Abstract
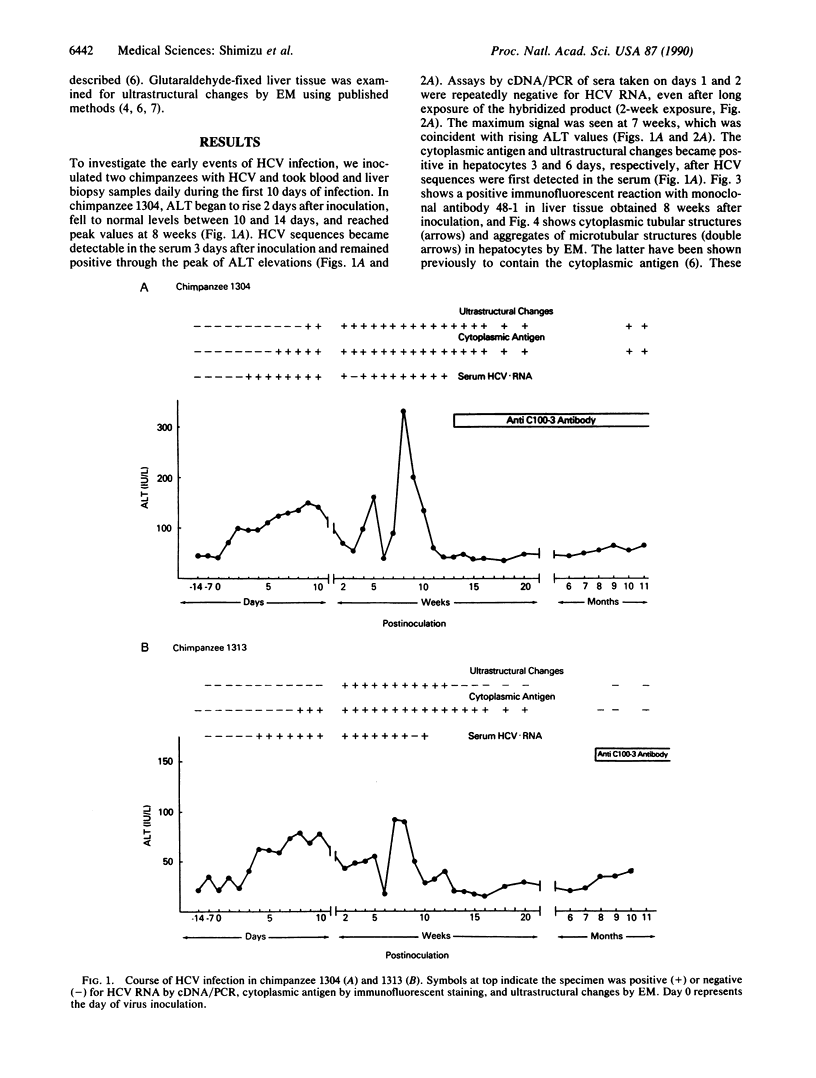
The cytoplasmic antigen and ultrastructural changes we described previously for chimpanzees (Pan troglodytes) infected with hepatitis C virus (HCV) or with hepatitis D virus have recently been shown to be indirect measures of viral replication and appear to represent a host response to the expression or action of interferon. The time of appearance of these changes in hepatocytes during HCV infection, when compared with similar changes in hepatitis D virus infection, suggests a very early replicative phase for HCV. To investigate the early events in HCV infection, we infected two chimpanzees with HCV and obtained blood and liver biopsy samples from them daily during the first 10 days of infection. The early stage of infection with regard to HCV replication, antigen expression, and ultrastructural changes was similar in both chimpanzees. When tested by cDNA/polymerase chain reaction, HCV sequences became detectable in the serum as early as 3 days after inoculation and remained positive through the peak of aminotransferase elevations. In one chimpanzee the peak of virus production appeared to be 7 weeks after inoculation, which was coincident with rising enzyme values. The cytoplasmic antigen, detected by immunofluorescence, and ultrastructural changes, detected by electron microscopy, became positive in hepatocytes 3 and 6 days, respectively, after HCV sequences were first detected in serum. Circulating anti-HCV appeared 13 weeks and 32 weeks after inoculation, respectively, in the chimpanzees. These data indicate a very early replicative phase for HCV and a potentially long period of infectivity before the appearance of anti-HCV.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter H. J., Purcell R. H., Holland P. V., Popper H. Transmissible agent in non-A, non-B hepatitis. Lancet. 1978 Mar 4;1(8062):459–463. doi: 10.1016/s0140-6736(78)90131-9. [DOI] [PubMed] [Google Scholar]
- Bradley D. W., Maynard J. E., Popper H., Cook E. H., Ebert J. W., McCaustland K. A., Schable C. A., Fields H. A. Posttransfusion non-A, non-B hepatitis: physicochemical properties of two distinct agents. J Infect Dis. 1983 Aug;148(2):254–265. doi: 10.1093/infdis/148.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Weiner A. J., Overby L. R., Kuo G., Houghton M., Bradley D. W. Hepatitis C virus: the major causative agent of viral non-A, non-B hepatitis. Br Med Bull. 1990 Apr;46(2):423–441. doi: 10.1093/oxfordjournals.bmb.a072408. [DOI] [PubMed] [Google Scholar]
- Feinstone S. M., Alter H. J., Dienes H. P., Shimizu Y., Popper H., Blackmore D., Sly D., London W. T., Purcell R. H. Non-A, non-B hepatitis in chimpanzees and marmosets. J Infect Dis. 1981 Dec;144(6):588–598. doi: 10.1093/infdis/144.6.588. [DOI] [PubMed] [Google Scholar]
- Feinstone S. M., Mihalik K. B., Kamimura T., Alter H. J., London W. T., Purcell R. H. Inactivation of hepatitis B virus and non-A, non-B hepatitis by chloroform. Infect Immun. 1983 Aug;41(2):816–821. doi: 10.1128/iai.41.2.816-821.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L. F., Alling D., Popkin T., Shapiro M., Alter H. J., Purcell R. H. Determining the size of non-A, non-B hepatitis virus by filtration. J Infect Dis. 1987 Oct;156(4):636–640. doi: 10.1093/infdis/156.4.636. [DOI] [PubMed] [Google Scholar]
- Kamimura T., Ponzetto A., Bonino F., Feinstone S. M., Gerin J. L., Purcell R. H. Cytoplasmic tubular structures in liver of HBsAg carrier chimpanzees infected with delta agent and comparison with cytoplasmic structures in non-A, non-B hepatitis. Hepatology. 1983 Sep-Oct;3(5):631–637. doi: 10.1002/hep.1840030502. [DOI] [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Purcell R. H. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviviruses as well as members of two plant virus supergroups. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2057–2061. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y. K., Feinstone S. M., Purcell R. H., Alter H. J., London W. T. Non-A, non-B hepatitis: ultrastructural evidence for two agents in experimentally infected chimpanzees. Science. 1979 Jul 13;205(4402):197–200. doi: 10.1126/science.451589. [DOI] [PubMed] [Google Scholar]
- Shimizu Y. K., Oomura M., Abe K., Uno M., Yamada E., Ono Y., Shikata T. Production of antibody associated with non-A, non-B hepatitis in a chimpanzee lymphoblastoid cell line established by in vitro transformation with Epstein-Barr virus. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2138–2142. doi: 10.1073/pnas.82.7.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y. K., Purcell R. H. Cytoplasmic antigen in hepatocytes of chimpanzees infected with non-A, non-B hepatitis virus or hepatitis delta virus: relationship to interferon. Hepatology. 1989 Nov;10(5):764–768. doi: 10.1002/hep.1840100503. [DOI] [PubMed] [Google Scholar]
- Shimizu Y. K., Purcell R. H., Gerin J. L., Feinstone S. M., Ono Y., Shikata T. Further studies by immunofluorescence of the monoclonal antibodies associated with experimental non-A, non-B hepatitis in chimpanzees and their relation to D hepatitis. Hepatology. 1986 Nov-Dec;6(6):1329–1333. doi: 10.1002/hep.1840060618. [DOI] [PubMed] [Google Scholar]
- Weiner A. J., Kuo G., Bradley D. W., Bonino F., Saracco G., Lee C., Rosenblatt J., Choo Q. L., Houghton M. Detection of hepatitis C viral sequences in non-A, non-B hepatitis. Lancet. 1990 Jan 6;335(8680):1–3. doi: 10.1016/0140-6736(90)90134-q. [DOI] [PubMed] [Google Scholar]