||||||||
| γ-Gurjunene | 1477 | 1475 | 0.4 | ||||||||
| γ-Muurolene | 1476 | 1478 | 0.8 | 0.7 | |||||||
| Widdra-2,4(14)-diene | 1483 | 1481 | 6.5 | ||||||||
| Germacrene D | 1484 | 1484 | 19.9 | 8.9 | 0.9 | 5.9 | 1.2 | 0.2 | 5.5 | ||
| β-Selinene | 1485 | 1489 | 2.2 | 20.3 | 12.1 | 0.3 | |||||
| Valencene | 1493 | 1491 | 1.1 | ||||||||
| cis-β-Guaiene | 1493 | 1492 | 8.3 | 3.0 | 5.2 | ||||||
| trans-Muurola-4(14),5-diene | 1491 | 1493 | 1.1 | ||||||||
| 2-Tridecanone | 1497 | 1495 | 7.3 | 30.0 | |||||||
| Viridiflorene | 1493 | 1496 | 9.8 | ||||||||
| Bicyclogermacrene | 1500 | 1500 | 29.6 | 10.4 | 5.3 | ||||||
| α-Muurolene | 1499 | 1500 | 1.7 | 1.0 | 0.1 | ||||||
| Germacrene A | 1504 | 1503 | 0.2 | 2 | |||||||
| β-Bisabolene | 1509 | 1505 | 0.6 | ||||||||
| δ-Amorphene | 1509 | 1511 | 0.8 | 2.0 | |||||||
| E,E-α-Farnesene | 1510 | 1508 | 0.3 | ||||||||
| γ-Cadinene | 1517 | 1513 | 0.9 | 5.9 | 1.6 | 3.1 | 1.7 | 0.1 | 0.4 | 0.7 | |
| 7-epi-α-Selinene | 1517 | 1520 | 4.5 | 14.8 | 9.0 | ||||||
| β-Cadinene | 1519 | 1518 | 0.7 | 0.6 | |||||||
| cis-Calamenene | 1523 | 1521 | 0.6 | ||||||||
| δ-Cadinene | 1526 | 1522 | 1.4 | 13.8 | 6.6 | 4.7 | 0.6 | 3.9 | 0.2 | 0.4 | 3.7 |
| trans-Cadina-1,4-diene | 1532 | 1533 | 0.6 | 0.2 | 0.2 | ||||||
| α-Cadinene | 1537 | 1538 | 0.1 | ||||||||
| α-Calacorene | 1542 | 1544 | 0.9 | 1.5 | 0.2 | ||||||
| Elemol | 1549 | 1549 | 0.1 | ||||||||
| Germacrene B | 1558 | 1559 | 1.6 | 3.3 | 0.8 | 0.4 | 0.7 | ||||
| E-Nerolidol | 1564 | 1561 | 1.4 | 2.5 | 3.9 | ||||||
| β-Calacorene | 1564 | 1564 | 0.7 | 0.5 | |||||||
| γ-Asarone | 1576 | 1572 | 0.4 | ||||||||
| Spathulenol | 1579 | 1577 | 1.4 | 0.5 | 1.0 | 6 | 8.5 | 15.3 | |||
| Caryophyllene oxide | 1585 | 1582 | 1.0 | 2.0 | 12.4 | 0.9 | 2.0 | ||||
| Globulol | 1592 | 1590 | 0.9 | ||||||||
| Viridiflorol | 1597 | 1592 | 1.2 | ||||||||
| Carotol | 1586 | 1594 | 2.7 | ||||||||
| 6-Methoxyelemicin | 1601 | 1595 | 6.3 | ||||||||
| Guaiol | 1597 | 1600 | 1.2 | 4.0 | 5.2 | ||||||
| Humulene epoxide II | 1608 | 1608 | 1.0 | 1.7 | |||||||
| Z-Asarone | 1625 | 1616 | 3.4 | ||||||||
| 1,10-di-epi-Cubenol | 1620 | 1618 | 3.0 | 1.4 | |||||||
| Junenol | 1620 | 1618 | 0.6 | ||||||||
| 1-epi-Cubenol | 1628 | 1627 | 3.8 | 0.4 | 3.3 | ||||||
| Muurola-4,10(14)-dien-1β-ol | 1628 | 1630 | 3.3 | ||||||||
| epi-α-Cadinol | 1642 | 1638 | 2.0 | 0.5 | |||||||
| allo-Aromadendrene epoxide | 1632 | 1639 | 1.4 | ||||||||
| Caryophylla-4(12),8(13)-dien-5β-ol | 1636 | 1639 | 3.2 | ||||||||
| α-Muurolol | 1642 | 1644 | 7.8 | 0.9 | 1.8 | ||||||
| Cubenol | 1646 | 1645 | 2.2 | 1.5 | |||||||
| α-Cadinol | 1652 | 1652 | 2.2 | 1.8 | 1.5 | 1.8 | |||||
| Valerianol | 1654 | 1656 | 6.8 | ||||||||
| Selin-11-en-4α-ol | 1657 | 1658 | 3.1 | 20.6 | |||||||
| 7-epi-α-Eudesmol | 1658 | 1662 | 4.2 | ||||||||
| Bulnesol | 1662 | 1666 | 29.5 | ||||||||
| 14-Hydroxy-(Z)-caryophyllene | 1666 | 1666 | 2.3 | 1.5 | 0.9 | 2.1 | |||||
| E-Asarone | 1683 | 1675 | 3.6 | 8.8 | |||||||
| β-Sinensal | 1699 | 1699 | 0.6 | ||||||||
| E,E-Farnesylacetate | 1843 | 1843 | 10.1 | ||||||||
| Isohibaene | 1922 | 1923 | 0.7 | ||||||||
| Kaurene | 2034 | 2034 | 34.0 |
RICalc = based on DB-5ms capillary column and alkane standards (C8-C32). RILit = based on Adams library. * Literature data (Takaku et al., 2007 [22]).
The main compounds identified in O. caudata oils were bicyclogermacrene (29.6%), germacrene D (19.9%), β-caryophyllene (9.6%), α-pinene (9.8%), and β-pinene (9.7%) in the leaves (Cau-L) and δ-cadinene (13.8%), germacrene D (8.9%), and α-muurulol (7.8%) in the branches (Cau-B). β-Caryophyllene, germacrene-D, α-pinene, and β-pinene were the principal common constituents of the EOs of leaves of Ocotea floribunda, O. holdridgeana, O. meziana, O. sinuata, O. tonduzii, O. valeriana, O. veraguensis, O. whitei, and two new undescribed Ocotea species [22].
In addition, these chemical compounds were identified in the leaves in other Lauraceae species. The EO of leaves of Endlicheria arenosa was dominated by bicyclogermacrene (42.2%), germacrene D (12.5%), and β-caryophyllene (10.1%) [23]. Similarly, the EO of Nectandra leucantha showed as the main compounds bicyclogermacrene (28.4%), germacrene A (7.3%), α-pinene (6.6%), and β-pinene (4.6%) [24]. The sesquiterpene δ-cadinene identified in the EO of branches was detected as the main compound in EOs of leaf and bark of Beilschmiedia madang (17.0% and 20.5%, respectively) and was associated with germacrene D in the oil of barks of Beilschmiedia glabra [25,26].
The EO of O. cujumary leaves (Cuj-L) was rich in β-caryophyllene (22.2%) and caryophyllene oxide (12.4%), followed by 2-tridecanone (7.3%) and δ-cadinene (6.6%). However, the EO of the branches (Cuj-B) was dominated by 2-tridecanone (30.0%), limonene (20.5%), and β-caryophyllene (8.1%). The occurrence of fatty acid derivatives such as 2-tridecanone in Lauraceae species is not very common. However, β-caryophyllene, caryophyllene oxide, and δ-cadinene were the main compounds of the EO oil from leaves of Cryptocarya mandioccana [27].
The oils of O. caniculata showed predominance of sesquiterpenes of selinane and caryophyllane skeletons. In the leaves (Can-L), the main compounds were β-selinene (20.3%), β-caryophyllene (18.9%), 7-epi-α-selinene (14.3%), and bicyclogermacrene (10.4%); in the branches (Can-B), selin-11-en-4-α-ol (20.6%), β-selinene (12.1%), 7-epi-α-selinene (9.0%), β-caryophyllene (7.1%), and the phenylpropanoid (E)-asarone (8.8%) were identified. Interestingly, the leaf essential oil of O. foetens, an endemic species of the Macronesian region (Canary Islands, Spain, and Madeira Islands, Portugal), had very little concentration of monoterpenoids or sesquiterpenoids, but was rich in ethyl p-coumarate [28].
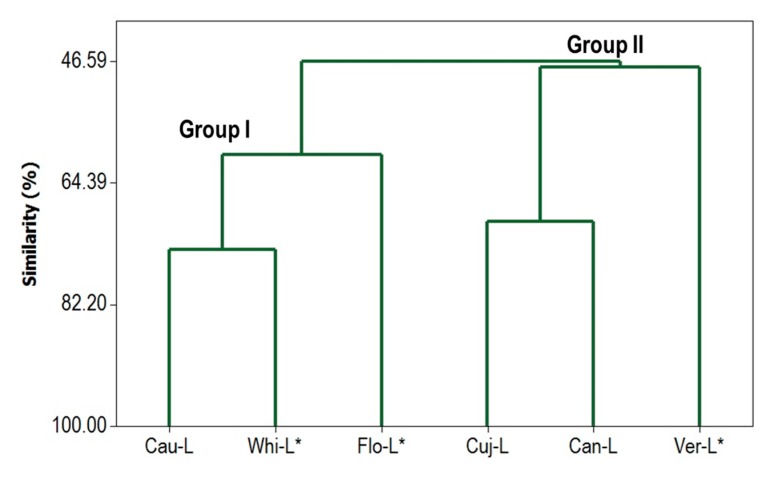
In order to differentiate between the analyzed Ocotea samples, a hierarchical cluster analysis using the chemical constituents was carried out and the resulting dendrogram is shown in Figure 2. According to these results, the samples were grouped in two main groups with similarity of 46.6%. Group I is characterized by oils from O. caudata, O. whitei, and O. floribunda, which present high amounts of α-pinene (9.8–22.5%) and β-pinene (7.3–21.3%). A higher similarity (74.1%) was found with O. caudata and O. whitei due the proportions of α-pinene, β-pinene, and β-caryophyllene (see Table 2). The main compound of O. floribunda oil was the diterpene kaurene (34.0%) and it decreased its similarity level. Group II includes O. cujumary, O. caniculata, and O. veraguensis oils, the samples Cuj-L and Can-L present similar amounts of β-caryophyllene (22.2% and 18.9%, respectively). A lower similarity was found in sample Ver-L, which was rich in bulnesol (29.0%) and p-cymene (19.8%).
Figure 2.
Dendrogram with Complete Linkage and Correlation Coefficient Distance. * Literature data (Takaku et al., 2007 [21]).
2.2. Antimicrobial and Cytotoxic Activities
All essential oils displayed high antimicrobial activity against E. coli (minimum inhibitory concentration (MIC) < 19.5 μg·mL−1). Cau-L and Cuj-L oils showed notable activity against S. epidermis and B. cereus (Table 3). It is not obvious what component(s) are responsible for the activity against E. coli. Most essential oil components show only marginal activity against this organism. The antibacterial activities against the other bacteria are consistent with the activities of the essential oil components (Table 3). On the other hand, the cytotoxic activity against MCF-7 cells did not display variation in the median inhibitory concentration (IC50) values among all samples with an average of 63.0 μg·mL−1 (Table 3). The main compounds germacrene D, bicyclogermacrene, β-caryophyllene, α-pinene, β-pinene, caryophyllene oxide, β-selinene, 7-epi-α-selinene have been reported as antimicrobial and cytotoxic [29,30,31,32,33]. The observed cytotoxicities of some of the essential oil components are consistent with the cytotoxicities of the essential oils themselves (Table 3).
Table 3.
Antimicrobial and cytotoxic activities of Ocotea essential oils and some major essential oil components. MIC, minimum inhibitory concentration; IC50, median inhibitory concentration.
| Material | MIC (μg·mL−1) | IC50 (μg·mL−1) | ||||
|---|---|---|---|---|---|---|
| P. aer | E. coli | S. epi | S. aur | B. cer | MCF-7 | |
| Can-L | 1250.0 | 19.5 | 625.0 | 625.0 | 625.0 | 63.9 ± 3.7 |
| Cau-L | 1250.0 | 19.5 | 625.0 | 625.0 | 312.5 | 64.0 ± 3.7 |
| Cuj-L | 1250.0 | 19.5 | 312.5 | 625.0 | 312.5 | 67.7 ± 3.7 |
| α-Pinene | 625 | 312 | - | 312 | 625 | 69.5 ± 1.3 |
| β-Pinene | 1250 | 625 | - | 625 | 312 | 71.2 ± 2.0 |
| Limonene | 1250 | 625 | - | 312 | 625 | 77.4 ± 1.2 |
| β-Caryophyllene | 1250 | 312 | - | 312 | 156 | 59.4 ± 5.1 |
| α-Humulene | 1250 | 625 | - | 312 | 312 | 26.5 ± 5.6 |
| Germacrene D | 1250 | 625 | - | 156 | 625 | 69.6 ± 2.5 |
| Caryophyllene oxide | 1250 | 1250 | - | 1250 | 156 | 73.4 ± 3.7 |
| Gentamicin control | 1.22 | 2.44 | <19.5 | 0.61 | 1.22 | - |
| Tingenone control | <19.5 | <19.5 | - | 2.44 | 1.22 | 16.8 ± 1.7 |
P. aer (Pseudomonas aeruginosa), E. coli (Escherichia coli), S. epi (Staphylococcus epidermidis), S. aur (Staphylococcus aureus), B. cer (Bacillus cereus).
The EOs of leaves of Endlicheria arenosa, which are rich in bicyclogermacrene (42.2%), germacrene D (12.5%), and β-caryophyllene (10.1%), showed the same IC50 value against Escherichia coli [23]. The EO from the leaves of Ruilopezia bracteosa (Asteraceae), with higher amounts of α-pinene (24.3%), 7-epi-α-selinene (9.1%), and β-pinene (8.5%), showed antibacterial activity against Gram-positive and Gram-negative bacteria [34].
Terpenoids from plant oils prevent tumor cell proliferation through necrosis or induction of apoptosis [35]. Germacrene D is reported as cytotoxic against cervical carcinoma (HeLa), leukemia (HL-60), colon carcinoma (HCT), breast adenocarcinoma (SKBr), and melanoma (A2058) [36]. β-Caryophyllene and caryophyllene oxide displayed activity against several tumor cells such as breast cancer (MCF-7), colon cancer (HCT-116), and human prostate (PC-3) [37,38]. The values of IC50 of bicyclogermacrene, α-pinene, and β-pinene against MCF-7 cells were 19.0, 30.7, and 80.2 µg/mL, respectively [24,39].
2.3. Phylogenetic Analysis
MatK from chloroplast genes has been used as a marker for the construction of plant phylogenies, due to its rapid evolution and their ubiquitous presence in plants [40]. The sequences have a size of about 1550 bp and encode the maturase K enzyme [41].
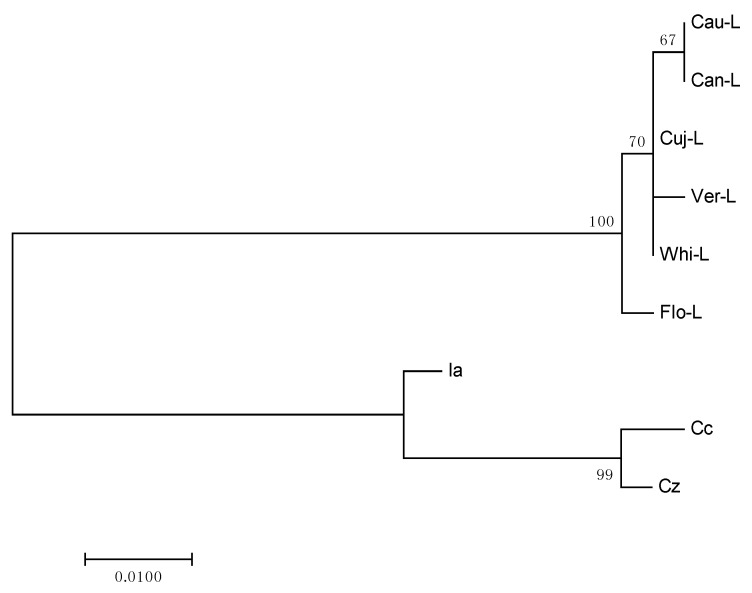
Lauraceae species used in this study formed a defined group in the phylogenetic analysis, and the results of the phylogenetic tree were robust and supported by bootstrap value (=100). Species of Calycanthaceae were used as outgroup; this corroborates with the taxonomic classification and supports the topology of the tree (Figure 3).
Figure 3.
Molecular Phylogenetic analysis by Maximum Likelihood method for matK sequence of species of Lauraceae and Calycanthaceae (Outgroup).
The genetic distances estimated for Lauraceae species were lower. The values ranged from 0 to 0.003 to Cau-L, Cuj-L, Can-L and from 0 to 0.009 to Ver-L, Flo-L and Whi-L.
The tree shows that the geographically closest species are grouped: Cau-L, Cuj-L, and Can-L were collected in Caxiuanã National Forest and Ver-L, Flo-L, and Whi-L were collected in Monteverde, Costa Rica. Although there may be differences in the composition of essential oils in plants of the same species with the same geographical location, the results of phylogenetic tree showed a good correlation with classes of chemical compounds. The species Cau-L and Can-L are rich in sesquiterpene hydrocarbons and the samples Cuj-L, Ver-L, and Whi-L showed similar amounts of monoterpene hydrocarbons. A higher difference could be observed for sample Flo-L, which had diterpenes as its main compound class. Our results support the hypothesis that matK gene is reasonably useful in phylogenetic reconstructions at high taxonomic levels (to order or family), but shows poorer reliability with lower taxonomic levels of classification (to genus or species) [42,43].
3. Materials and Methods
3.1. Plant Material
Leaves and branches of O. caudata (Nees) Mez, O. cujumary Mart., and O. caniculata (Rich.) Mez were collected in Caxiuanã National Forest, Marajó Island, and their vouchers were deposited in the Herbarium of Museum Paraense Emílio Goeldi, Belém, Pará state, Brazil (Table 1).
3.2. Essential Oil Extraction
Leaves and branches were air-dried, pulverized, and subjected to hydrodistillation using a Clevenger-type apparatus (100 g, 3 h). The essential oils were dried over anhydrous sodium sulfate, and the essential oil yields were calculated on the basis of the dry weight of plant material. The moisture contents of the samples were calculated after phase separation using a Dean–Stark trap (5 g, 60 min) using toluene as the solvent phase.
3.3. Gas Chromatographic–Mass Spectral Analysis
The volatile compositions were analyzed by gas chromatography using an Agilent 6890 GC with an HP-5 ms column, and mass spectrometry with an Agilent 5973 mass selective detector (MSD) operated in the electron impact (EI) mode with electron energy = 70 eV. The scan range was 40–400 atomic mass units (amu) and the scan rate was 3.99 scans/s. The data were processed with an Agilent ChemStation data system. The gas chromatography (GC) column was a fused silica capillary with a (5% phenyl)-polymethylsiloxane stationary phase that had a film thickness of 0.25 μm and a length of 30 m, and an internal diameter of 0.25 mm. Helium was the carrier gas with a flow rate of 1.0 mL/min and a column head pressure of 48.7 kPa. The injector temperature was 200 °C and the detector temperature was 280 °C. The GC oven temperature was programed to start with an initial temperature of 40 °C, which was held for 10 min. The temperature was then increased at a rate of 3 °C/min to 200 °C, and then increased at 2 °C/min to 220 °C. A 1-μL injection of a solution (0.2% w/v in CH2Cl2) of the sample was performed using a splitless injection technique. The percentages of each component were based on total ion current and are reported without standardization. Individual components were identified by comparison of both their mass spectra and GC retention data with authentic compounds present in commercial libraries [44] and our own in-house library.
3.4. Antibacterial Assay
The antimicrobial activity of EOs was determined against Bacillus cereus (ATCC No. 14579), Escherichia coli (ATCC No. 10798), Pseudomonas aeruginosa (ATCC No. 27853), Staphylococcus aureus (ATCC No. 29213), and Staphylococcus epidermidis (ATCC No. 12228), using the microbroth serial dilution method as previously reported [45]. Thus, 50 μL of 1% w/v solution of the samples in dimethylsulfoxide (DMSO) was placed in the top well of 96-well microtiter plates and 50 μL of cation-adjusted Mueller Hinton broth (CAMHB) was added. The sample solutions were then serially diluted (1:1) by transferring 50 μL of sample-CAMHB mixture to the next lane and adding 50 μL fresh CAMHB to obtain a concentration range from 2500 to 12.5 μg·mL−1. The bacteria were harvested from a fresh culture and added to each well at a concentration of approximately 1.5 × 108 colony forming units (CFU)·mL−1. The plates were incubated at 37 °C for 24 h and the final minimum inhibitory concentrations (MICs) were determined as the lowest concentrations free of turbidity. The antibiotic gentamicin was used as positive control and DMSO solvent was used as negative control.
3.5. Cytotoxicity Assay
MCF-7 human mammary adenocarcinoma cells (ATCC No. HTB-22) were cultured in RPMI (Roswell Park Memorial Institute) 1640 medium supplemented with 10% fetal bovine serum (FBS), 30 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer), NaHCO3, and penicillin streptomycin. The cytotoxicity of the essential oils on MCF-7 cells was determined using the 96-well MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay as reported previously [45]. Cells were seeded into 96-well cell culture plates at a concentration of 1.2 × 104 cells/well and a volume of 100 μL in each well. The plate was then labeled and incubated at 37 °C and 5% CO2 for 48 h. After 48 h, the cells reached 70–80% confluent growth. The supernatant fluid was gently aspirated (without touching the bottom of the well to avoid removing cells) and replaced with 100 μL growth medium that contained either 1.0 or 0.5 μL of essential oil (1% in DMSO), to give final concentrations of 100 and 50 μg·mL−1. The plate was then incubated at 37 °C and 5% CO2 for 48 h. After 48 h, the liquid was gently aspirated from each well. In a tube, 10 mL feeding medium was mixed with 2 mL of MTT stock solution (and was protected from light). Into each well, 100 µL of the MTT solution was added and the pre-read absorbance was immediately measured spectrophotometrically at 570 nm (using a Molecular Devices Spectra Max Plus 384 microplate reader). Formazan crystals were formed over the course of 4 h at 37 °C and 5% CO2. After 4 h, DMSO was used to dissolve the purple crystals. The amount of MTT-formazan produced was determined spectrophotometrically at 570 nm. Growing medium, DMSO, and tingenone (100 μg·mL−1) served as negative, compound, and positive controls, respectively. Solutions were added to wells in eight replicates. Average absorbances, standard deviations, and percent kill ratios (% killoil/% killcontrol) were calculated. Median inhibitory concentrations (IC50) were determined using the Reed-Muench method [46].
3.6. Multivariate Statistical Analysis of Chemical Composition
Hierarchical cluster analysis was carried out to organize and cluster the essential oils according to their main volatile constituents. Complete linkage and absolute correlation coefficient distance was chosen to determine similarity. Agglomerative hierarchical clustering was utilized for clustering the essential oils. The MINITAB 14.0 software was used to statistically analyze all data.
3.7. DNA Isolation, PCR Amplification, and Sequencing
Genomic DNA material was extracted from dried leaf tissue of each plant using E.Z.N.A® Poly-Gel DNA Extraction Kit (Omega Bio-tek, Norcross, GA, USA) according to the protocol given by the company. For amplification of matK region, PCR was performed in a 50 µL reaction mixture that consisted of 36.5 µL of nanofiltrated (NF) water, 5.0 µL of 10× Advantage Buffer, 1.0 µL of deoxynucleotide (DNTP), 0.5 µL of Taq-DNA polymerase, 5.0 µL template DNA, 1.0 µL of matK-Lau001 (F-5’-TCCTTTCTTGAGCGAACACA-3’), and 1.0 µL of matK-Lau002 (R-5’-CTGACAAATCGGACCGAAAC-3’) primers purchased from Eurofins MWG Operon (Huntsville, AL, USA). Amplifications conditions for matK primers consisted of an initial denaturation step for 2 min at 95 °C, followed by 40 cycles of 30 s at 95 °C, 30 s at 61 °C, 60 s at 68 °C, and 60 s at 72 °C for final extension. Amplifications were performed in an Eppendorf MasterCycler Pro thermocycler, the PCR products were visualized in agarose gel (1%), purified, and then they were sent to Eurofins MWG Operon (Huntsville, AL, USA) for sequencing.
3.8. Phylogenetic Analysis
The matK sequences of O. caudata (Nees) Mez, O. cujumary Mart., and O. caniculata (Rich.) were assembled and edited using FinchTV (biomatters Ltd., Auckland, New Zealand). Sequences were checked against those in GenBank using the BLAST algorithm. The matK sequences from O. caudata (Nees) Mez, O. cujumary Mart., and O. caniculata (Rich.) were aligned with sequences from O. floribunda (gbEU153866.1), O. veraguensis (gb.JQ589849.1), and O. whitei (gb. JQ589831.1) available in GenBank. The sequences were edited using BioEdit V7.2.5 [47] and the alignment was performed using the Clustal W [48]. The distance genetic and Phylogenetic Tree was determined using Mega 7 Program [49]. The evolutionary history was inferred using the maximum likelihood method and the evolutionary distances were computed using the Kimura 2-parameter model with 10,000 replicates. The outgroups used for the construct phylogenetic tree were the matK sequences from Calycanthus chinensis (Cc) (gb|AY525339.1), Idiospermum australiense (Ia) (gi71891392), and Chimonanthus zhejiangensis (Cz) (gb|AY525341.1) belonging to the order Laurales and Calycanthaceae family.
4. Conclusions
In this present study, the main compounds identified in the essential oils of species collected in Caxiuanã Forest were terpenoid hydrocarbons and oxygenated terpenoids, which had good antimicrobial and cytotoxic activities. The main class of chemical compounds displayed a good correlation with chloroplast DNA region (matK gene) analysis. However, the analysis of phylogenetic data and specific chemical compounds showed differences. These results suggest that matK gene was not efficient for representing this relationship.
Acknowledgments
The authors are grateful to CNPq-Programa “Ciências sem fronteiras” (Grant No. 233761/2014-4) for financial support.
Abbreviations
| matK | Megakaryocyte-Associated Tyrosine Kinase |
| MIC | Minimum Inhibitory Concentration |
| RICalc. | Calculated retention index |
| RILit | Literature retention index |
| IC50 | Median Inhibitory Concentration |
| GC | Gas chromatography |
| DMSO | Dimethylsulfoxide |
| RPMI | Roswell Park Memorial Institute medium |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| HEPES | 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid buffer |
| PCR | Polymerase Chain Reaction |
Author Contributions
Joyce Kelly da Silva, Leland J. Cseke, and William N. Setzer conceived and designed the experiments; Joyce Kelly da Silva, Rafaela da Trindade, Rebecca S. Miller, and Noura S. Dosoky performed the experiments; Joyce Kelly da Silva, Edith Cibelle Moreira, and William N. Setzer analyzed the data; José Guilherme Maia, Leland J. Cseke, and William N. Setzer contributed reagents/materials/analysis tools; Joyce Kelly da Silva, Rafaela da Trindade, Edith Cibelle Moreira, and William N. Setzer wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data and in the decision to publish the results.
References and Notes
- 1.Rohwer J.G. Lauraceae. In: Kubitzki K., Rohwer J.G., Bittrich V., editors. The Families and Genera of Vascular Plants. Volume 2. Springer; Berlin, Germany: 1993. pp. 366–391. [Google Scholar]
- 2.Quinet A., Baitello J.B., Moraes P.L.R., Alves F.M., Assis L. Lauraceae. Lista de Espécies da flora do Brasil. Jardim Botânico do Rio de Janeiro. [(accessed on 7 March 2017)]; Available online: http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB143.
- 3.Institute of Research Rio de Janeiro Botanical Garden Lauraceae. Flora do Brasil 2020 em Construção. Jardim Botânico do Rio de Janeiro. [(accessed on 7 March 2017)]; Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB143.
- 4.Van der Werff H. A synopsis of Ocotea (Lauraceae) in Central America and southern Mexico. Ann. Mo. Bot. Gard. 2002;89:429–451. doi: 10.2307/3298602. [DOI] [Google Scholar]
- 5.Marques C.A. Importância econômica da família Lauraceae Lindl. Florest. Ambient. 2001;8:195–206. [Google Scholar]
- 6.David J.M., Yoshida M., Gottlieb O.T. Phenylpropanoid-catechins from bark of Ocotea porosa. Phytochemistry. 1994;35:545–546. doi: 10.1016/S0031-9422(00)94800-3. [DOI] [Google Scholar]
- 7.David J.M., Yoshida M., Gottlieb O.R. Neolignans from bark and leaves of Ocotea porosa. Phytochemistry. 1994;36:491–499. doi: 10.1016/S0031-9422(00)97102-4. [DOI] [Google Scholar]
- 8.Barrera E.D.C., Suárez L.E.C. Aporphine Alkaloids from Leaves of Ocotea macrophylla (Kunth) (Lauraceae) from Colombia. Biochem. Syst. Ecol. 2009;37:522–524. doi: 10.1016/j.bse.2009.05.002. [DOI] [Google Scholar]
- 9.Garcez W.S., Yoshida M., Gottlieb O.R. Benzylisoquinoline alkaloids and flavonols from Ocotea vellosiana. Phytochemistry. 1995;39:815–816. doi: 10.1016/0031-9422(94)00961-R. [DOI] [Google Scholar]
- 10.Chavez J.P., Gottlieb O.R., Yoshida M. 10-Desmethyl-1-methyl-eudesmanes from Ocotea corymbosa. Phytochemistry. 1995;39:849–852. doi: 10.1016/0031-9422(94)00977-2. [DOI] [Google Scholar]
- 11.Ballabenia V., Tognolinia M., Bertonia S., Brunib R., Guerrinib A., Ruedac G.M., Barocellia E. Antiplatelet and antithrombotic activities of essential oil from wild Ocotea quixos (Lam.) Kosterm. (Lauraceae) calices from Amazonian Ecuador. Pharmacol. Res. 2007;55:23–30. doi: 10.1016/j.phrs.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Chaverri C., Díaz C., Cicció J.F. Chemical Analysis of Essential Oils from Ocotea gomezii W.C. Burger and Ocotea morae Gómez-Laur. (Lauraceae) Collected at “Reserva Biológica Alberto M. Brenes” in Costa Rica and their Cytotoxic Activity on Tumor Cell Lines. J. Braz. Chem. Soc. 2011;22:741–745. doi: 10.1590/S0103-50532011000400018. [DOI] [Google Scholar]
- 13.Morais L.C.S.L., Barbosa-Filho J.M., Almeida R.N. Central depressant effects of reticuline extracted from Ocotea duckei in rats and mice. J. Ethnopharmacol. 1998;62:57–61. doi: 10.1016/S0378-8741(98)00044-0. [DOI] [PubMed] [Google Scholar]
- 14.Fournet A., Ferreira M.E., Arias A.R., Guy I., Guinaudeau H., Heinzen H. Phytochemical and antiprotozoal activity of Ocotea lancifolia. Fitoterapia. 2007;78:382–384. doi: 10.1016/j.fitote.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Montrucchio D.P., Miguel O.G., Zanin S.M., da Silva G.A., Cardoso A.M., Santos A.R. Antinociceptive effects of a chloroform extract and the alkaloid dicentrine isolated from fruits of Ocotea puberula. Planta Med. 2012;78:1543–1548. doi: 10.1055/s-0032-1315026. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y., Cheng E., Rakotondraib L.H., Brodie P.J., Applequist W., Randrianaivo R., Rakotondrafara A., Ratsimbason M., Rasamison V.E., Kingston D.G.I. Antiproliferative compounds from Ocotea macrocarpa from the Madagascar dry forest. Tetrahedron Lett. 2015;56:3630–3632. doi: 10.1016/j.tetlet.2015.01.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa I.F., Calixto S.D., Heggdorne de Araujo M., Konno T.U., Tinoco L.W., Guimarães D.O., Lasunskaia E.B., Leal I.R., Muzitano M.F. Antimycobacterial and Nitric Oxide Production Inhibitory Activities of Ocotea notata from Brazilian Restinga. Sci. World J. 2015;2015:1–9. doi: 10.1155/2015/947248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballabeni V., Tognolini M., Giorgio C., Bertoni S., Bruni R., Barocelli E. Ocotea quixos Lam. essential oil: In vitro and in vivo investigation on its anti-inflammatory properties. Fitoterapia. 2010;81:289–295. doi: 10.1016/j.fitote.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 19.MMA (Ministério do Meio Ambiente). Instrução Normativa n°.6 de 23/09/2008. Reconhece as espécies da flora brasileira ameaçadas de extinção.
- 20.Martins E.M., Martinelli G., Arbetman M.P., Lamont R.W., Simões-Araújo J.L., Powell D., Ciampi-Guillardi M., Baldauf C., Quinet A., Galisa P., et al. Development and characterization of microsatellite loci for Ocotea species (Lauraceae) threatened with extinction. Genet. Mol. Res. 2014;13:5138–5142. doi: 10.4238/2014.July.7.6. [DOI] [PubMed] [Google Scholar]
- 21.Rahali F.Z., Lamine M., Gargouri M., Rebey I.B., Hammami M., Sellami I.H. Metabolite profiles of essential oils and molecular markers analysis to explore the biodiversity of Ferula communis: Towards conservation of the endemic giant fennel. Phytochemistry. 2016;124:58–67. doi: 10.1016/j.phytochem.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Takaku S., Haber W.A., Setzer W.N. Leaf essential oil composition of 10 species of Ocotea (Lauraceae) from Monteverde, Costa Rica. Biochem. Syst. Ecol. 2007;35:525–532. doi: 10.1016/j.bse.2007.02.003. [DOI] [Google Scholar]
- 23.Da Silva J.K.R., da Trindade R.C., Maia J.G., Setzer W.N. Chemical Composition, Antioxidant, and Antimicrobial Activities of Essential Oils of Endlicheria arenosa (Lauraceae) from the Amazon. Nat. Prod. Commun. 2016;11:695–698. [PubMed] [Google Scholar]
- 24.Grecco S.S., Martins E.G., Girola N., de Figueiredo C.R., Matsuo A.L., Soares M.G., Bertoldo B.C., Sartorelli P., Lago J.H. Chemical composition and in vitro cytotoxic effects of the essential oil from Nectandra leucantha leaves. Pharm. Biol. 2015;53:133–137. doi: 10.3109/13880209.2014.912238. [DOI] [PubMed] [Google Scholar]
- 25.Salleh W.M., Ahmada F., Yen K.H., Zulkifli R.M. Chemical Compositions and Biological Activities of Essential Oils of Beilschmiedia glabra. Nat. Prod. Commun. 2015;10:1297–1300. [PubMed] [Google Scholar]
- 26.Salleh W.M., Ahmada F., Yen K.H. Chemical Compositions and Biological Activities of Essential Oils of Beilschmiedia glabra. Arch. Pharm. Res. 2015;38:485–493. doi: 10.1007/s12272-014-0460-z. [DOI] [PubMed] [Google Scholar]
- 27.Telascrea M., de Araújo C.C., Marques M.O.M., Facanali R., de Moraes P.L.R., Cavalheiro A.J. Essential oil from leaves of Cryptocarya mandioccana Meisner (Lauraceae): Composition and intraspecific chemical variability. Biochem. Syst. Ecol. 2007;35:222–227. doi: 10.1016/j.bse.2006.09.015. [DOI] [Google Scholar]
- 28.Pino J.A., Fernandes P., Marbot R., Sá Fontinha S. Chemical composition of the leaf oil of Ocotea foetens (Alt.) Benth. et Hook. from Madeira. J. Essent. Oil Res. 2004;16:131–132. doi: 10.1080/10412905.2004.9698673. [DOI] [Google Scholar]
- 29.Schmidt J.M., Noletto J.A., Vogler B., Setzer W.N. Abaco bush medicine: Chemical composition of the essential oils of four aromatic medicinal plants from Abaco Island, Bahamas. J. Herbs Spices Med. Plants. 2007;12:43–65. doi: 10.1300/J044v12n03_04. [DOI] [Google Scholar]
- 30.Wright B.S., Bansal A., Moriarity D.M., Takaku S., Setzer W.N. Cytotoxic leaf essential oils from Neotropical Lauraceae: Synergistic effects of essential oil components. Nat. Prod. Commun. 2007;2:1241–1244. [Google Scholar]
- 31.Owolabi M.S., Ogundajo A., Yusuf K.O., Lajide L., Villanueva H.E., Tuten J.A., Setzer W.N. Chemical composition and bioactivity of the essential oil of Chromolaena odorata from Nigeria. Rec. Nat. Prod. 2010;4:72–78. [Google Scholar]
- 32.Sobral M.V., Xavier A.L., Lima T.C., de Sousa D.P. Antitumor activity of monoterpenes found in essential oils. Sci. World J. 2014;2014:953451. doi: 10.1155/2014/953451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gautam N., Mantha A.K., Mittal S. Essential Oils and Their Constituents as Anticancer Agents: A Mechanistic View. BioMed Res. Int. 2014;2014:154106. doi: 10.1155/2014/154106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alarcón L., Peña A., Velascd J., Baptista J.G., Rojas L., Aparicio R., Usubillaga A. Chemical composition and antibacterial activity of the essential oil of Ruilopezia bracteosa. Nat. Prod. Commun. 2015;10:655–656. [PubMed] [Google Scholar]
- 35.Raut J.S., Karuppayil S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014;62:250–264. doi: 10.1016/j.indcrop.2014.05.055. [DOI] [Google Scholar]
- 36.Da Silva E.B.P., Matsuo A.L., Figueiredo C.R., Chaves M.H., Sartorelli P., Lago J.H.G. Chemical constituents and cytotoxic evaluation of essential oils from leaves of Porcelia macrocarpa (Annonaceae) Nat. Prod. Commun. 2013;8:277–279. [PubMed] [Google Scholar]
- 37.El Hadri A., Del Río M.A.G., Sanz J., Coloma A.G., Idaomar M., Ozonas B.R., González J.B., Reus M.I.S. Cytotoxic activity of α-humulene and transcaryophyllene from Salvia officinalis in animal and human tumor cells. An. R. Acad. Nac. Farm. 2010;76:343–356. [Google Scholar]
- 38.Park K.R., Nam D., Yun H.M., Jang H.J., Sethi G., Cho S.K., Ahn K.S. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011;312:178–188. doi: 10.1016/j.canlet.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Santana J.S., Sartorelli P., Guadagnin R.C., Matsuo A.L., Figueiredo C.R., Soares M.G., da Silva A.M., Lago J.H. Essential oils from Schinus terebinthifolius leaves—Chemical composition and in vitro cytotoxicity evaluation. Pharm. Biol. 2012;50:1248–1253. doi: 10.3109/13880209.2012.666880. [DOI] [PubMed] [Google Scholar]
- 40.Hilu K.W., Liang H. The MATK gene: Sequence variation and application in plant systematics. Am. J. Bot. 1997;84:830–839. doi: 10.2307/2445819. [DOI] [PubMed] [Google Scholar]
- 41.Neuhaus H., Link G. The chloroplast tRNALys (UUU) gene from mustard (Sinapis alba) contains a class II intron potentially coding for a maturase-related polypeptide. Curr. Genet. 1987;11:251–257. doi: 10.1007/BF00355398. [DOI] [PubMed] [Google Scholar]
- 42.Chase M.W., Cowan R.S., Hollingsworth P.M., Berg C., Madrinan S., Petersen G., Seberg O., Jorgsensen T., Camero K.M., Carine M., et al. A Proposal for a Standardised Protocol to Barcode All Land Plants. Taxon. 2007;56:295–299. [Google Scholar]
- 43.Lahaye R., van der Bank M., Bogarin D., Warner J., Pupulin F., Gigot G., Maurin O., Duthoit S., Barraclough T.G., Savolainen V. DNA barcoding the floras of biodiversity hotspots. Proc. Natl. Acad. Sci. USA. 2008;105:2923–2928. doi: 10.1073/pnas.0709936105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. p. 804. [Google Scholar]
- 45.Da Silva J.K.R., Gomes M.V.S., Maia J.G.S., Dosoky N.S., Setzer W.N. Chemical composition and in vitro biological activities of essential oil chemotypes of Licaria rigida (Kosterm.) Kosterm. (Lauraceae) Int. J. Appl. Res. Nat. Prod. 2016;9:1–9. [Google Scholar]
- 46.Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Hygeine. 1938;27:493–497. [Google Scholar]
- 47.Hall T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- 48.Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]