Abstract
Background:
Thromboelastography (TEG) is a global test of coagulation which analyzes the whole coagulation process. TEG is popular in trauma, liver transplant, and cardiac surgeries, but studies in sepsis are limited. We have assessed the utility of TEG for evaluating coagulopathy in nonbleeding patients with sepsis.
Materials and Methods:
A prospective, observational study was done in 12-bedded Intensive Care Unit (ICU) of a tertiary care hospital in North India, during May 2014-November 2014. After ethical clearance, all patients at ICU admission with sepsis were included in the study. Exclusion criteria were age <18 years, plasma/platelet transfusion before admission, patients on oral antiplatelets/anticoagulants, or with underlying hematological disorders. At admission, blood samples for TEG were analyzed by kaolin-based TEG analyzer within an hour of collecting 2.7 ml citrated blood from arterial line. TEG parameters included reaction time (R), K time (K), alpha angle (a), maximum amplitude (MA), coagulation index (CI), and lysis index (LY 30).
Results:
In TEG, mean values of R, K, a, MA, CI, and LY30 were 6.45 ± 2.59 (min), 1.67 ± 0.96 (min), 66.37 ± 10.44 (0), 67.08 ± 10.33 (mm), 0.63 ± 3.46, and 2.23 ± 4.08 (%), respectively. In conventional coagulation assay (CCA), mean values of international normalized ratio (INR), platelet, and fibrinogen were 1.63 ± 0.57, 153.96 ± 99.16 (×103 /mm3), and 301.33 ± 112.82 (mg/dl), respectively. In those with deranged INR (INR ≥1.6), 60% were normocoagulable and 20% were hypercoagulable. Similarly, 81% patients with thrombocytopenia (platelet count <1,00,000/mL) were normocoagulable.
Conclusion:
TEG could differentiate among normocoagulant, hypocoagulant, hypercoagulant states (unlike CCAs). Patients with septic shock had trend toward hypocoagulant state while those without shock had trend toward hypercoagulant state.
Keywords: Coagulopathy, sepsis, septic shock, thromboelastography
INTRODUCTION
Sepsis is the manifestation of complex interactions between invading microorganisms and the immune, inflammatory, and coagulation responses of the host.[1] Coagulopathy in sepsis is a risk factor for mortality. Conventional coagulation assays (CCAs) include prothrombin time/international normalized ratio (INR), activated partial thromboplastin time (aPTT), platelet count (PLT), and fibrinogen. CCAs are routinely used to diagnose coagulation disorders in sepsis and also guide the transfusion practices. However, CCAs have many limitations.[2] These tests do not evaluate the whole coagulation process as they are done in plasma without the cellular component (platelets and tissue bearing cells) and not in whole blood. Besides, they fail to diagnose hypercoagulable states and the fibrinolytic system. To overcome these shortcomings, global tests of coagulation such as thromboelastography (TEG) and rotational thromboelastometry (ROTEM) have emerged which analyze the whole blood (both plasma and cellular components) and closely resemble in vivo hemostasis. They define elaborately the dynamics of clot formation (development, stabilization, and dissolution of clot).[3] Both TEG and ROTEM provide pertinent information about adhesiveness, elasticity, and physical properties of clot from both dynamic and global perspectives. Clot strength and fibrinolysis can also be analyzed by TEG (unlike CCAs). These tests have already gained popularity as a monitoring tool for hemostasis and transfusion in the fields of trauma and major surgeries such as liver transplantation and cardiac surgeries.[4] This study was planned to evaluate the utility of TEG in assessing the hemostatic dysfunction in nonbleeding patients with sepsis.
MATERIALS AND METHODS
Study design and setting
This study was a prospective, single-center, observational study done in 12-bedded Intensive Care Unit (ICU) of a tertiary care hospital in North India, over a period of 6 months (May 2014-November 2014), after approval from the local Ethics Committee. A written and informed consent was obtained from all patients or their relatives before enrollment in the study.
Study eligibility
All patients with sepsis admitted to the ICU over a period of 6 months were included in this study. Patients with preexisting hematological disorders (congenital, malignancy, liver dysfunction), age ≤18 years of age, on antiplatelets/anticoagulants, history of transfusion of blood products up to 24 h before taking blood samples were excluded from the study. The patients included in the study were divided into two groups for analysis: Group 1 (septic shock) and Group 2 (sepsis).
Blood sampling and thromboelastography analysis
At the time of admission to the ICU, 2.7 ml blood was collected from arterial line in a nonheparinized syringe and collected in a citrate vial and immediately sent to the department of transfusion medicine. TEG analysis was done in the transfusion medicine laboratory by trained personnel using a kaolin-based thromboelastograph analyzer (TEG Haemoscope 5000, USA) within 1 h of collection of the sample. TEG parameters included reaction time (R): time to initial fibrin formation up to 2 mm, K time (K): time to clot formation up to 20 mm, alpha angle (a): speed of clot formation, maximum amplitude (MA): measurement of clot strength, lysis index (LY 30%): clot dissolution (percentage decrease in amplitude 30 min post-MA), and coagulation index (CI): an index representative of the entire coagulation process. The normal reference values of TEG parameters (as provided by the manufacturer) for kaolin-activated citrated samples were taken as R = 2-8 min, K = 1-3 min, alpha (a) angle = 55-78°, MA = 51-69 mm, CI -3 to +3, and LY 30% = 0-8.
Definition of coagulopathy
On the basis of individual TEG parameters, hypocoagulability was defined as prolonged R and K times and/or decreased alpha and MA below the reference values. Conversely, hypercoagulability was defined as shortened R and K times and/or increased alpha and MA values above the reference values. With respect to CI on TEG, hypocoagulability was defined as CI <−3 and hypercoagulability as CI >3.
On the basis of CCAs, coagulopathy was defined as one of the following criteria: INR ≥1.6, thrombocytopenia defined by PLT ≤100,000/mL, fibrinogen levels in blood ≤100 mg/dl.
Data collection
Additional data recorded at admission to the ICU were demographic profile of the patients, severity scores such as sequential organ failure assessment (SOFA) and acute physiology and chronic health evaluation II (APACHE II) scores, investigations routinely done as standard of care in the ICU such as hemoglobin, hematocrit, total leukocyte count (TLC), procalcitonin, blood urea nitrogen (BUN), serum creatinine, total and direct bilirubin, serum albumin, serum aspartate aminotransferase (SGOT), serum alanine aminotransferase (SGPT), CCAs which included INR, aPTT, PLTs, and fibrinogen levels. Type and number of blood products transfused, use of antiplatelet and anticoagulant medications, need for mechanical ventilation, length of the ICU stay, and outcome at the ICU discharge were also recorded.
Statistical analysis
SPSS 17 software was used (SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc.). Continuous variables were expressed as mean ± standard deviation and comparison of means was done by Student's t-test. Categorical data were expressed as frequencies and percentages. Chi-square test was used to analyze relationships between categorical data. Significance was established when P < 0.05. A formal power calculation was not performed for this exploratory study due to unavailability of relevant data at the time of the study design.
RESULTS
During the study period, 104 patients got admitted to the ICU, out of which 87 patients were in sepsis. Of these 87 patients, 55 were included in the study. The remaining 32 patients were excluded from the study owing to a history of transfusion of blood products 24 h before the ICU admission (n = 15), the presence of obvious bleeding manifestation (n = 12), and a known hematological disorder (n = 5).
Baseline demographic and laboratory data
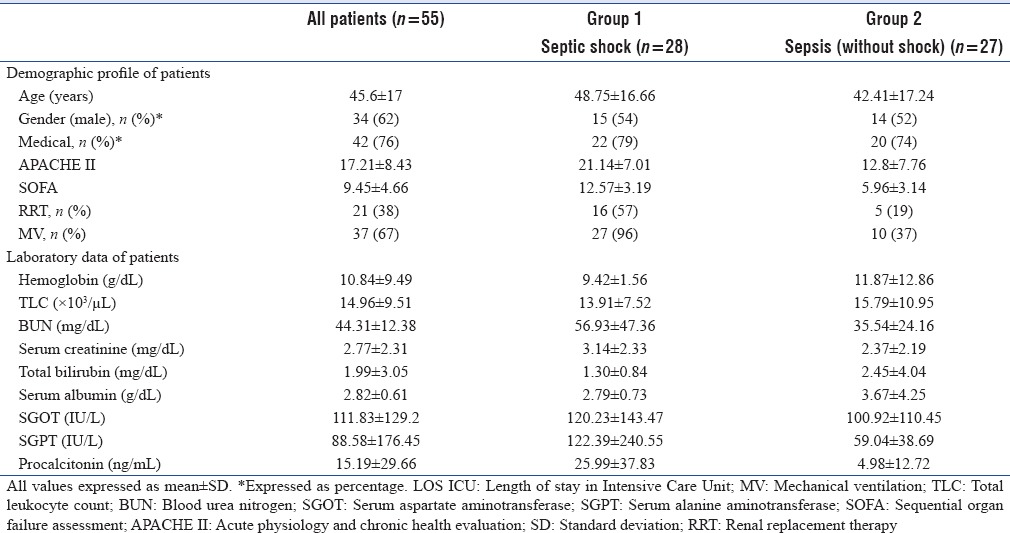
Mean age of included patients was 45.6 (±17) years, 62% were males, 76% had medical illness, and 60% had comorbidities (such as diabetes mellitus, hypertension, ischemic heart disease, and chronic kidney disease). Primary source of illness was respiratory system, followed by neurological, urosepsis, and tropical illness. Mean APACHE II was 17.21 ± 8.43, and SOFA score was 9.45 ± 4.66. The laboratory data included hemoglobin, TLC, BUN, serum creatinine, total and direct bilirubin, SGOT, SGPT, and procalcitonin [Table 1].
Table 1.
Demographic profile and laboratory data of patients at Intensive Care Unit admission
Conventional coagulation assay parameters at admission
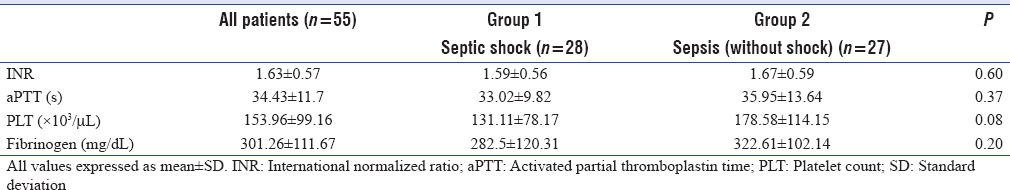
Among CCAs, mean values for INR, aPTT, platelet, and fibrinogen were 1.63 ± 0.57, 34.43 ± 11.7 (s), 153.96 ± 99.16 (×103 /mL), and 301.33 ± 112.82 (mg/dl), respectively [Table 2].
Table 2.
Conventional coagulation assays of patients at Intensive Care Unit admission
Thromboelastography parameters at admission
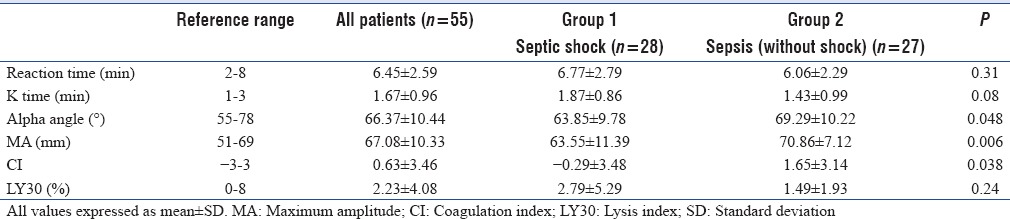
On TEG analysis, mean values of R, K, a, MA, CI, and LY30 parameters were 6.45 ± 2.59 (min), 1.67 ± 0.96 (min), 66.37 ± 10.44 (°), 67.08 ± 10.33 (mm), 0.63 ± 3.46, and 2.23 ± 4.08 (%), respectively. Moreover, on assessing individual TEG values, we found that patients with septic shock had lower alpha, MA, and CI values) while those without shock had higher alpha, MA, and CI values [Table 3].
Table 3.
Thromboelastography parameters of patients at Intensive Care Unit admission
Overall classification of coagulation status of patients by conventional coagulation assay and thromboelastography
TEG (based on CI): Overall, 56% patients were normocoagulable, 27% hypercoagulable, and 17% hypocoagulable. On further analysis, in septic shock group, 61% patients had normal coagulation state, 14% had hypercoagulability, and 25% patients had a hypocoagulable profile while in patients without shock, 50% had normal coagulation state, 43% were hypercoagulable, and 7% had hypocoagulant profile [Figure 1].
Figure 1.
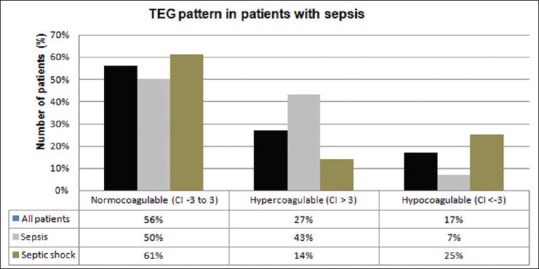
Coagulation status of patients based on thromboelastography
CCA (based on INR): Overall, 37% patients were hypocoagulable, and 63% were normocoagulable [Figure 2].
Figure 2.
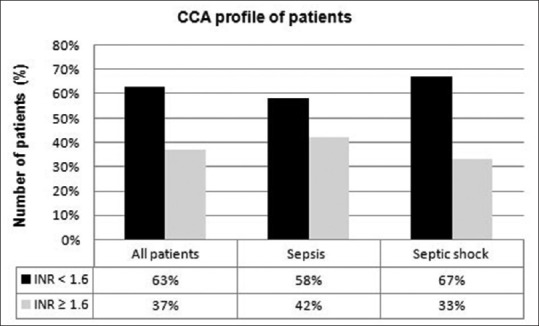
Coagulation status of patients based on conventional coagulation assays
Thromboelastography pattern (based on coagulation index) in patients with and without coagulopathy
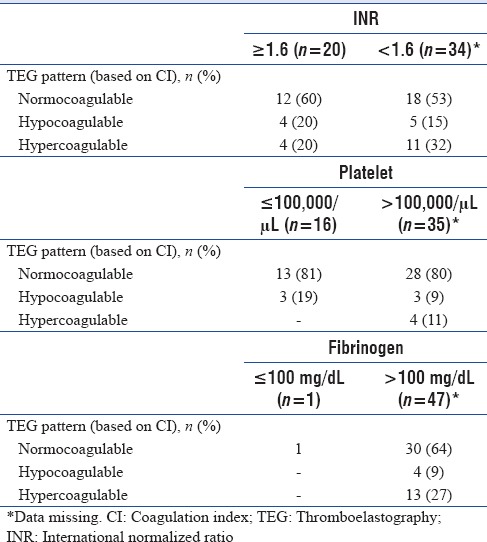
In patients with normal INR, we found that 15% patients were hypocoagulable and 32% were hypercoagulable and in those with deranged INR (INR ≥1.6), 60% were normocoagulable and 20% were hypercoagulable. Similarly, 81% patients with thrombocytopenia were normocoagulable and in patients with normal fibrinogen levels, 9% were hypocoagulable and 27% were hypercoagulable [Table 4].
Table 4.
Thromboelastography patterns (based on coagulation index) in patients with and without coagulopathy
Follow-up
At the ICU discharge, 55% patients had survived and the mean length of ICU stay was 23.35 ± 27.97 days.
DISCUSSION
Coagulation abnormalities are pivotal in causing microcirculatory dysfunction and multiorgan failure in sepsis.[1,5] Sepsis-induced coagulopathy ranges from a subtle activation of coagulation to a more severe condition known as disseminated intravascular coagulation (DIC), which is characterized by simultaneous widespread microvascular thrombosis and profuse bleeding from various sites.[6] Early diagnosis and management of sepsis-induced coagulopathy can influence the outcome. TEG has been used for many years in the fields of trauma and major surgeries.[7,8,9,10,11,12] TEG is emerging as a promising tool in sepsis also. However, the studies available on TEG in sepsis are limited in number, are observational in design, have small and heterogeneous patient populations, and lack a universally validated definition of coagulopathy (hypocoagulability and hypercoagulability).[13]
In our study, we have assessed the spectrum of hemostatic dysfunction in patients with sepsis by using TEG. We found three coagulation profiles in our patients on the basis of CI: normocoagulable (56%), hypercoagulable (27%), and hypocoagulable (17%) profiles [Figure 1]. On assessing individual TEG values, we also observed that patients with septic shock had trend toward a hypocoagulant state while those without shock had trend toward a hypercoagulant state [Table 3]. These findings may be useful in the management of patients with sepsis. Hypercoagulability has also been seen in previous studies of TEG in sepsis. Kiliç et al. in 2014 studied 21 patients with systemic inflammatory response syndrome (SIRS)-sepsis and compared them with 34 patients without SIRS-sepsis. They noticed that patients with SIRS-sepsis had hypercoagulability as compared to the control group.[14] Luckner et al. in 2008 studied a case series of patients with sepsis who were planned for emergency laparotomy and had significantly deranged standard coagulation tests with a putatively increased risk of bleeding as per these coagulation assays. However, TEG was suggestive of hypercoagulability in these patients and surgery could be performed with minimal blood loss.[15] Sivula et al. in 2009 assessed the role of thromboelastometry in severe sepsis and found a trend towards hypercoagulability in patients without DIC and a trend towards hypocoagulability in those with overt DIC.[16] The initial phase of sepsis is characterized by the formation of microvascular thrombi and the later phase manifests as a hypocoagulant phase secondary to consumptive coagulopathy. Hence, sepsis is a dynamic process, and therefore, both the timing of test and severity of sepsis can influence the results of TEG.
However, by CCAs, only two states of coagulation could be analyzed (normocoagulant or hypocoagulant) [Figure 2]. These observations may be explained by the fact that CCAs assess only the plasmatic part of coagulation cascade and fail to reflect a patient's coagulation profile accurately. On the contrary, global tests of coagulation such as rotational TEG (TEG®) analyze both plasmatic and cellular components of blood, and therefore, they can provide a comprehensive assessment of the whole coagulation process (clot initiation, propagation, and degradation).[3]
Classification of patients into coagulation patterns can be helpful in prognostication of the bleeding risk, severity of sepsis, and outcome of patients. TEG clot strength profile was also studied by Ostrowski et al. in fifty patients with severe sepsis, wherein they divided the patients into three groups at admission (on the basis of MA): normocoagulable MA (48%), hypercoagulable MA (30%), and hypocoagulable MA (22%). Patients progressing to hypocoagulability were found to have higher mortality.[17] Haase et al. found an association between progressive hypocoagulability and an increased risk of bleeding and mortality in patients with severe sepsis.[18] CI was used in our study for studying the whole coagulation profile of patients because CI is an index calculated from all the TEG parameters (including MA) and thus, is better than individual parameters such as MA.
Besides hyper- and hypo-coagulability, involvement of fibrinolytic system can also be detected by TEG. LY30% was the parameter used for this purpose in our study. Adamzik et al. in 2010 studied 56 patients with severe sepsis and compared them with 52 postoperative patients (controls). They found that LY was significantly increased in patients with severe sepsis (suggestive of an early involvement of fibrinolysis in patients with severe sepsis).[19] In our study, we did not find raised LY. It is possible that LY60% (percentage decrease in amplitude 60 min post-MA) could have been deranged, but this parameter was not analyzed in our study. Hyperfibrinolysis has been associated with high mortality in acute traumatic coagulopathy,[20] but studies correlating hypo- or hyper-fibrinolysis and with the outcome of sepsis are not available.
The strength of our study is that it has been prospectively conducted and is one of the few studies done for the assessment of hemostatic dysfunction in sepsis using TEG. The limitations are that it is an observational study with a small sample size. Reference values used for TEG analysis (provided by manufacturer) may not be applicable to patients with sepsis. Sequential measurements of TEG parameters were not done (due to financial constraints) and comparison of TEG profile with bleeding events or transfusion requirement and outcome has not been done.
CONCLUSION
Identification of coagulation disorders is essential in sepsis because coagulopathy is a risk factor for mortality. Based on the above findings, we suggest that in comparison to CCA, TEG can provide a wider spectrum of coagulation abnormalities. Larger trials are needed to assess the utility of TEG for prognostication of outcome and for guiding transfusion practices or antithrombotic therapies in sepsis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Levi M, Cate HT. Current concepts: Disseminated intravascular coagulation. N Engl J Med. 1999;341:586–92. doi: 10.1056/NEJM199908193410807. [DOI] [PubMed] [Google Scholar]
- 2.Segal JB, Dzik WH. Transfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: An evidence-based review. Transfusion. 2005;45:1413–25. doi: 10.1111/j.1537-2995.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 3.Bolliger D, Seeberger MD, Tanaka KA. Principles and practice of thromboelastography in clinical coagulation management and transfusion practice. Transfus Med Rev. 2012;26:1–13. doi: 10.1016/j.tmrv.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Afshari A, Wikkelsø A, Brok J, Møller AM, Wetterslev J. Thrombelastography (TEG) or thromboelastometry (ROTEM) to monitor haemotherapy versus usual care in patients with massive transfusion. Cochrane Database Syst Rev. 2011;3:CD007871. doi: 10.1002/14651858.CD007871.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 6.Wada H, Matsumoto T, Yamashita Y. Diagnosis and treatment of disseminated intravascular coagulation (DIC) according to four DIC guidelines. J Intensive Care. 2014;2:15. doi: 10.1186/2052-0492-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.da Luz LT, Nascimento B, Rizoli S. Thrombelastography (TEG®): Practical considerations on its clinical use in trauma resuscitation. Scand J Trauma Resusc Emerg Med. 2013;21:29. doi: 10.1186/1757-7241-21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schöchl H, Nienaber U, Maegele M, Hochleitner G, Primavesi F, Steitz B, et al. Transfusion in trauma: Thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care. 2011;15:R83. doi: 10.1186/cc10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avidan MS, Alcock EL, Da Fonseca J, Ponte J, Desai JB, Despotis GJ, et al. Comparison of structured use of routine laboratory tests or near-patient assessment with clinical judgement in the management of bleeding after cardiac surgery. Br J Anaesth. 2004;92:178–86. doi: 10.1093/bja/aeh037. [DOI] [PubMed] [Google Scholar]
- 10.Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999;88:312–9. doi: 10.1097/00000539-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Vucelic D, Miljic P, Antonijevic N, Milicevic M. The role of rotational thromboelastometry in real time assessment of haemostasis in surgical settings. Srp Arh Celok Lek. 2010;138(Suppl 1):43–9. doi: 10.2298/sarh10s1043v. [DOI] [PubMed] [Google Scholar]
- 12.Ives C, Inaba K, Branco BC, Okoye O, Schochl H, Talving P, et al. Hyperfibrinolysis elicited via thromboelastography predicts mortality in trauma. J Am Coll Surg. 2012;215:496–502. doi: 10.1016/j.jamcollsurg.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Müller MC, Meijers JC, Vroom MB, Juffermans NP. Utility of thromboelastography and/or thromboelastometry in adults with sepsis: A systematic review. Crit Care. 2014;18:R30. doi: 10.1186/cc13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiliç Y, Topçu I, Bambal H, Civi M. Thromboelastography in the evaluation of coagulation disorders in patients with sepsis. Turk J Med Sci. 2014;44:267–72. doi: 10.3906/sag-1210-99. [DOI] [PubMed] [Google Scholar]
- 15.Luckner G, Mayr VD, Fries DR, Innerhofer P, Jochberger S, Hasibeder WR, et al. Uncovering hypercoagulability in sepsis using ROTEM thromboelastometry: A case series. Open Crit Care Med J. 2008;1:1–6. [Google Scholar]
- 16.Sivula M, Pettilä V, Niemi TT, Varpula M, Kuitunen AH. Thromboelastometry in patients with severe sepsis and disseminated intravascular coagulation. Blood Coagul Fibrinolysis. 2009;20:419–26. doi: 10.1097/MBC.0b013e32832a76e1. [DOI] [PubMed] [Google Scholar]
- 17.Ostrowski SR, Windeløv NA, Ibsen M, Haase N, Perner A, Johansson PI. Consecutive thrombelastography clot strength profiles in patients with severe sepsis and their association with 28-day mortality: A prospective study. J Crit Care. 2013;28:317.e1–11. doi: 10.1016/j.jcrc.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Haase N, Ostrowski SR, Wetterslev J, Lange T, Møller MH, Tousi H, et al. Thromboelastography in patients with severe sepsis: A prospective cohort study. Intensive Care Med. 2015;41:77–85. doi: 10.1007/s00134-014-3552-9. [DOI] [PubMed] [Google Scholar]
- 19.Adamzik M, Eggmann M, Frey UH, Görlinger K, Bröcker-Preuss M, Marggraf G, et al. Comparison of thromboelastometry with procalcitonin, interleukin 6, and C-reactive protein as diagnostic tests for severe sepsis in critically ill adults. Crit Care. 2010;14:R178. doi: 10.1186/cc9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll RC, Craft RM, Langdon RJ, Clanton CR, Snider CC, Wellons DD, et al. Early evaluation of acute traumatic coagulopathy by thrombelastography. Transl Res. 2009;154:34–9. doi: 10.1016/j.trsl.2009.04.001. [DOI] [PubMed] [Google Scholar]


