Abstract
Aims:
Sedation, as it is often required in critical care, is associated with immobilization, prolonged ventilation, and increased morbidity. Most sedation protocols are based on benzodiazepines. The presented study analyzes the benefit of benzodiazepine-free sedation.
Methods:
In 2008, 134 patients were treated according to a protocol using benzodiazepine and propofol (Group 1). In 2009, we introduced a new sedation strategy based on sufentanil, nonsteroidal anti-inflammatory drugs, neuroleptics, and antidepressants, which was applied in 140 consecutive patients (Group 2). Depth of sedation, duration of mechanical ventilation, duration of Intensive Care Unit, and hospital stay were analyzed.
Results:
Group 1 had both a longer duration of deep sedation (18.7 ± 2.5 days vs. 12.6 ± 1.85 days, P = 0.031) and a longer duration of controlled ventilation (311, 35 ± 32.69 vs. 143, 96 ± 20.76 h, P < 0.0001) than Group 2. Ventilator days were more frequent in Group 1 (653, 66 ± 98.37 h vs. 478, 89 ± 68.92 h, P = 0.128).
Conclusions:
The benzodiazepine-free sedation protocol has been shown to significantly reduce depth of sedation and controlled ventilation. Additional evidence is needed to ascertain reduction of ventilator days which would not only be of benefit for the patient but also for the hospital Management.
Keywords: Analgesia, benzodiazepine, critical care, intensive care unit, midazolam, sedation
INTRODUCTION
Sedation and analgesia are required in most mechanically ventilated patients.[1,2] Thus, stress-induced reactions such as agitation, anxiety and pain, hypermetabolism, sodium and water retention, hypertension, tachycardia, and impaired wound healing are reduced or prevented.[3,4,5,6] Insufficient or excessively deep sedation can lead to increased morbidity and prolonged stay in the Intensive Care Unit (ICU) and consecutively to increased costs.[7,8,9,10]
Ten years ago, in Europe, the most commonly used medication for continuous sedation was midazolam. In 2002, the Society of Critical Care Medicine recommended combining it with propofol for short-term and the use of lorazepam for long-term sedation.[11,12] Adequate analgesia should also be applied since there are indications that 70% of intensive care patients recall severe pain during treatment whereas 70%-90% of ICU nurses and physicians consider their patients pain-free.[6,13] Most patients do not ask for analgesia despite the presence of moderate to even severe pain while the medical staff is concerned about possible adverse physiologic response to increased dosage.[13]
Several studies report the increasing use of sedation protocols, tools for scoring the level of sedation, and a target end-point of light sedation that enables patients to interact with their environment.[1,2,14,15,16,17] This goal-oriented sedation management allows earlier spontaneous breathing, quicker weaning, and shorter ICU stay.[12,18,19,20,21] The ability to sedate the patients deeply for necessary interventions with short-acting medication so that the patient quickly regains responsiveness and cooperation is retained.[12,18] However, the optimal strategy in sedation management is still controversial and differs widely.[22,23,24,25,26,27,28]
The primary goal of the presented study was to evaluate the influence of midazolam on the weaning period analyzing the “time to extubation” in postoperative mechanically ventilated patients in comparison to patients treated with the innovative benzodiazepine-free concept. A new analgesic, nonbenzodiazepine sedation concept allowing quicker weaning was introduced in the ICU during the study period. The new concept also uses a standardized protocol for analgesic sedation with the option of situational adaptation.
METHODS
The period of the presented study included 2 years, from January 2008 to December 2009. All patients treated with mechanical ventilation on the surgical ICU were analyzed. Patients admitted between January and December 2008 formed Group 1 whereas the following went into Group 2. The essential treatment change was the introduction of the new sedation protocol on 1 January, 2009.
As a standard procedure, early percutaneous tracheotomy within 2 days after surgery was performed to prevent tube-associated comorbid conditions. Responsive patients were asked to indicate pain levels on a visual analog scale (ranging from 0 [no pain] to 10 [maximum] on a linear scale) to monitor the effects of the analgesic therapy aiming at ratings of less than four points.
All patients received daily neurological examination using or the Richmond Agitation Sedation Scale (RASS)[55,56] [Table 1] or the Ramsay Sedation Scale (RSS)[57] [Table 2] to direct sedation management as quickly as possible to ratings of RASS 0 or RSS 2-3. Propofol was substantially reduced or ceased earliest continuing analgesic sedation with intravenous (iv) sufentanil (25-200 mg/h).
Table 1.
Richmond Agitation Sedation Scale (RASS)

Table 2.
Ramsay Sedation Scale (RSS)

Additional nonsteroidal analgesics such as metamizole or paracetamol were prescribed to maximum doses of 4 × 1 g/day. The use of antidepressants and neuroleptics protecting the patient from posttraumatic stress syndrome and ease adaptation was determined on an individual basis (amitriptyline 25-150 mg/day iv, haloperidol 4 × 5 mg/day [up to 40 mg/day], sertraline 25-200 mg/day). Patients who qualified for regional anesthesia were adequately informed and given the consent, treated accordingly.
The parameters “eventual use of midazolam” and “duration of mechanical ventilation >72 h” were appreciated since in 92% of hospitals surveyed, midazolam was the preferred sedative for long-term sedation.[1,12] To represent these findings, subgroups were distinguished. Group 1 >72+ comprised all 2008 patients with both mechanical ventilation for more than 72 h and midazolam treatment. Group 2 >72− comprised all 2009 patients mechanically ventilated for >72 h not treated with midazolam.
The data set comprised patient age, gender, surgical indication, eventual use of regional anesthesia, duration of hospital stay (ICU/total hospital stay), and outcome (survival/death). Furthermore, type and route of ventilation (bilevel positive airway pressure [BIPAP], continuous positive airway pressure [CPAP], or noninvasive ventilation/through tube or tracheotomy) as well as duration of deep sedation (RASS − 5/−6 or RSS 6/5/4) and duration of low sedation or alertness were reported.
The study data were analyzed using SPSS software (Statistical Product and Services Solutions, version 16.0, SPSS Inc, Chicago, IL, USA). Results are presented as mean values with the ± standard error of the mean values. Fisher's exact test as well as Student's t-test was used to calculate significant differences. Statistical significance was defined for a P < 0.05.
RESULTS
The study group consisted of 274 patients with mechanical ventilation on surgical ICU from January 2008 to December 2009. The number of patients assigned to Group 1 (January 2008 to December 2008) was 134 which was almost equal to the number of patients entered into Group 2 (January 2009 to December 2009).
Subgroups as explained before were identified as “1 >72+” consisting of 36 patients (13 female, 23 male, mean age of 69.0 ± 11.1 years) and “2 >72−” including 50 patients (17 female, 33 male, mean age of 69.9 ± 13.6 years).
There were no differences between the groups regarding gender, age, “mechanical ventilation days,” “death during mechanically ventilation,” “tracheotomy,” or “use of regional anesthetics” [Table 3]. The mean postoperative duration of total mechanical ventilation as well as BIPAP and CPAP ventilation is shown in Table 4; only the duration of BIPAP ventilation differed between groups.
Table 3.
Outcome parameters

Table 4.
Duration of mechanical ventilation in hours

Extubation was successful in 50% of the patients after 643 h in “Group 1 >72+” and after 386 h in “Group 2 >72−” [Figure 1] (P = 0.128). Successful transfer from BIPAP to pressure support (CPAP) ventilation was possible in 50% of the patients after 246 h in “Group 1 >72+” and 101 h in “Group 2 >72−” (P < 0.001) [Figure 2]. Extubation was performed successfully in 50% of the patients on CPAP ventilation after 307 h in “Group 1 >72+” and after 341 h in “Group 2 >72−” (P = 0.7) [Figure 3].
Figure 1.

Kaplan–Meier analysis of the total duration of mechanical ventilation
Figure 2.

Kaplan–Meier analysis of the duration of bilevel positive airway pressure ventilation
Figure 3.

Kaplan–Meier analysis of the duration of continuous positive airway pressure ventilation
Deep sedation defined by RASS-scores >−5/−4 or RSS-scores <6/5/4 was significantly longer in subgroup 1 >72+ (18.7 ± 2.5 days) than in subgroup 2 >72− (12.6 ± 1.8 days) (P = 0.031). Fifty percent of patients demonstrated no “deep sedation” after 15 days in Group 1 >72+ and after 8 days in Group 2 >72− [Figure 4]. The mean duration of stay in surgical ICU was 35.3 ± 4.3 days in Group 1 >72+ and 33.2 ± 2.9 days in Group 2 >72− (not significant [n.s.]). The mean duration of hospital stay was 57.0 ± 9.8 days in Group 1 >72 + and 64.3 ± 8.1 days in Group 2 >72− (n.s.). The Kaplan-Meier curves show that 50% of patients were transferred from the ICU after 30 days in subgroup 1 >72+ and after 33 days in subgroup 2 >72− (P = 0.879) [Figure 5]. No significant difference could be shown when analyzing the duration of hospital stay. 50% of the patients of Group 1 >72+ were discharged after 45 days and after 60 days in Group 2 >72− (P = 0.606) [Figure 6].
Figure 4.
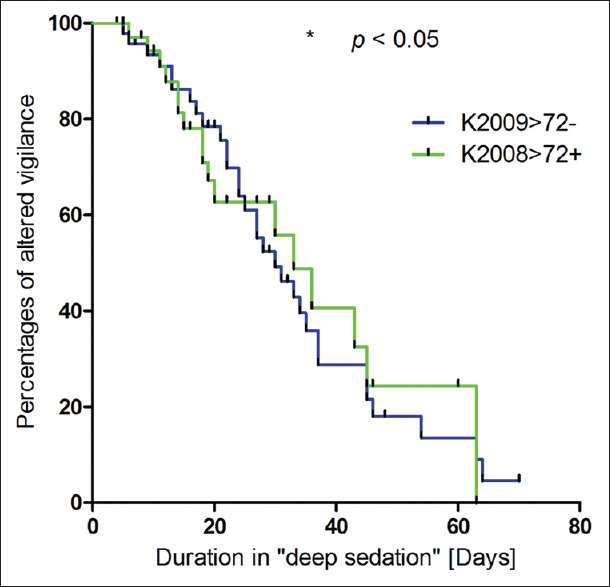
Kaplan–Meier analysis of the duration in “deep sedation”
Figure 5.

Kaplan–Meier analysis of duration of stay in the Intensive Care Unit
Figure 6.

Kaplan–Meier analysis of duration of stay in the hospital
Early mobilization, weight loss, and the initiation of enteral nutrition were not different in the two study groups.
DISCUSSION
The presented data demonstrate that a barbiturate – free sedation strategy significantly reduces the duration of controlled ventilation and improves patient's vigilance. A significant reduction in the total of ventilation days has not been found which may be attributable to the old average age of the patients analyzed.
Cooperating in physiotherapy was limited due to the age of the patients despite lighter sedation. Length of stay in the ICU or in the hospital was not reduced because interventions became necessary and/or secondary illness occurred significantly delaying the date of discharge. Whether our sedation concept shortens overall hospital stay in younger patients should be the object of further study.
Sedatives and analgesics facilitate mechanical ventilation and are used to treat anxiety and agitation. However, both substance categories can also have negative effects on ICU outcome,[29,30,31] including delirium, prolonged mechanical ventilation, withdrawal symptoms, and immunosuppression.[30,32,33,34,35] Delirium and acute brain dysfunction are independent predictors of mortality, hospital length of stay, and cognitive dysfunction even months after discharge.[36] Prolonged mechanical ventilation and immobility in combination with critical illness lead to muscle atrophy, especially in older, sarcopenic patients.[37] In a prospective study of 109 ARDS patients, survivors had a body weight loss of 18%.[38] Immobility also induces insulin resistance which may contribute to neuromuscular injury and impair microvascular function. Immobilization also increases cytokine levels and produces reactive oxygen species, resulting in further muscle weakness.[37] Many CT scans are performed because the patient's cognitive status is inaccessible. We, therefore, intended to reduce the duration of ventilation by introducing a benzodiazepine-free protocol based on opioids and additional medication allowing the combination with a mental assessment score.
Standardization of ICU procedures can decrease the duration of sedation and ventilation and thus substantially reduce length of stay in hospital and overall treatment costs.[39,40,41] A survey of 261 German hospitals found that 43% had a verbal policy for analgesia and sedation and 21% a written protocol.[6] In British ICUs, the number standard verbal or written protocols were present in 51% and 43%, respectively.[17] Twenty-five years previously, the results were similar.[42] Pain Assessment tools are important although less frequently used than sedation assessment tools.[43,44] A French multicenter study found that only 50% of the patients treated with analgesics and sedatives were systematically assessed for pain.[14] Poor pain control results in excessive use of sedatives and consecutively in prolonged stay in the ICU.[45]
Standardized protocols for sedation and pain control as established in our ICU are an effective tool both in the day-to-day work and scientific assessment of therapy quality and outcome.
Sedation can be assessed by the RASS [Table 1] or the RSS [Table 2]. Patient assessment by RSS is common in German ICUs.[1] The consistent use of validated sedation scales such as the RSS or RASS can reduce the duration of mechanical ventilation and the length of stay on ICU as demonstrated by recently published reviews,[46,47] evidence that is fully in line with the findings in our study. Interestingly, patients in the 2008 group had to stay in the hospital for 60 days, whereas the 2009 group was discharged already after 45 days though duration of controlled ventilation was significantly shorter in the 2008 cohort.
The overall goal of improving patient care by the implementation of standard operating procedures is best achieved by adequate training of all personnel involved and by adaptation of the guidelines to the local hospital environment.[1]
The drug of choice in short-term sedation is propofol whereas midazolam and lorazepam are recommended for long-term sedation.[11] Propofol was the most commonly used agent for sedation of a duration up to 24 h as well as during ventilation, while midazolam served for longer-term sedation of more than 72 h according to findings from ICUs in Germany.[6] 92% of the hospitals agreed that the expected length of sedation played a decisive role in selecting the medication.[6] Lorazepam was not used in this study due to higher costs.[6] Carson et al. compared the pharmacokinetics of propofol and benzodiazepines with favorable results for propofol.[48]
American guidelines recommend fentanyl, hydromorphine, and morphine for analgesia in all phases.[11] A German study found that fentanyl and sufentanil were used most often for up to 72 h and for weaning from ventilation.[6] Fentanyl was the preferred analgesic for more than 72 h.[6] Alfentanil was the most used analgesic in a British survey from 2000,[17] in a Danish study, the preferred drugs were morphine (94%), fentanyl (76%), and sufentanil (46%).[49] In our institution, we prefer sufentanil because of its great analgesic potency and assessable pharmacokinetics and pharmacodynamics. Elsewhere, the use of fentanyl decreased while sufentanil was increasingly applied in all stages of sedation.[1]
Data from all the cited studies show that there is conflicting evidence about the length and depth of sedation for mechanically ventilated patients. We introduced our new sedation concept to avoid the negative characteristics of benzodiazepines as there are the unfavorable halftime and severe side effects. The lighter sedation also improves the cooperation with the physiotherapist and allows easier assessment of vigilance and pain. Since we had not introduced delirium screening at the time when we changed our sedation strategy, we have no sound data concerning the incidence of delirium.
The additional use of regional anesthetics is recommended in the German guidelines on analgesia and sedation.[50] Epidural anesthesia was more frequently performed for longer than 72 h when compared to the initial survey several years ago.[1] Although there was no increase in the use of regional anesthesia in our study, several meta-analyses demonstrated that epidural anesthesia can reduce intensive care stays and the incidence of cardiac and pulmonary complications.[51,52]
New Danish data show that a “no-sedation” protocol significantly reduces ventilator days, ICU stay, and hospital stay while increasing urinary output. ICU mortality was reduced and there were no long-term psychological sequels.[53,54] This no-sedation approach is comparable to our protocol in that it was based on morphine analgesia; haloperidol was given if delirium was diagnosed, whereas ICU and hospital stay durations were not significantly reduced in our study. This might be due to the fact that the Danish study treated patients in a medical/surgical ICU, whereas our study population only consisted of patients after major abdominal, thoracic, or vascular surgery. Larger prospective trials are needed to establish whether a no-sedation strategy is superior to contemporary protocols.
Limitations of the study
Patient's data in our study were assessed in retrospect and the cohort was rather small. Nevertheless, we were able to demonstrate significant differences with respect to the duration of mechanical ventilation. Electrophysiological investigations to identify critical illness neuropathy or myopathy were not performed and we did not screen patients for symptoms of delirium during the observation period.
CONCLUSIONS
The study indicates that the presented benzodiazepine-free sedation protocol as a standard procedure allows a shortening of the duration of mechanical ventilation with all resulting benefits such as, earlier extubation and, improved cooperation in physiotherapy resulting in earlier mobilization. However, further studies are needed to determine whether the length of stay in ICU and the incidence of delirium can be reduced by this protocol.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Martin J, Franck M, Sigel S, Weiss M, Spies C. Changes in sedation management in German Intensive Care Units between 2002 and 2006: A national follow-up survey. Crit Care. 2007;11:R124. doi: 10.1186/cc6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Connor M, Bucknall T, Manias E. Sedation management in Australian and New Zealand Intensive Care Units: Doctors’ and nurses’ practices and opinions. Am J Crit Care. 2010;19:285–95. doi: 10.4037/ajcc2009541. [DOI] [PubMed] [Google Scholar]
- 3.Koepke JP. Effect of environmental stress on neural control of renal function. Miner Electrolyte Metab. 1989;15:83–7. [PubMed] [Google Scholar]
- 4.Bonica JJ. Importance of effective pain control. Acta Anaesthesiol Scand Suppl. 1987;85:1–16. doi: 10.1111/j.1399-6576.1987.tb02665.x. [DOI] [PubMed] [Google Scholar]
- 5.Lewis KS, Whipple JK, Michael KA, Quebbeman EJ. Effect of analgesic treatment on the physiological consequences of acute pain. Am J Hosp Pharm. 1994;51:1539–54. [PubMed] [Google Scholar]
- 6.Martin J, Parsch A, Franck M, Wernecke KD, Fischer M, Spies C. Practice of sedation and analgesia in German Intensive Care Units: Results of a national survey. Crit Care. 2005;9:R117–23. doi: 10.1186/cc3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–7. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 8.Freire AX, Afessa B, Cawley P, Phelps S, Bridges L. Characteristics associated with analgesia ordering in the Intensive Care Unit and relationships with outcome. Crit Care Med. 2002;30:2468–72. doi: 10.1097/00003246-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Riker RR, Fraser GL. Adverse events associated with sedatives, analgesics, and other drugs that provide patient comfort in the Intensive Care Unit. Pharmacotherapy. 2005;25(5 Pt 2):8S–18S. doi: 10.1592/phco.2005.25.5_part_2.8s. [DOI] [PubMed] [Google Scholar]
- 10.Gehlbach BK, Kress JP. Sedation in the Intensive Care Unit. Curr Opin Crit Care. 2002;8:290–8. doi: 10.1097/00075198-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–41. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Martin J, Franck M, Fischer M, Spies C. Sedation and analgesia in German Intensive Care Units: How is it done in reality? Results of a patient-based survey of analgesia and sedation. Intensive Care Med. 2006;32:1137–42. doi: 10.1007/s00134-006-0214-6. [DOI] [PubMed] [Google Scholar]
- 13.Whipple JK, Lewis KS, Quebbeman EJ, Wolff M, Gottlieb MS, Medicus-Bringa M, et al. Analysis of pain management in critically ill patients. Pharmacotherapy. 1995;15:592–9. doi: 10.1002/j.1875-9114.1995.tb02868.x. [DOI] [PubMed] [Google Scholar]
- 14.Mehta S, Burry L, Fischer S, Martinez-Motta JC, Hallett D, Bowman D, et al. Canadian survey of the use of sedatives, analgesics, and neuromuscular blocking agents in critically ill patients. Crit Care Med. 2006;34:374–80. doi: 10.1097/01.ccm.0000196830.61965.f1. [DOI] [PubMed] [Google Scholar]
- 15.Guldbrand P, Berggren L, Brattebö G, Mälstam J, Rönholm E, Winsö O. Scandinavian Critical Care Trials Group. Survey of routines for sedation of patients on controlled ventilation in Nordic Intensive Care Units. Acta Anaesthesiol Scand. 2004;48:944–50. doi: 10.1111/j.1399-6576.2004.00445.x. [DOI] [PubMed] [Google Scholar]
- 16.Egerod I, Christensen BV, Johansen L. Trends in sedation practices in Danish Intensive Care Units in 2003: A national survey. Intensive Care Med. 2006;32:60–6. doi: 10.1007/s00134-005-2856-1. [DOI] [PubMed] [Google Scholar]
- 17.Murdoch S, Cohen A. Intensive care sedation: A review of current British practice. Intensive Care Med. 2000;26:922–8. doi: 10.1007/s001340051282. [DOI] [PubMed] [Google Scholar]
- 18.Tonner PH, Weiler N, Paris A, Scholz J. Sedation and analgesia in the Intensive Care Unit. Curr Opin Anaesthesiol. 2003;16:113–21. doi: 10.1097/00001503-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Schaffrath E, Kuhlen R, Tonner PH. Analgesia and sedation in intensive care medicine. Anaesthesist. 2004;53:1111–30. doi: 10.1007/s00101-004-0773-2. [DOI] [PubMed] [Google Scholar]
- 20.Putensen C, Zech S, Wrigge H, Zinserling J, Stüber F, Von Spiegel T, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med. 2001;164:43–9. doi: 10.1164/ajrccm.164.1.2001078. [DOI] [PubMed] [Google Scholar]
- 21.Tung A, Rosenthal M. Patients requiring sedation. Crit Care Clin. 1995;11:791–802. [PubMed] [Google Scholar]
- 22.Elliott R, McKinley S, Aitken LM, Hendrikz J. The effect of an algorithm-based sedation guideline on the duration of mechanical ventilation in an Australian Intensive Care Unit. Intensive Care Med. 2006;32:1506–14. doi: 10.1007/s00134-006-0309-0. [DOI] [PubMed] [Google Scholar]
- 23.Bucknall TK, Manias E, Presneill JJ. A randomized trial of protocol-directed sedation management for mechanical ventilation in an Australian Intensive Care Unit. Crit Care Med. 2008;36:1444–50. doi: 10.1097/CCM.0b013e318168f82d. [DOI] [PubMed] [Google Scholar]
- 24.Egerod I, Christensen BV, Johansen L. Nurses’ and physicians’ sedation practices in Danish ICUs in 2003 A national survey. Intensive Crit Care Nurs. 2006;22:22–31. doi: 10.1016/j.iccn.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Martin E, Ramsay G, Mantz J, Sum-Ping ST. The role of the alpha2-adrenoceptor agonist dexmedetomidine in postsurgical sedation in the Intensive Care Unit. J Intensive Care Med. 2003;18:29–41. doi: 10.1177/0885066602239122. [DOI] [PubMed] [Google Scholar]
- 26.Samuelson KA, Larsson S, Lundberg D, Fridlund B. Intensive care sedation of mechanically ventilated patients: A national Swedish survey. Intensive Crit Care Nurs. 2003;19:350–62. doi: 10.1016/s0964-3397(03)00065-x. [DOI] [PubMed] [Google Scholar]
- 27.Soliman HM, Mélot C, Vincent JL. Sedative and analgesic practice in the Intensive Care Unit: The results of a European survey. Br J Anaesth. 2001;87:186–92. doi: 10.1093/bja/87.2.186. [DOI] [PubMed] [Google Scholar]
- 28.Mehta S, Meade MO, Hynes P, Filate WA, Burry L, Hallett D, et al. A multicenter survey of Ontario Intensive Care Unit nurses regarding the use of sedatives and analgesics for adults receiving mechanical ventilation. J Crit Care. 2007;22:191–6. doi: 10.1016/j.jcrc.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): A randomised controlled trial. Lancet. 2008;371:126–34. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 30.Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114:541–8. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- 31.Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, et al. Lorazepam is an independent risk factor for transitioning to delirium in Intensive Care Unit patients. Anesthesiology. 2006;104:21–6. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Hughes CG, Pandharipande PP. Review articles: The effects of perioperative and Intensive Care Unit sedation on brain organ dysfunction. Anesth Analg. 2011;112:1212–7. doi: 10.1213/ANE.0b013e318215366d. [DOI] [PubMed] [Google Scholar]
- 33.Zapantis A, Leung S. Tolerance and withdrawal issues with sedation. Crit Care Nurs Clin North Am. 2005;17:211–23. doi: 10.1016/j.ccell.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Galley HF, Dubbels AM, Webster NR. The effect of midazolam and propofol on interleukin-8 from human polymorphonuclear leukocytes. Anesth Analg. 1998;86:1289–93. doi: 10.1097/00000539-199806000-00030. [DOI] [PubMed] [Google Scholar]
- 35.Nseir S, Makris D, Mathieu D, Durocher A, Marquette CH. Intensive Care Unit-acquired infection as a side effect of sedation. Crit Care. 2010;14:R30. doi: 10.1186/cc8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGrane S, Pandharipande PP. Sedation in the Intensive Care Unit. Minerva Anestesiol. 2012;78:369–80. [PubMed] [Google Scholar]
- 37.Fan E. Critical illness neuromyopathy and the role of physical therapy and rehabilitation in critically ill patients. Respir Care. 2012;57:933–44. doi: 10.4187/respcare.01634. [DOI] [PubMed] [Google Scholar]
- 38.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–93. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 39.Brattebø G, Hofoss D, Flaatten H, Muri AK, Gjerde S, Plsek PE. Effect of a scoring system and protocol for sedation on duration of patients’ need for ventilator support in a surgical Intensive Care Unit. BMJ. 2002;324:1386–9. doi: 10.1136/bmj.324.7350.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacLaren R, Plamondon JM, Ramsay KB, Rocker GM, Patrick WD, Hall RI. A prospective evaluation of empiric versus protocol-based sedation and analgesia. Pharmacotherapy. 2000;20:662–72. doi: 10.1592/phco.20.7.662.35172. [DOI] [PubMed] [Google Scholar]
- 41.Mascia MF, Koch M, Medicis JJ. Pharmacoeconomic impact of rational use guidelines on the provision of analgesia, sedation, and neuromuscular blockade in critical care. Crit Care Med. 2000;28:2300–6. doi: 10.1097/00003246-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 42.Bion JF, Ledingham IM. Sedation in intensive care – A postal survey. Intensive Care Med. 1987;13:215–6. [PubMed] [Google Scholar]
- 43.Mehta S, McCullagh I, Burry L. Current sedation practices: Lessons learned from international surveys. Crit Care Clin. 2009;25:471. doi: 10.1016/j.ccc.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Wøien H, Stubhaug A, Bjørk IT. Analgesia and sedation of mechanically ventilated patients – A national survey of clinical practice. Acta Anaesthesiol Scand. 2012;56:23–9. doi: 10.1111/j.1399-6576.2011.02524.x. [DOI] [PubMed] [Google Scholar]
- 45.Rozendaal FW, Spronk PE, Snellen FF, Schoen A, van Zanten AR, Foudraine NA, et al. Remifentanil-propofol analgo-sedation shortens duration of ventilation and length of ICU stay compared to a conventional regimen: A centre randomised, cross-over, open-label study in the Netherlands. Intensive Care Med. 2009;35:291–8. doi: 10.1007/s00134-008-1328-9. [DOI] [PubMed] [Google Scholar]
- 46.Fraser GL, Riker RR. Sedation and analgesia in the critically ill adult. Curr Opin Anaesthesiol. 2007;20:119–23. doi: 10.1097/ACO.0b013e32808255b4. [DOI] [PubMed] [Google Scholar]
- 47.Fraser GL, Riker RR. Comfort without coma: Changing sedation practices. Crit Care Med. 2007;35:635–7. doi: 10.1097/01.CCM.0000254072.83323.4E. [DOI] [PubMed] [Google Scholar]
- 48.Carson SS, Kress JP, Rodgers JE, Vinayak A, Campbell-Bright S, Levitt J, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med. 2006;34:1326–32. doi: 10.1097/01.CCM.0000215513.63207.7F. [DOI] [PubMed] [Google Scholar]
- 49.Christensen BV, Thunedborg LP. Use of sedatives, analgesics and neuromuscular blocking agents in Danish ICUs 1996/97. A national survey. Intensive Care Med. 1999;25:186–91. doi: 10.1007/s001340050814. [DOI] [PubMed] [Google Scholar]
- 50.Martin J, Heymann A, Bäsell K, Baron R, Biniek R, Bürkle H, et al. Evidence and consensus-based German guidelines for the management of analgesia, sedation and delirium in intensive care – Short version. Ger Med Sci. 2010;8:Doc02. doi: 10.3205/000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brodner G, Van Aken H, Hertle L, Fobker M, Von Eckardstein A, Goeters C, et al. Multimodal perioperative management – Combining thoracic epidural analgesia, forced mobilization, and oral nutrition – Reduces hormonal and metabolic stress and improves convalescence after major urologic surgery. Anesth Analg. 2001;92:1594–600. doi: 10.1097/00000539-200106000-00049. [DOI] [PubMed] [Google Scholar]
- 52.Rodgers A, Walker N, Schug S, McKee A, Kehlet H, van Zundert A, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: Results from overview of randomised trials. BMJ. 2000;321:1493. doi: 10.1136/bmj.321.7275.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strøm T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: A randomised trial. Lancet. 2010;375:475–80. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 54.Strøm T, Stylsvig M, Toft P. Long-term psychological effects of a no-sedation protocol in critically ill patients. Crit Care. 2011;15:R293. doi: 10.1186/cc10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: Validity and reliability in adult Intensive Care Unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 56.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, et al. Monitoring sedation status over time in ICU patients: Reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–91. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 57.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]


