Abstract
Background:
Atherosclerosis (AS) is an inflammatory disease. Inflammation was considered to play a role in the whole process of AS. This study aimed to analyze the relationships of inflammatory factors and risk factors with different target organ damages (TOD) in essential hypertension (EH) patients and to explore its clinical significance.
Methods:
A total of 294 EH patients were selected and divided into four groups according to their conditions of TOD. Forty-eight healthy subjects were selected as control. The clinical biochemical parameters, serum amyloid A, serum tryptase, and lipoprotein-associated phospholipase A2 (Lp-PLA2) in each group were detected, and the related risk factors were also statistically analyzed.
Results:
Fibrinogen (Fbg) was the most significant independent risk factor in acute coronary syndrome (ACS) group (odds ratio [OR]: 22.242, 95% confidence interval [CI]: 6.458–76.609, P < 0.001) with the largest absolute value of the standardized partial regression coefficient B’ (b’: 1.079). Lp-PLA2 was the most significant independent risk factor in stroke group (OR: 13.699, 95% CI: 5.236–35.837, P < 0.001) with b’ = 0.708. Uric acid (UA) was the most significant independent risk factor in renal damage group (OR: 15.307, 95% CI: 4.022–58.250, P < 0.001) with b’ = 1.026.
Conclusions:
Fbg, Lp-PLA2, and UA are the strongest independent risk factors toward the occurrence of ACS, ischemic stroke, and renal damage in EH patients, thus exhibiting the greatest impacts on the occurrence of ACS, ischemic stroke, and renal damage in EH patients, respectively.
Keywords: Hypertension, Inflammatory Cytokines, Target Organ Damage
Introduction
Hypertension is a global problem, which occurs not only in developed countries, but also gradually in the developing countries. In China, the prevalence rate of hypertension is 29.6%, and the awareness, treatment and control rates among all hypertensive participants are 42.6%, 34.1%, and 9.3%, respectively. In treated hypertensive participants, the rate of blood pressure reaching standard is 27.4%.[1] In clinical practice, essential hypertension (EH) cannot be effectively controlled, leading to the specific target organ damage (TOD) such as heart disease, cerebrovascular disease, vascular dementia, renal failure, atherosclerotic vascular disease, and retinopathy[2] TOD caused by EH is a complex process involving biochemistry, inflammations, and hemodynamics.[3] It is found that atherosclerosis (AS) is the pathogenesis of the occurrence of different TOD in EH, and the inflammation hypothesis is also considered as the main mechanism of AS.[4]
Whether EH-AS associated inflammatory markers and high-risk factors can be used for diagnosis, risk stratification and targeted therapy against different TOD is the direction of current clinical studies. Furthermore, the relationships of EH-induced different TOD with inflammations and many risk factors have not been reported. This study investigated the serum inflammatory factors, clinical biochemical indices, and cardiovascular risk factors in healthy people, simple EH patients, and the EH patients accompanied with various TOD, aiming to analyze the relationships of inflammatory factors and related factors with different TOD in these EH patients.
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Shanxi Provincial People's Hospital. Informed written consent was obtained from all patients prior to their enrollment in this study.
Subjects
A total of 294 EH patients hospitalized in the Department of Cardiology, Shanxi Provincial People's Hospital, from June 2013 to December 2014 were selected, and according to the TOD criteria, these patients were further divided into five groups: Group A (patients with simple EH, 79 patients, aging 57.4 ± 13.0 years), Group B (EH combined with acute coronary syndrome (ACS), 85 patients, aging 58.6 ± 11.0 years), Group C (EH combined with stroke, 88 patients, aging 58.5 ± 12.6 years), Group D (EH combined with renal damage, 42 patients, aging 58.2 ± 13.5 years), and Group E (48 healthy controls, aging 57.4 ± 11.3 years).
Inclusion criteria
Diagnostic criteria of EH: According to the Chinese EH Prevention Guide (2011 revised edition), the diagnostic criteria of hypertension is systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg (without using any antihypertensive drugs, and the blood pressure is measured three times in different days), or the hypertension patients taking antihypertensive drugs. The EH classification were divided into three levels: Level 1, SBP 140–159 mmHg and/or DBP 90–99mmHg; Level 2, SBP 160–179 mmHg and/or DBP 100–109 mmHg; and Level 3, SBP ≥180 mmHg and/or DBP ≥110 mmHg.
Diagnostic criteria of essential hypertension combined with acute coronary syndrome
A group of clinical syndrome in which AS progresses to myocardial ischemia, including (1) ST-elevation myocardial infarction (STEMI) patients: occurred electrocardiogram (ECG) changes (persistent ST-segment elevation ≥0.1 mV, new Q-wave or new left bundle branch block), and/or troponin or creatine kinase-MB (CK-MB was increased. (2) Unstable angina/non-STEMI patients: occurred 10 min or more ischemic discomfort combined with ST-segment depression ≥1 mV when at rest, and/or troponin or CK-MB was increased. After admission, the coronary angiography using the Judkin's method revealed the stenosis (>50%) of more than one major coronary arteries. Diagnostic criteria of EH combined with acute ischemic stroke occurred acute stroke and thus being hospitalized within 2 weeks. The brain magnetic resonance imaging or computed tomography confirmed ischemic stroke.
Diagnostic criteria of essential hypertension combined with chronic kidney disease
With persistent hypertension-caused renal structural and functional changes, followed by persistent albuminuria (urinary albumin >300 mg/d), and (or) renal insufficiency (glomerular filtration rate < 60 ml·min−1·1.73 m−2); or the renal biopsy revealed the certain pathological changes.
Exclusion criteria
Secondary EH, EH combined with diabetes, autoimmune diseases, myocarditis, endocarditis, rheumatic heart disease, cancer, cirrhosis, acute and chronic infections, or thyroid dysfunction.
Collection of general clinical information
All the patients were enquired detailed disease histories, and their general situations, laboratory biochemical tests, and imaging results were set as the clinical baseline data. Fibrinogen (Fbg), cystatin, uric acid (UA), homocysteine (Hcy), and high-sensitivity C-reactive protein (hs-CRP) were routinely inspected after admission. Lipoprotein associated phospholipase A 2 (Lp-PLA2), serum amyloid A (SAA), and serum serum tryptase (TPS) were inflammatory factors associated with EH and AS. The determination of AS plaques was based on the ultrasound findings of carotid and femoral arteries.
Specimen collection and detection of serum inflammatory factors
A volume of 5 ml of fasting venous blood was sampled from each patient on the 2nd day of admission, and the healthy control were sampled 5 ml of venous blood during their physical inspection process. After naturally stood for 0.5 h, the blood specimen was centrifuged for 15 min (r = 5 cm, 3000 r/min); the serum was then separated, and the upper layer was placed in one Eppendorf tube for the detection using enzyme-linked immunosorbent assay (ELISA). The associated detection kits of SAA, TPS, and Lp-PLA2 were purchased from Westang Company (Shanghai, China). SAA, TPS, and Lp-PLA2 were measured by the enzyme-labeled instrument (Bio-Rad iMark; Bio-Rad, California Hercules, USA).
Statistical analysis
Statistical analysis was performed using SPSS (version 13.0, SPSS Inc., Chicago, IL, USA). The measurement data (normal distribution) were expressed as mean ± standard deviation (SD); the statistical analysis used analysis of variance (ANOVA), and the pairwise comparison used the least significant difference test under the conditions of meeting the homogeneity of variance, otherwise the Dunnett T3 comparison was performed; the nonnormally distributed measurement data were expressed as median (interquartile range), and the statistical analysis used the total random nonparametric test. The count data were expressed as rate, and performed the Chi-square test, with a P < 0.05 considered statistically significant. To obtain the cut-off values of Lp-PLA2, SAA, and TPS, we use the receiver-operating characteristic (ROC) curve of SPSS version 13.0. The logistic regression was performed to analyze the risk factors of different TOD, followed by the step-wise selection method and maximum likelihood ratio test; the introduced level α was set as 0.05 with the exclusion level α as 0.10; A value of P < 0.05 was considered statistically significant.
Results
Comparison of general clinical information
There existed no statistically significant differences in the age, smoking history, blood glucose, triglycerides, high-density lipoprotein, total cholesterol, low-density lipoprotein (LDL-C), and white blood cell count among the four groups (P > 0.05). On admission, the body mass index, gender, history of EH, EH grading, AS plaque, Fbg, UA, Hcy, hs-CRP, Lp-PLA2, SAA, and TPS of Group B–D were significantly higher than Group A (P < 0.05). The levels of serum creatinine and blood urea nitrogen in Group D were significantly higher than other groups [P < 0.05, Table 1].
Table 1.
Comparison of general clinical information among the five groups
| Items | Group E (n = 48) | Group A (n = 79) | Group B (n = 85) | Group C (n = 88) | Group D (n = 42) | Statistics values | P |
|---|---|---|---|---|---|---|---|
| Age (years) | 57.35 ± 11.29 | 57.44 ± 13.03 | 58.62 ± 11.04 | 58.51 ± 12.63 | 58.24 ± 13.45 | 0.166 | 0.956 |
| BMI (kg/m2) | 23.40 ± 2.86 | 26.19 ± 2.77 | 28.42 ± 3.07* | 28.20 ± 3.39*,† | 24.72 ± 2.52*,† | 32.121 | <0.001 |
| Fasting plasma glucose (mmol/L) | 5.09 ± 0.88 | 5.35 ± 1.68 | 5.46 ± 1.58 | 5.60 ± 1.55 | 5.36 ± 1.47 | 0.947 | 0.437 |
| CHO (mmol/L) | 4.47 ± 1.02 | 4.34 ± 1.09 | 4.36 ± 1.17 | 4.16 ± 1.05 | 4.46 ± 1.19 | 0.852 | 0.493 |
| LDL-C (mmol/L) | 2.73 ± 0.81 | 2.71 ± 0.82 | 2.68 ± 0.93 | 2.69 ± 0.79 | 2.75 ± 0.90 | 0.061 | 0.993 |
| TG (mmol/L) | 1.68 ± 0.96 | 1.65 ± 0.90 | 1.84 ± 0.83 | 1.54 ± 0.75 | 1.67 ± 1.14 | 1.250 | 0.290 |
| HDL-C (mmol/L) | 1.28 ± 0.56 | 1.18 ± 0.30 | 1.15 ± 0.32 | 1.15 ± 0.32 | 1.08 ± 0.30 | 1.937 | 0.104 |
| WBC (×109) | 6.13 ± 1.98 | 6.21 ± 1.70 | 6.54 ± 1.70 | 6.72 ± 1.87 | 6.42 ± 1.86 | 1.224 | 0.301 |
| Male, n (%) | 31 (64.60) | 37 (46.80) | 53*,† (62.35) | 55*,† (62.5) | 28* (66.67) | 8.190 | 0.042 |
| Smoking, n (%) | 5 (10.42) | 39 (49.37) | 37 (43.53) | 39 (44.32) | 19 (45.24) | 0.659 | 0.833 |
| Serum creatinine (µmol/L) | 79.10 ± 20.79 | 77.07 ± 20.20 | 82.85 ± 20.38 | 81.22 ± 18.43 | 165.37 (88.85, 382.11)* | 22.889 | <0.001 |
| BUN (µmol/L) | 5.41 ± 1.41 | 6.65 ± 12.84 | 6.00 ± 3.55 | 5.30 ± 1.89 | 10.46 (6.36, 20)* | 11.715 | <0.001 |
| Family history, n (%) | 3 (6.25) | 41 (51.90) | 49 (81.18) | 46 (47.73) | 1.716 | 0.424 | |
| History of EH (years) | – | 4 (1, 10) | 9 (3, 12.5)*,† | 7 (3, 9)*,† | 6.5 (2.75, 14.5) | 17.374 | <0.001 |
| EH grading, n (%) | |||||||
| Level 1 | – | 9 (11.39) | 2 (2.35) | 14 (15.91) | 0 | 29.176 | <0.001 |
| Level 2 | – | 25 (31, 65) | 21 (24.71) | 35 (39.77) | 7 (16.67) | ||
| Level 3 | – | 45 (56.96) | 61*,† (72.94) | 39*,† (44.32) | 35*,† (83.33) | ||
| With AS plaque, n (%) | 12 (25.00) | 67 (84.81) | 42*,† (49.41) | 52*,† (59.09) | – | 25.478 | <0.001 |
| Fibrinogen (g/L) | 2.27 ± 0.44 | 2.79 ± 0.54 | 3.55 ± 0.64*,† | 4.24 ± 1.13* | 4, 52 ± 0.72* | 171.041 | <0.001 |
| Cystatin (mg/L) | 0.80 ± 0.17 | 0.92 ± 0.26 | 1.05 ± 0.33* | 1.08 ± 0.44* | 2.06 ± 1.02* | 50.599 | <0.001 |
| UA (µmol/L) | 287.83 ± 95.06 | 313.81 ± 93.08 | 366.15 ± 74.46* | 379.29 ± 89.00*,† | 427.61 ± 139.36*,† | 17.509 | <0.001 |
| Homocysteine (µmol/L) | 13.10 ± 3.22 | 18.76 ± 3.98 | 22.06 ± 5.17* | 21.06 ± 6.04* | 23.06 ± 6.78* | 30.387 | <0.001 |
| hs-CRP (mg/L) | 0.95 (0.46, 1.77) | 2.01 (1.27, 3.54) | 3.79 (2.71, 5.08)*,† | 5.18 (6.62, 7.11)*,† | 10.01 (5.75, 13.25)*,† | 60.991 | <0.001 |
| Lp-PLA2 (μg/L) | 249.97 ± 101.72 | 343.89 ± 91.25 | 399.17 ± 91.42*,† | 395.52 ± 62.60*,† | 381.98 ± 59.53*,† | 31.228 | <0.001 |
| SAA (ng/ml) | 680.37 ± 229.58 | 1016.28 ± 239.29 | 1188.29 ± 267.22* | 1153.10 ± 290.50* | 1125.39 ± 293.65* | 33.284 | <0.001 |
| TPS (ng/ml) | 3.75 ± 0.72 | 5.73 ± 1.75 | 8.08 ± 2.10*,† | 8.08 ± 2.61* | 6.92 ± 3.12* | 42.557 | <0.001 |
*P<0.05 compared with Group E; †P<0.05 compared with Group A. Data were presented as mean ± SD or n (%) or median (P25, P75). Group A: patients with simple EH; Group B: EH combined with acute coronary syndrome; Group C: EH combined with stroke; Group D: EH combined with renal damage; Group E: healthy controls. BMI: Body mass index; CHO: Total cholesterol; LDL-C: Low-density lipoprotein-cholesterol; TG: Triglyceride; HDL-C: High-density lipoprotein-cholesterol; WBC: White blood cell count; BUN: Blood urea nitrogen; UA: Uric acid; hs-CRP: High-sensitivity C-reactive protein; Lp-PLA2: Lipoprotein associated phospholipase A2; SAA: Serum amyloid A; TPS: Serum tryptase; AS: Atherosclerosis; SD: Standard deviation; EH: Essential hypertension.
Comparison of essential hypertension-related risk factors with inflammatory factors using logistic regression analysis
The cut-off values of Lp-PLA2, SAA, and TPS were obtained from ROC curve [Figure 1]. The assignment of ACS-related clinical data, risk factors, and inflammatory factors in EH patients is shown in Table 2. The logistic regression analysis revealed seven independent risk factors that had significance toward the occurrence of ACS in these EH patients, namely Fbg, hs-CRP, TPS, Lp-PLA2, and EH classification and AS plaque, among which Fbg was the most significant independent risk factor in group ACS (odds ratio [OR]: 22.242, 95% confidence interval [CI]: 6.458–76.609, P = 0.001), indicating that the risk of the occurrence of ACS in EH patients with Fbg ≥4.98 g/L was 22 times than those with Fbg <4.98 g/L. The standardized partial regression coefficient of Fbg, B’, also had the largest absolute value (b’: 1.079), indicating that among the 8 assignments, Fbg had biggest impacts toward the occurrence of ACS in EH patients [Table 3].
Figure 1.
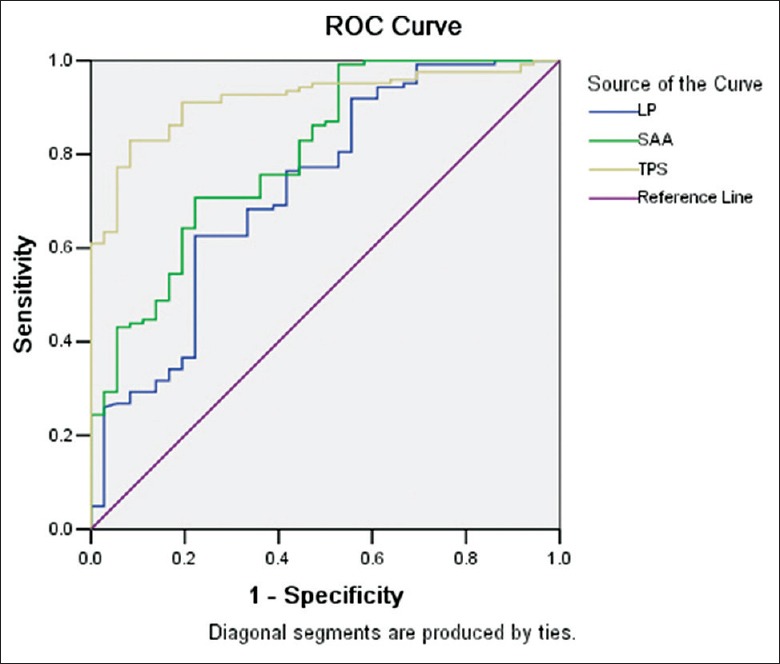
ROC curve of inflammatory factors. Lp-PLA2: Area under ROC curve, 0.730; cut-off, 358.33; sensitivity, 62.6%; specificity, 77.8%. SAA: Area under ROC curve, 0.802; cut-off, 997.72; sensitivity, 70.7%; specificity, 77.8%. TPS: Area under ROC curve, 0.918; cut-off, 5.83; sensitivity, 82.9%; specificity, 91.7%. ROC: Receiver-operating characteristic; Lp-PLA2: Lipoprotein associated phospholipase A2; SAA: Serum amyloid A; TPS: Serum tryptase.
Table 2.
Assignment table of CHD-ACS-related risk factors and inflammatory factors in EH patients
| Factors | Variable | Explanation of assignment |
|---|---|---|
| Gender | X1 | Male = 1, female = 2 |
| History of blood pressure (years) | X2 | <10 = 1, ≥10 = 2 |
| Hypertension grading | X3 | Level 1 = 1, Level 2 = 2, Level 3 = 3 |
| AS plaque | X4 | Yes = 1, no = 2 |
| Fibrinogen (g/L) | X5 | <4.98 = 1, ≥4.98 = 2 |
| hs-CRP (mg/L) | X6 | <3 = 1, ≥3 = 2 |
| Lp-PLA2 (µ/L) | X7 | <358.33 = 1, ≥358.33 = 2 |
| TPS (ng/ml) | X8 | <5.83 = 1, ≥5.83 = 2 |
| CHD-ACS | Y | EH = 0, EH with CHD-ACS = 1 |
The reference value of fibrinogen is 2.38–4.98 g/L, so we choose the right value of the reference value as the point of stratification. When the value of hs-CRP is bigger than 3 mg/L, one can suffer the CVD easier, so we choose 3 mg/L as the point of stratification. We use the ROC curve of SPSS 13.0 obtain the cut-off values of Lp-PLA2, SAA, TPS, so we choose the cut-off values of Lp-PLA2, SAA, TPS as the point of stratification, respectively. hs-CRP: High-sensitivity C-reactive protein; Lp-PLA2: Lipoprotein associated phospholipase A2; SAA: Serum amyloid A; TPS: Serum tryptase; AS: Atherosclerosis; EH: Essential hypertension; CHD-ACS: Coronary heart disease-acute coronary syndrome; CVD: Cardiovascular disease; ROC: Receiver-operating characteristic.
Table 3.
Logistic regression statistical results in Group A and B
| Variables | B | SE | Wald χ2 | P | OR | 95% CI | b’ |
|---|---|---|---|---|---|---|---|
| EH classification | 1.439 | 0.489 | 8.670 | 0.003 | 4.218 | 1.618–10.993 | 0.388 |
| Fibrinogen | 3.102 | 0.631 | 24.168 | <0.001 | 22.242 | 6.458–76.609 | 1.079 |
| Lp-PLA2 | 1.279 | 0.546 | 5.493 | 0.019 | 3.592 | 1.233–10.467 | 0.385 |
| hs-CRP | 1.767 | 0.579 | 9.325 | 0.002 | 5.853 | 1.833–18.193 | 0.564 |
| TPS | 2.956 | 0.636 | 21.620 | <0.001 | 19.230 | 5.530–66.865 | 1.037 |
| AS plaque | 2.431 | 0.653 | 13.840 | <0.001 | 11.367 | 3.159–40.905 | 0.875 |
OR: Odds ratio; CI: Confidence interval; SE: Standard error; hs-CRP: High-sensitivity C-reactive protein; Lp-PLA2: Lipoprotein associated phospholipase A2; TPS: Serum tryptase; AS: Atherosclerosis; EH: Essential hypertension.
The assignment of stroke-related clinical data, risk factors, and inflammatory factors in EH patients is shown in Table 4. The logistic regression analysis revealed four independent risk factors that had significance toward the occurrence of stroke in these EH patients, among which Lp-PLA2 was the most important independent risk factor (OR: 13.699, 95% CI: 5.236–35.837, P < 0.0001), indicating that the risk of the occurrence of stroke in EH patients with Lp-PLA2 ≥358.33 μg/L was 13.6 times than those with Lp-PLA2 <358.33 μg/L. The absolute B’ value of Lp-PLA2 was also the largest (b’: 0.708), indicating that among the 8 assignments, Lp-PLA2 had biggest impacts toward the occurrence of stroke in EH patients [Table 5].
Table 4.
Assignment table of ischemic stroke risk factors and inflammatory factors in EH patients
| Factors | Variable | Explanation of assignment |
|---|---|---|
| Gender | X1 | Male = 1, female = 2 |
| BMI (kg/m2) | X2 | <25 = 1, ≥25 = 2 |
| History of blood pressure (years) | X3 | <10 = 1, ≥10 = 2 |
| Hypertension grading | X4 | Level 1 = 1, Level 2 = 2, Level 3 = 3 |
| AS plaque | X5 | Yes = 1, no = 2 |
| UA (µmol/L) | X6 | <410 = 1, ≥410 = 2 |
| hs-CRP (mg/L) | X7 | <3 = 1, ≥3 = 2 |
| Lp-PLA2 (µ/L) | X8 | <358.33 = 1, ≥358.33 = 2 |
| Ischemic stroke | Y | EH = 0, EH with ischemic stroke = 1 |
The reference value of BMI is 18–25 kg/m2 and the reference value of UA is 150–410 µmol/L, so we choose the right value of the reference value as the point of stratification, resepectively. hs-CRP: High-sensitivity C-reactive protein; Lp-PLA2: Lipoprotein associated phospholipase A2; AS: Atherosclerosis; BMI: Body mass index; UA: Uric acid; EH: Essential hypertension.
Table 5.
Logistic regression statistical results in Group A and C
| Variables | B | SE | Wald χ2 | P | OR | 95% CI | b’ |
|---|---|---|---|---|---|---|---|
| BMI | 1.137 | 0.447 | 6.478 | 0.011 | 3.117 | 1.299–7.481 | 0.280 |
| UA | 1.721 | 0.533 | 10.443 | 0.001 | 5.590 | 1.968–15.877 | 0.506 |
| hs-CRP | 1.437 | 0.418 | 11.814 | 0.001 | 4.210 | 1.855–9.554 | 0.331 |
| Lp-PLA2 | 2.617 | 0.491 | 28.454 | <0.001 | 13.699 | 5.236–35.837 | 0.708 |
OR: Odds ratio; CI: Confidence interval; SE: Standard error; hs-CRP: High-sensitivity C-reactive protein; Lp-PLA2: Lipoprotein associated phospholipase A2; UA: Uric acid; BMI: Body mass index.
The assignments of risk factors and related inflammatory factors in EH combined with renal damage are shown in Table 6. The Logistic regression analysis revealed three independent risk factors that had significance toward the occurrence of renal damage in EH patients, among which UA were the most significant independent risk factors, and also was the most important independent risk factor (OR: 15.307, 95% CI: 4.022–58.250, P < 0.001), indicating that the risk of the occurrence of renal damage in EH patients with UA ≥410 μmol/L was 17 times than those with UA <410 μmol/L. The absolute value of B’ of UA was the largest (b’: 1.026), suggesting that UA had the largest impacts on the occurrence of renal damage in EH patients [Table 7].
Table 6.
Assignments of five risk factors and related inflammatory factors of EH-renal damage
| Factors | Variable | Explanation of assignment |
|---|---|---|
| BMI (kg/m2) | X1 | <25 = 1, ≥25 = 2 |
| Hypertension grading | X2 | Level 1 = 1, Level 2 = 2, Level 3 = 3 |
| UA (µmol/L) | X3 | <410 = 1, ≥410 = 2 |
| hs-CRP (mg/L) | X4 | <3 = 1, ≥3 = 2 |
| Lp-PLA2 (µg/L) | X5 | <358.33 = 1, ≥358.33 = 2 |
| Kidney damage | Y | EH = 0, EH with kidney damage = 1 |
hs-CRP: High-sensitivity C-reactive protein; Lp-PLA2: Lipoprotein associated phospholipase A2; UA: Uric acid; BMI: Body mass index; EH: Essential hypertension.
Table 7.
Logistic regression statistical results in Group A and D
| Variables | B | SE | Wald χ2 | P | OR | 95% CI | b’ |
|---|---|---|---|---|---|---|---|
| UA | 2.728 | 0.682 | 16.009 | <0.001 | 15.307 | 4.022–58.250 | 1.026 |
| Lp-PLA2 | 2.344 | 0.627 | 13.959 | <0.001 | 10.427 | 3.048–35.667 | 0.810 |
| hs-CRP | 2.104 | 0.603 | 12.193 | <0.001 | 8.199 | 2.517–26.710 | 0.699 |
OR: Odds ratio; CI: Confidence interval; SE: Standard error; hs-CRP: High-sensitivity C-reactive protein; Lp-PLA2: Lipoprotein associated phospholipase A2; UA: Uric acid.
Discussion
During the occurrence and development of EH-induced TOD, the involvement of chronic inflammation and AS may be the important cardiovascular features toward most TOD cases.[3] It has been proven that chronic inflammation may be involved in the origin and development of EH, as well as TOD in the heart, brain, kidney, and blood vessels.[4] These TOD cases also participate in the development of EH. Predicting future cardiovascular events in patients with AS is a challenge for cardiologists. In the recent years, with the growing understanding about the occurrence and development of AS, the inflammation hypothesis is considered as the main mechanism of AS, starting from the permeability changes of arterial endothelial functions caused by EH and hypercholesterolemia, followed by the adhesion and infiltration of inflammatory cells which representative the inflammatory reactions of AS. It has been found in a large number of experimental studies that different stages of AS inflammatory responses can be quantitatively monitored by inflammatory markers, and these inflammatory markers may reflect the pathophysiological changes in different AS stages.[5] The meta-analysis of clinical studies strongly suggests that inflammation involves in the whole process of the starting and development of AS, so inflammatory markers are meaningful predictors that can provide significance for the occurrence, development, and prognosis of AS.[6,7] In clinical practice, it has been proved that EH is the major risk factor leading to the formation and development of AS, as well as causing various degrees of TOD. Although EH itself is undoubtedly a major factor toward hypertension-TOD, certain evidence also indicate that some related inflammatory markers are also crucial in the mechanisms of TOD. Furthermore, targeted intervening inflammatory markers will prevent and reduce the risks of TOD and overall cardiovascular events.[8,9] Whether there exist the levels of significance among TOD-related risk factors and inflammatory markers in EH patients, and what will their clinical application significance be still need further studies.
This study investigated the relationships between various risk factors and chronic inflammatory markers in EH with different TOD. The results showed Fbg, hs-CRP, SAA, TPS, and AS plaque were important independent risk factors for the occurrence of CHD-ACS in EH patients, among which Fbg was the strongest independent risk factor toward CHD-ACS in EH, indicating that the risk of the occurrence of ACS in EH patients with Fbg ≥4.98 g/L was 22 times than those with Fbg < 4.98 g/L. The absolute value of B’ of Fbg is also the largest (B’: 1.079), indicating that among the 8 assignments, Fbg had biggest impacts toward the occurrence of ACS in EH patients.
There is evidence showing that coronary plaque rupture and thrombosis lead to the occurrence of ACS. The occurrence of ACS in EH patients is through a variety of mechanisms that lead to AS plaque rupture and thrombosis. Studies have found that plasma Fbg and its degradation products can stimulate the proliferation and migration of vascular smooth muscle cells, suggesting that in the early formation stage of AS plaques, Fbg is involved.[9,10] As a pro-coagulation protein with short half-life, Fbg not only participates in the chronic inflammatory responses in AS in the circulation but also effectively participates in the clinical course of ACS. hs-CRP, SAA, TPS, interleukin-6 (IL-6), and matrix metalloproteinases play important roles in the local inflammatory reactions of AS plaque. The local inflammatory responses of AS can activate such macrophage-secreted inflammatory factors as IL-6, Lp-PLA2, SAA, or TPS, and these inflammatory factors can promote the synthesis and secretion of Fbg by liver cells, thus resulting in the plasma Fbg level to be increased.[11] These inflammatory factors also can activate Fbg and platelet, thus promoting arterial thrombosis. The results of this study suggest that EH associated with high levels of plasma Fbg can trigger the occurrence of ACS. Fbg, hs-CRP and other three inflammatory factors can act as independent risk factors of ACS. The combined detection of Fbg, hs-CRP or other inflammatory markers may improve their prediction significance toward ACS. It is showed that the plasma concentrations of Fbg and Lp-PLA2 are related to the severity of coronary heart disease,[12] and high plasma Fbg in the circulation is associated with cardiovascular events.[13]
hs-CRP is a potential biomarker and recognized inflammatory marker toward plaque instability. However, hs-CRP is synthesized in the livers, and it is still unclear that whether plasma hs-CRP is the plaque-specific inflammatory marker or systemic inflammatory marker, namely its tissue-specificity remains unclear. Lp-PLA2 is a specific inflammatory marker of vascular plaque because it is generated within intra-plaque macrophages, and presents inside the atherosclerotic plaques with the form of Lp-PLA2 enzyme. Lp-PLA2 and LDL-C combine and exist in the blood circulation. It can be a meaningful biomarker toward vascular repair, and if its measurements can be standardized, it can be used as an independent clinical inflammatory marker for the plaques in intracranial arterial stenosis and coronary artery. Studies have shown that in Chinese patients with hypertension, Lp-PLA2 is significantly correlated with the occurrence of ischemic stroke in intracranial AS stenosis (ICAS).[14] The patients with high Lp-PLA2 show more severe intracranial lesions. Lp-PLA2 is independently associated with ICAS (OR: 2.3; 95% CI: 1.14–4.64). ICAS is a common cause of ischemic stroke in Asians, whereas in whites, extracranial arterial stenosis is more common. The prophylactic therapy with Statins can significantly reduce the concentration and activity of Lp-PLA2, so high activities of Lp-PLA2 may be used as a useful indicator for identifying the vascular events in ICAS diseases.
Higher levels of Lp-PLA2 are closely related to acute or recurrent ischemic cerebrovascular events.[15] One multivariate regression analysis showed that the increased Lp-PLA2 concentration and/or activity can predict potential recurrent stroke and transient ischemic attack (TIA), indicating that Lp-PLA2 is an important early predictor toward recurrent stroke and TIA.[5,16] In Chinese people, Lp-PLA2 is related to the nonstroke-associated hypertension-ICAS patients, a series of long-term studies further clarify the specific impacts of Lp-PLA2 toward asymptomatic ICAS. Lp-PLA2 has become a new bio-predictor and exhibits stronger ability in predicting stroke than cardiovascular disease.[17] The clinical features of symptomatic carotid plaque are strongly correlated with Lp-PLA2 level and related tissue oxidative stress substances, inflammation and plaque instability. These findings strongly support that Lp-PLA2 plays important roles in the pathophysiological and clinical manifestations of cerebrovascular diseases.[18,19] This paper revealed, from performing logistic regression analysis toward the 8 risk factors in 88 cases of ischemic cerebrovascular events, 4 significant independent risk factors toward stroke in EH patients, among which Lp-PLA2 is the most important independent risk factor (OR: 13.699, 95% CI: 5.236–35.837, P < 0.0001), with B’ as 0.708, indicating its highest risk toward stroke. The occurrence of stroke in EH patients with Lp-PLA2 ≥358.33 μg/L was 13.6 times than those with Lp-PLA2 < 358.33 μg/L, indicating that Lp-PLA2 is an important independent risk factor toward ischemic cerebrovascular events.
EH often causes damages to specific organs such as heart disease, cerebrovascular disease, vascular dementia, renal failure, atherosclerotic vascular disease, or retinopathy.[3] Although EH itself is undoubtedly a major factor in hypertension-TOD, it is still clearly proven than a number of related inflammatory markers play crucial roles in different TOD mechanisms, and interventions targeting these inflammatory markers will prevent and reduce TOD and overall cardiovascular risks.[3,20] It was reported that chronic inflammation can reduce the reactivity of anterior glomerular vessels, increase the release of renin, activate the afferent renal nerve, and enhance the vasoconstriction, thus promoting glomerular dysfunction and proteinuria.[21] In addition, serum UA can increase the levels of oxygen free radicals and CRP. hs-CRP is a recognized inflammatory marker, and it has been confirmed that inflammations are involved in the whole process of CKD occurring in EH; furthermore, CKD is an independent risk factor for cardiovascular disease and mortality.[21] Most researches indicated that an elevated serum UA level can independently predict the development of CKD. Increasing the UA level in rats can lead to glomerular hypertension and renal disease such as the development of arteriolosclerosis and tubulointerstitial fibrosis.[22] Our measurement results of inflammatory markers in the EH patients with acute exacerbation of CKD show that UA had the largest absolute value of standardized partial regression coefficient (b’: 1.026), indicating that UA has the greatest influence toward the occurrence of CKD in EH. The results further show that the roles of inflammations in the pathogenesis of CKD, and the clinical deterioration of CKD may be secondary to the UA-participated inflammatory process.
In conclusion, AS associated EH is a disease with its occurrence and development being involved in various high-risk factors and chronic inflammations, which often causes TOD in the heart, brain, kidney, or blood vessels clinically. Focusing on the pathogenesis and high-risk factors of different TOD will better prevent and targeted-intervene the occurrence of TOD in EH patients.
Financial support and sponsorship
This work was supported by the Science and Technology Key Project of Shanxi Province, China (No. 20120313018-8).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
References
- 1.Wang J, Zhang L, Wang F, Liu L, Wang H China National Survey of Chronic Kidney Disease Working Group. Prevalence, awareness, treatment, and control of hypertension in China: Results from a national survey. Am J Hypertens. 2014;27:1355–61. doi: 10.1093/ajh/hpu053. doi: 10.1093/ajh/hpu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun NL, Huo Y, Wang JG, Li NF, Tao J, Li Y, et al. Consensus of Chinese specialists on diagnosis and treatment of resistant hypertension. Chin Med J. 2015;128:2102–8. doi: 10.4103/0366-6999.161395. doi: 10.4103/0366-6999.161395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubattu S, Pagliaro B, Pierelli G, Santolamazza C, Castro SD, Mennuni S, et al. Pathogenesis of target organ damage in hypertension: Role of mitochondrial oxidative stress. Int J Mol Sci. 2014;16:823–39. doi: 10.3390/ijms16010823. doi: 10.3390/ijms16010823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dikalov SI, Ungvari Z. Role of mitochondrial oxidative stress in hypertension. Am J Physiol Heart Circ Physiol. 2013;305:H1417–27. doi: 10.1152/ajpheart.00089.2013. doi: 10.1152/ajpheart.00089.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis – An inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. doi: 10.1056/nejm199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.Choi H, Tostes RC, Webb RC. Mitochondrial aldehyde dehydrogenase prevents ROS-induced vascular contraction in angiotensin-II hypertensive mice. J Am Soc Hypertens. 2011;5:154–60. doi: 10.1016/j.jash.2011.02.005. doi: 10.1016/j.jash.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langlois M, Duprez D, Delanghe J, De Buyzere M, Clement DL. Serum Vitamin C concentration is low in peripheral arterial disease and is associated with inflammation and severity of atherosclerosis. Circulation. 2001;103:1863–8. doi: 10.1161/01.cir.103.14.1863. doi.org/10.1161/01.CIR.103.14.1863. [DOI] [PubMed] [Google Scholar]
- 8.Nadar SK, Tayebjee MH, Messerli F, Lip GY. Target organ damage in hypertension: Pathophysiology and implications for drug therapy. Curr Pharm Des. 2006;12:1581–92. doi: 10.2174/138161206776843368. doi: 10.2174/138161206776843368. [DOI] [PubMed] [Google Scholar]
- 9.Dawood T, Schlaich MP. Mediators of target organ damage in hypertension: Focus on obesity associated factors and inflammation. Minerva Cardioangiol. 2009;57:687–704. [PubMed] [Google Scholar]
- 10.Perlini S, Grassi G. Hypertension-related target organ damage: Is it a continuum? J Hypertens. 2013;31:1083–5. doi: 10.1097/HJH.0b013e32836157da. doi: 10.1097/hjh.0b013e32836157da. [DOI] [PubMed] [Google Scholar]
- 11.Ernst E, Resch KL. Fibrinogen as a cardiovascular risk factor: A meta-analysis and review of the literature. Ann Intern Med. 1993;118:956–63. doi: 10.7326/0003-4819-118-12-199306150-00008. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 12.Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: An individual participant meta-analysis. JAMA. 2005;294:1799–809. doi: 10.1001/jama.294.14.1799. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 13.Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, et al. Emerging Risk Factors Collaboration. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–20. doi: 10.1056/NEJMoa1107477. doi: 10.1056/nejmoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puddu P, Puddu GM, Galletti L, Cravero E, Muscari A. Mitochondrial dysfunction as an initiating event in atherogenesis: A plausible hypothesis. Cardiology. 2005;103:137–41. doi: 10.1159/000083440. doi: 10.1159/000083440. [DOI] [PubMed] [Google Scholar]
- 15.Delgado P, Chacón P, Penalba A, Pelegri D, García-Berrocoso T, Giralt D, et al. Lipoprotein-associated phospholipase A(2) activity is associated with large-artery atherosclerotic etiology and recurrent stroke in TIA patients. Cerebrovasc Dis. 2012;33:150–8. doi: 10.1159/000334193. doi: 10.1159/000334193. [DOI] [PubMed] [Google Scholar]
- 16.Ding X, Li C, Yu K, Gao A, Xiao L, Peng F, et al. Different risk factors between intracranial and extracranial atherosclerotic stenosis in Asian population: A systematic review and meta-analysis. Int J Neurosci. 2014;124:834–40. doi: 10.3109/00207454.2013.879580. doi: 10.3109/00207454.2013.879580. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Zhang J, Qian Y, Tang X, Ling H, Chen K, et al. Association of Lp-PLA2 Mass and aysmptomatic intracranial and extracranial arterial stenosis in hypertension patients. PLoS One. 2015;10:e0130473. doi: 10.1371/journal.pone.0130473. doi: 10.1371/journal.pone.0130473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin J, Zheng H, Cucchiara BL, Li J, Zhao X, Liang X, et al. Association of Lp-PLA2-A and early recurrence of vascular events after TIA and minor stroke. Neurology. 2015;85:1585–91. doi: 10.1212/WNL.0000000000001938. doi: 10.1212/wnl.0000000000001938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JS, Nah HW, Park SM, Kim SK, Cho KH, Lee J, et al. Risk factors and stroke mechanisms in atherosclerotic stroke: Intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke. 2012;43:3313–8. doi: 10.1161/STROKEAHA.112.658500. doi: 10.1161/strokeaha. 112.658500. [DOI] [PubMed] [Google Scholar]
- 20.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–69. doi: 10.1161/01.CIR.0000095676.90936.80. doi: 10.1161/01.cir.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 21.Sun X, He J, Ji XL, Zhao YM, Lou HY, Song XX, et al. Association of chronic kidney disease with coronary heart disease and stroke risks in patients with type 2 diabetes mellitus: An observational cross-sectional study in Hangzhou, China. Chin Med J. 2017;130:57–63. doi: 10.4103/0366-6999.196564. doi: 10.4103/0366-6999.196564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson RJ, Nakagawa T, Jalal D, Sánchez-Lozada LG, Kang DH, Ritz E. Uric acid and chronic kidney disease: Which is chasing which? Nephrol Dial Transplant. 2013;28:2221–8. doi: 10.1093/ndt/gft029. doi: 10.1093/ndt/gft029. [DOI] [PMC free article] [PubMed] [Google Scholar]


