Abstract
Background:
As the use of colistin to treat carbapenem-resistant Gram-negative infections increases, colistin resistance is being increasingly reported in Indian hospitals.
Materials and Methods:
Retrospective chart review of clinical data from patients with colistin-resistant isolates (minimum inhibitory concentration >2 mcg/ml). Clinical profile, outcome, and antibiotics that were used for treatment were analyzed.
Results:
Twenty-four colistin-resistant isolates were reported over 18 months (January 2014-June 2015). A history of previous hospitalization within 3 months was present in all the patients. An invasive device was used in 22 (91.67%) patients. Urine was the most common source of the isolate, followed by blood and respiratory samples. Klebsiella pneumoniae constituted 87.5% of all isolates. Sixteen (66.6%) were considered to have true infection, whereas eight (33.3%) were considered to represent colonization. Susceptibility of these isolates to other drugs tested was tigecycline in 75%, chloramphenicol 62.5%, amikacin 29.17%, co-trimoxazole 12.5%, and fosfomycin (sensitive in all 4 isolates tested). Antibiotics that were used for treatment were combinations among the following antimicrobials-tigecycline, chloramphenicol, fosfomycin, amikacin, ciprofloxacin, co-trimoxazole, and sulbactam. Among eight patients who were considered to have colonization, there were no deaths. Bacteremic patients had a significantly higher risk of death compared to all nonbacteremic patients (P = 0.014).
Conclusions:
Colistin resistance among Gram-negative bacteria, especially K. pneumoniae, is emerging in Indian hospitals. At least one-third of isolates represented colonization only rather than true infection and did not require treatment. Among patients with true infection, only 25% had a satisfactory outcome and survived to discharge. Fosfomycin, tigecycline, and chloramphenicol may be options for combination therapy.
Keywords: Colistin resistance, combination therapy, outcome
INTRODUCTION
Hospital-associated infections caused by multidrug-resistant (MDR) Gram-negative bacteria (GNB), especially Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, represent a growing problem worldwide. Over the last decade, an increase in carbapenem-resistant isolates has resulted in the widespread use of combination therapy with colistin as the backbone of the regimen.[1] However, colistin resistance is beginning to emerge, raising with it the specter of untreatable Gram-negative infection resulting in difficult antibiotic therapy.[2] We analyzed the epidemiological and clinical profile and outcome of patients with colistin-resistant Gram-negative isolates at a tertiary care hospital in India. We conducted a retrospective chart review of patients admitted at a 700-bed tertiary care hospital, from whom colistin-resistant GNB were isolated.
MATERIALS AND METHODS
We identified all colistin-resistant Gram-negative bacilli (minimum inhibitory concentration (MIC) >2 mcg/ml) from our Microbiology Department between January 2014 and June 2015. Organisms that are intrinsically resistant to colistin such as Stenotrophomonas, Burkholderia, Proteus, Serratia, Morganella, and Providencia are not tested and were excluded. Susceptibility testing for colistin was done by VITEK 2, and MIC was reported based on Clinical and Laboratory Standards Institute guidelines. The isolates were also tested against piperacillin-tazobactam, gentamicin, amikacin, ceftazidime, cefoperazone-sulbactam, cefepime, imipenem, meropenem, ciprofloxacin, trimethoprim/sulfamethoxazole, chloramphenicol, and tigecycline using VITEK 2. Fosfomycin sensitivity was checked manually.
Patient records were then analyzed for variables such as age, sex, comorbidities, Intensive Care Unit (ICU) stay, presence of indwelling devices, prior antibiotic exposure within 3 months, colonization versus true infection, antibiotics used, clearance of infection, and outcome. Acute Physiology and Chronic Health Evaluation (APACHE) score and Charlson Comorbidity Index were also calculated to corroborate with outcome. Outcome of patients, who were considered to have true infection and treated, was analyzed.
Clearance from the Ethics Committee of the hospital was obtained before the commencement of the study.
RESULTS
Twenty-four colistin-resistant isolates were reported over 18 months (January 2014-June 2015). Colistin MIC was >16 mcg/ml in 14 isolates (58.33%), 8-16 mcg/ml in 3 isolates (12.50%), and 4-8 mcg/ml in 7 (29.17%) isolates. All these isolates were resistant to cephalosporins, piperacillin-tazobactam, cefoperazone-sulbactam, and carbapenems. The mean age of the patients was 58.33 and median length of stay was 24.5 days. The average APACHE II score was 12.66. Comorbidities present were diabetes in 12 (50%) isolates, chronic kidney disease (CKD) in 12 (50%), chronic liver disease in 6 (25%), heart disease in 3 (12.5%), and chronic obstructive pulmonary disease in 1 (4.17%) isolate.
Previous hospitalization within 3 months was noted in all 24 patients. An invasive device was used in 22 (91.67%) patients. Urine (8 [33%]) was the most common source of the isolate, followed by blood (6 [25%]), respiratory (5 [20.8%]), pus (4 [16.67%]), and cerebrospinal fluid (1 [4.17%]). K. pneumoniae constituted 21 of the 24 (87.5%) organisms, with one isolate each of Escherichia coli, Enterobacter, and Acinetobacter. No colistin-resistant Pseudomonas isolate was encountered. Nineteen of these patients had positive cultures from one other site (pus, respiratory sample, urine, and blood), of which nine were carbapenem-resistant GNB.
Antibiotic exposure in a previous hospitalization (within 3 months) was analyzed [Figure 1]: colistin was used in 1 patient, carbapenem in 11, and beta-lactam-beta-lactamase inhibitor (BL-BLI) in 16 patients. Antibiotics that were used in their current admission before isolating a colistin-resistant organism were colistin in 15 patients (62.5%), carbapenem in 19 (79.17%), BL-BLI in 9 (37.5%), and tigecycline in 12 (50%) patients.
Figure 1.
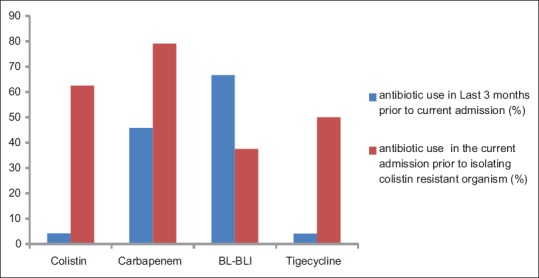
Antibiotic exposure in the past 3 months
Sensitivity of isolates to other drugs tested was shown in Figure 2: tigecycline 18/24 (75%), doxycycline 5/24 (20.8%), chloramphenicol 15/24 (62.5%), amikacin 7/24 (29.17%), and co-trimoxazole 3/24 (12.5%). Ciprofloxacin was sensitive in only one isolate among the 24 isolates, whereas fosfomycin was tested in 4 isolates only and was susceptible in all.
Figure 2.
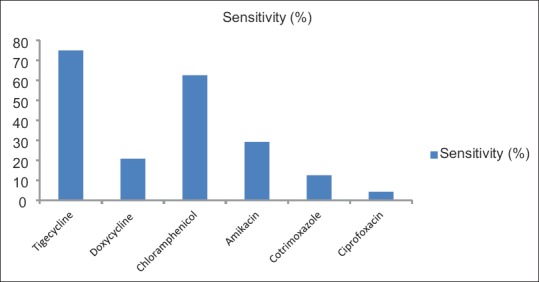
Sensitivity of the colistin-resistant isolates to other drugs tested
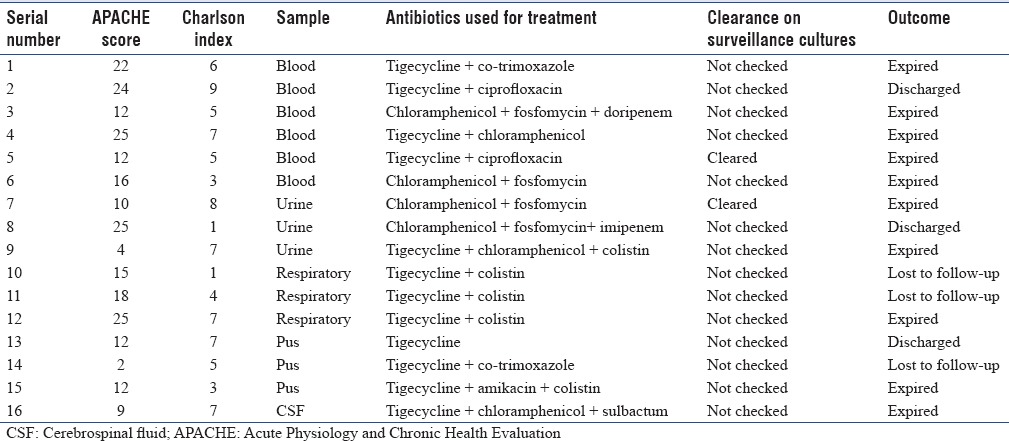
Sixteen patients (66.6%) were considered to have true infection, whereas eight (33.3%) were considered as colonization and were not treated. Among eight patients who were considered to have colonization, there were no deaths and seven (87.5%) improved. Antibiotics that were used for treatment were combinations of tigecycline, chloramphenicol, fosfomycin, amikacin, ciprofloxacin, co-trimoxazole, and sulbactam. Among 16 patients with true infection, 4 (25%) improved and were discharged, 9 (56.25%) expired, and 3 (18.75%) were transferred in poor condition for palliative care to other centers at family request. Among the six bacteremic cases, five patients expired and one improved. Among the nonbacteremic patients with true infections, 4/10 (40%) patients expired. Bacteremic patients had a significantly higher risk of death compared to all nonbacteremic patients (P = 0.014) though this was not significant after exclusion of colonized patients (P = 0.145). Antibiotic combinations used for treatment in those with true infection are shown in Table 1.
Table 1.
Patient profile, antibiotic combination used, and outcome
DISCUSSION
The increase in colistin use to treat carbapenem resistant bacteria unfortunately has resulted in the emegence of colistin resistance in GNB by definition considered as extensively drug resistant GNB.[3] Unfortunately, this has resulted in the emergence of colistin resistance in GNB, by definition considered drug-resistant GNB (extensively drug resistant).[4] The emergence of mobilized colistin resistance-1-mediated colistin resistance carried on plasmids further complicates the issue, and therefore, it is important to understand the epidemiology and define treatment approaches to colistin-resistant bacteria, while at the same time making efforts to limit spread.[5]
Initial reports of colistin resistance were in Acinetobacter isolates. As early as in 2001, a Spanish study by Valero et al. noted colistin resistance among Acinetobacter species.[6] Isolates from Greece also revealed resistance to polymyxin in 2007.[7] Data from the SENTRY study on global antimicrobial surveillance program between 2001 and 2004 showed polymyxin B resistance in Acinetobacter species ranging between 2.7% and 3.3%.[8] In 2011, a multicentric study from Kuwait pointed a relatively high rate of colistin resistance of 12% among the 250 Acinetobacter isolates studied.[9]
In 2011, an Indian study on colistin resistance among Acinetobacter in urine samples done during 2007-2008 revealed colistin resistance of around 3.5%.[10] Qureshi et al. report on a case series of patients with isolation of colistin-resistant and carbapenem-resistant A. baumannii (CRAB). In some of the cases described by the authors, the isolates have become truly pandrug resistant (PDR) with resistance seen to all tested antimicrobials.[11] In this study, we had only one Acinetobacter isolate: the majority were K. pneumoniae (87.5%), the other organisms being one isolate each of E. coli, Enterobacter, and Acinetobacter. Among carbapenem-resistant K. pneumoniae, 17% resistance rate to colistin has been reported in Taiwan.[12] In the study by Ghafur et al., 7 out of 13 clinical isolates were Klebsiella.[13] An Indian study by Goel et al. described 24 isolates of colistin-resistant K. pneumoniae in an oncology center located in eastern India during 2013-2014.[14] An Indian study to determine colistin resistance among Gram-negative isolates, with colistin MIC assessed by agar dilution method and without clinical data, showed that among 94 MDR isolates, 27 showed colistin resistance and among 9 K. Pneumoniae isolates 6 were colistin resistant.[15] Colistin resistance among other isolates in the above study was as follows: E. coli (9/48), P. Aeruginosa (3/10), A. baumannii (1/2), Proteus mirabilis (4/5), Enterobacter cloacae (1/3), Proteus rettgeri (2/2), and Salmonella enterica (1/4).
Transmission of colistin-resistant K. pneumoniae within hospital has always been a concern, and several outbreaks have been reported. In an acute general hospital in Italy between June and December 2011, 58 colistin-resistant K. pneumoniae isolates were recovered from 28 patients admitted to different wards but mainly in the ICUs.[16] An Euro surveillance study revealed that 43% of carbapenemase-producing K. pneumoniae isolates were resistant to colistin in Italy.[17] Several other outbreaks of colistin-resistant Klebsiella have been reported in hospitals, depicting both emergence of resistance and transmission of resistant clones of Klebsiella.[18,19] However, studies looking into clinical profile and outcome of these patients are scarce.[13]
The mean age of patients in our study was 58.3 years, which is comparable to the study by Ghafur et al.[13] Previous hospitalization within 3 months was noted in all our patients, and most had a prolonged current hospital stay before a positive culture of colistin resistant isolate; these factors are reflective of comorbidities, a “sick” state in general and antibiotic exposure. Previous hospitalization and stay in long-term care facility as a risk factor are noted in previous studies as well.[20] An invasive device was used in 22 (91.67%) patients, reflecting the underlying disease of the patient and the need for a portal of entry for infection to occur.
Prior antibiotic exposure is considered an important risk factor for colistin resistance. In our study, antibiotics used in previous hospitalizations within 3 months were BL-BLI (16 patients) followed by carbapenem (11 patients). Qureshi et al. found that colistin-resistant A. baumannii occurred almost exclusively among patients who had received colistin methane sulfate for treatment of carbapenem resistant (CRAB), colistin susceptible A. baumannii infection.[11] However, there are previous reports of independent emergence of colistin-resistant Enterobacteriaceae isolates without colistin exposure.[21] In this study done between 2009 and 2010 on 82 carbapenem resistance Enterobacteriaceae isolates, four were colistin resistant (3 Klebsiella and 1 E. cloacae) for whom no prior therapy with colistin was noted.
Among the drug tested for sensitivity, tigecycline 18/24 (75%) had a good susceptibility followed by chloramphenicol 15/24 (62.5%). Aminoglycosides 7/24 (29.17%), doxycycline 5/24 (20.8%), and co-trimoxazole 3/24 (12.5%) all had poor sensitivity. Ciprofloxacin was sensitive in only one isolate among the 24 isolates. Fosfomycin was tested in four isolates only and was sensitive in all. These are similar to two other studies from India.[13,14]
Tigecycline was the most common antibiotic (12/16) used in combination with other drugs, even used for bacteremic patients, due to lack of treatment options. Chloramphenicol was used in seven patients in combination with an another agent; the drug has variable sensitivity in different studies.[22,23,24] Fosfomycin was used as intravenously in four patients in whom it was found to be susceptible. Only one patient who received a combination of tigecycline and ciprofloxacin cleared bacteremia and improved. The treatment regimen for colistin-resistant A. baumannii infection associated with the lowest mortality rate was noted in his study by Qureshi et al. as a combination of colistin, a carbapenem, and ampicillin-sulbactam.[11] In the study by Tsiotutis, the drug that was commonly used was tigecycline.[25] High-dose tigecycline (200 mg daily) in combination with colistin has been tried.[26]
Another drug that has reemerged for use against drug-resistant GNB is fosfomycin. Falagas et al. systematically reviewed 17 studies (accounting for 5057 clinical isolates of Enterobacteriaceae) evaluating the antimicrobial activity and clinical effectiveness of fosfomycin for infections caused by MDR Enterobacteriaceae including ESBL. Eleven studies reported that at least 90% of the isolates were susceptible to fosfomycin.[27]
In our study, 66.6% were true infection whereas 33.3% were colonizers, similar to the study by Ghafur et al., in which 5 of 13 isolates (38%) were colonizers.[19] Among the eight patients who were considered to have colonization, there were no deaths and seven (87.5%) improved, highlighting the role of making this distinction. In nonbacteremic isolates, it is essential to differentiate true infection and colonization to prevent the unwarranted use of antibiotics, as patients with colonization in our study had a good outcome as compared to true infections. Six out of our 24 isolates were from blood, of whom 5 expired. Bacteremic patients carried a very high mortality of 83.3% which is similar to the other study from India where three out of four patients with PDR died.[13] High APACHE score, prolonged hospital stay, invasive devices, presence of diabetes and CKD, and blood stream infections were associated with mortality. Among the nonbacteremic patients with true infections, 4/10 (40%) patients expired. In the study by Goel et al., 21 out of 24 were true infections and 3 were colonizers; out of 24, 10 were bacteremic, overall mortality was 25% (6 out 24 expired), but the outcome of bacteremic patients was not stated.[14] Resistance to colistin is independently associated with worst outcomes and was observed in other studies as well.[28]
CONCLUSIONS
Colistin resistance among GNB, especially K. pneumoniae, is emerging in Indian hospitals. Recent hospitalization, prolonged current hospitalization (median of 24.5 days), presence of diabetes and CKD, use of invasive devices, and prior colistin exposure were all commonly seen. At least one-third of isolates represented colonization rather than true infection, highlighting the role of the clinician in making this distinction. Among patients with true infection, only 25% had a satisfactory outcome and survived to discharge, with bacteremia carrying an even poorer prognosis. Fosfomycin, tigecycline, and chloramphenicol may be options for combination therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, et al. Colistin: The re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 2.Antoniadou A, Kontopidou F, Poulakou G, Koratzanis E, Galani I, Papadomichelakis E, et al. Colistin-resistant isolates of Klebsiella pneumoniae emerging in intensive care unit patients: First report of a multiclonal cluster. J Antimicrob Chemother. 2007;59:786–90. doi: 10.1093/jac/dkl562. [DOI] [PubMed] [Google Scholar]
- 3.Bialvaei AZ, Samadi Kafil H. Colistin, mechanisms and prevalence of resistance. Curr Med Res Opin. 2015;31:707–21. doi: 10.1185/03007995.2015.1018989. [DOI] [PubMed] [Google Scholar]
- 4.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 5.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–8. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 6.Valero E, Sevillano D, Calvo A, García R, Leturia A, Gómez-Lus ML. Activity of new fluoroquinolones against clinical isolates of Acinetobacter baumannii. Rev Esp Quimioter. 2001;14:358–63. [PubMed] [Google Scholar]
- 7.Falagas ME, Bliziotis IA. Pandrug-resistant Gram-negative bacteria: The dawn of the post-antibiotic era? Int J Antimicrob Agents. 2007;29:630–6. doi: 10.1016/j.ijantimicag.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Gales AC, Jones RN, Sader HS. Global assessment of the antimicrobial activity of polymyxin B against 54 731 clinical isolates of Gram-negative bacilli: Report from the SENTRY antimicrobial surveillance programme (2001-2004) Clin Microbiol Infect. 2006;12:315–21. doi: 10.1111/j.1469-0691.2005.01351.x. [DOI] [PubMed] [Google Scholar]
- 9.Al-Sweih NA, Al-Hubail MA, Rotimi VO. Emergence of tigecycline and colistin resistance in Acinetobacter species isolated from patients in Kuwait hospitals. J Chemother. 2011;23:13–6. doi: 10.1179/joc.2011.23.1.13. [DOI] [PubMed] [Google Scholar]
- 10.Taneja N, Singh G, Singh M, Sharma M. Emergence of tigecycline and colistin resistant Acinetobacter baumanii in patients with complicated urinary tract infections in north India. Indian J Med Res. 2011;133:681–4. [PMC free article] [PubMed] [Google Scholar]
- 11.Qureshi ZA, Hittle LE, O’Hara JA, Rivera JI, Syed A, Shields RK, et al. Colistin-resistant Acinetobacter baumannii: Beyond carbapenem resistance. Clin Infect Dis. 2015;60:1295–303. doi: 10.1093/cid/civ048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu SK, Wu TL, Chuang YC, Lin JC, Fung CP, Lu PL, et al. National surveillance study on carbapenem non-susceptible Klebsiella pneumoniae in Taiwan: The emergence and rapid dissemination of KPC-2 carbapenemase. PLoS One. 2013;8:e69428. doi: 10.1371/journal.pone.0069428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghafur A, Vidyalakshmi PR, Murali A, Priyadarshini K, Thirunarayan MA. Emergence of pan-drug resistance amongst gram negative bacteria! The first case series from India. J Microbiol Infect Dis. 2014;4:86–91. [Google Scholar]
- 14.Goel G, Hmar L, Sarkar De M, Bhattacharya S, Chandy M. Colistin-resistant Klebsiella pneumoniae: Report of a cluster of 24 cases from a new oncology center in Eastern India. Infect Control Hosp Epidemiol. 2014;35:1076–7. doi: 10.1086/677170. [DOI] [PubMed] [Google Scholar]
- 15.Ramesh N, Gothandam KM, Prasanth M, Ramkumar S, Karthikeyan S, Bozdogan B, et al. Colistin susceptibility of gram-negative clinical isolates from Tamil Nadu, India. Asian Biomed. 2016;10:35–9. [Google Scholar]
- 16.Mammina C, Bonura C, Di Bernardo F, Aleo A, Fasciana T, Sodano C, et al. Ongoing spread of colistin-resistant Klebsiella pneumoniae in different wards of an acute general hospital, Italy, June to December 2011. Euro Surveill. 2012;17:pii:20248. [PubMed] [Google Scholar]
- 17.Monaco M, Giani T, Raffone M, Arena F, Garcia-Fernandez A, Pollini S, et al. Colistin resistance superimposed to endemic carbapenem-resistant Klebsiella pneumoniae: A rapidly evolving problem in Italy, November 2013 to April 2014. Euro Surveill. 2014;19:pii:20939. doi: 10.2807/1560-7917.es2014.19.42.20939. [DOI] [PubMed] [Google Scholar]
- 18.Bogdanovich T, Adams-Haduch JM, Tian GB, Nguyen MH, Kwak EJ, Muto CA, et al. Colistin-resistant, Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae belonging to the international epidemic clone ST258. Clin Infect Dis. 2011;53:373–6. doi: 10.1093/cid/cir401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchaim D, Chopra T, Pogue JM, Perez F, Hujer AM, Rudin S, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother. 2011;55:593–9. doi: 10.1128/AAC.01020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heudorf U, Farber D, Mischler D, Schade M, Zinn C, Nillius D, et al. Multidrug-Resistant Organisms (MDRO) in rehabilitation clinics in the Rhine-Main-district, Germany, 2014: Prevalence and risk factors. Rehabilitation (Stuttg) 2015;54:339–45. doi: 10.1055/s-0035-1559642. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Hu F, Zhang X, Xu X, Liu Y, Zhu D, et al. Independent emergence of colistin-resistant Enterobacteriaceae clinical isolates without colistin treatment. J Clin Microbiol. 2011;49:4022–3. doi: 10.1128/JCM.01233-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sood S. Chloramphenicol-A potent armament against Multi-Drug Resistant (MDR) gram negative bacilli? J Clin Diagn Res. 2016;10:DC01–3. doi: 10.7860/JCDR/2016/14989.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathur P, Behera B, Chaturvedi D, Misra MC. Chloramphenicol: Is old really gold? J Assoc Physicians India. 2010;58:584. [PubMed] [Google Scholar]
- 24.Civljak R, Giannella M, Di Bella S, Petrosillo N. Could chloramphenicol be used against ESKAPE pathogens? A review of in vitro data in the literature from the 21 st century. Expert Rev Anti Infect Ther. 2014;12:249–64. doi: 10.1586/14787210.2014.878647. [DOI] [PubMed] [Google Scholar]
- 25.Tsioutis C, Kritsotakis EI, Maraki S, Gikas A. Infections by pandrug-resistant gram-negative bacteria: Clinical profile, therapeutic management, and outcome in a series of 21 patients. Eur J Clin Microbiol Infect Dis. 2010;29:301–5. doi: 10.1007/s10096-009-0857-7. [DOI] [PubMed] [Google Scholar]
- 26.Humphries RM, Kelesidis T, Dien Bard J, Ward KW, Bhattacharya D, Lewinski MA. Successful treatment of pan-resistant Klebsiella pneumoniae pneumonia and bacteraemia with a combination of high-dose tigecycline and colistin. J Med Microbiol. 2010;59:1383–6. doi: 10.1099/jmm.0.023010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum beta-lactamase producing, Enterobacteriaceae infections: A systematic review. Lancet Infect Dis. 2010;10:43–50. doi: 10.1016/S1473-3099(09)70325-1. [DOI] [PubMed] [Google Scholar]
- 28.Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, et al. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect. 2013;19:E23–30. doi: 10.1111/1469-0691.12070. [DOI] [PubMed] [Google Scholar]


