Abstract
Background:
Previous studies have demonstrated that ultrasonography is the recommended imaging modality for preoperative staging of papillary thyroid carcinomas (PTCs). However, only a few studies have kept watch on preoperative evaluation of capsular invasion (CI) or extracapsular extension (ECE) and cervical lymph node metastasis using preoperative ultrasonography. This study aimed to investigate the relationship between the CI or ECE and the cervical lymph node metastasis in PTCs using preoperative ultrasonography and postoperative pathology in Chinese patients.
Methods:
The data of preoperative ultrasonography and postoperative pathology of 166 patients who had a definitive diagnosis of PTCs from October 2011 to July 2014 at Xuanwu Hospital, Beijing were collected and reviewed. Preoperative ultrasonic parameters of thyroid nodules were compared with those of postoperative pathological diagnoses. All the patients were divided into bilateral PTCs group (n = 42, 78 nodules) and unilateral PTCs group (n = 124, 124 nodules), and the data of the nodule sizes, CI or ECE, and cervical lymph node metastasis by preoperative ultrasonography were compared between two groups.
Results:
A total of 202 nodules of 166 patients which were confirmed by preoperative ultrasonography and postoperative pathology were analyzed. Hypoechogenicity (n = 201, 99.5%) and irregular margins (n = 167, 82.7%) were the main ultrasonic characteristics of PTCs. A significant moderate agreement between preoperative ultrasonic examination and postoperative pathology for CI or ECE (κ = 0.622, P < 0.001) was observed. The diagnostic sensitivity was 92.0%, and specificity was 71.1%. In bilateral PTCs group, 81.0% had CI or ECE, and 61.9% had cervical lymph node metastasis. In unilateral PTCs group, 76.6% had CI or ECE, and 58.1% had cervical lymph node metastasis. There were no significant differences in the incidence of CI or ECE and cervical lymph node metastasis between two groups (all P > 0.05).
Conclusions:
Ultrasonography was proved to be a valuable method for preoperative diagnosis of PTCs. Hypoechogenicity and irregular margins were strongly associated with PTCs. CI or ECE in unilateral PTCs strongly implied the cervical lymph node metastasis. Therefore, the cervical lymph nodes should be carefully examined by ultrasonography in patients with PTCs.
Keywords: Capsular Invasion, Papillary Thyroid Carcinomas, Ultrasonography
Introduction
Previous studies have demonstrated that papillary thyroid carcinomas (PTCs) accounts for approximately 80% of all thyroid neoplasms[1,2] and has shown a permanent increase in its incidence.[3] Despite a high survival rate (5- and 10-year overall survival of 90% and 95%, respectively[4]), patients with lymph node metastasis of PTCs have an increased rate of extrathyroidal invasion, local recurrence, and distant metastasis.[5,6,7]
Ultrasonography may provide a thorough evaluation of nodules, capsula invasion, and lymph nodes metastasis, which can significantly influence the extent of surgical resection. A ultrasound-based preoperative evaluation of the primary tumor's extent as well as lymph node involvement has become an essential procedure, which can modify the overall surgical approach in up to 40% of cases.[8,9] Several ultrasonographic characteristics of thyroid nodule have been found to correlate highly with malignancy, including irregular borders, microcalcifications, height greater than width, hypoechogenicity, and increased vascularity.[10,11,12,13,14] Only a few studies involved capsular invasion (CI) or extracapsular extension (ECE) of PTCs using ultrasonography. Some ultrasound doctors do not routinely assess CI or ECE and cervical lymph nodes during thyroid ultrasonography, and it was suggested that a series of ultrasonic operation standard should be gradually established.
Lymph node metastasis is associated with several predisposing risk factors, such as male gender, age older than 45 years, tumor size greater than 1 cm, and lymphovascular and extrathyroidal invasions.[15] Some studies demonstrated that massive ECE showed poor prognostic outcome.[16,17] However, only a few studies have kept watch on preoperative evaluation of CI or ECE and cervical lymph node metastasis using preoperative ultrasonography. The relation between CI or ECE and the cervical lymph node metastasis was still unconfirmed, especially for the bilateral PTCs. Therefore, the current study aimed to evaluate the relationship between the CI or ECE and the cervical lymph node metastasis in PTCs using preoperative ultrasonography and postoperative pathology in Chinese patients.
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Xuanwu Hospital, Beijing. Informed written consent was obtained from all patients prior to their enrollment in this study.
Study population
The data of preoperative ultrasonography findings and postoperative pathology of 166 patients from October 2011 to July 2014 at Xuanwu Hospital, Beijing were collected and reviewed. All patients who had a definitive diagnosis of PTCs underwent total thyroidectomy (n = 42) or lobectomy with isthmectomy (n = 124). We excluded patients with other pathologic types of thyroid malignancies. The patients who had no suspicious lesion on preoperative ultrasonic figures were also excluded from the study.
According to the preoperative ultrasonic examination and postoperative pathological diagnoses, the patients were divided into two groups: bilateral PTCs group (n = 42, 78 nodules) and unilateral PTCs group (n = 124, 124 nodules). Demographic features (such as gender and age) and ultrasonographic lymph node characteristics (including nodule size, numbers, position, echo, margin, calcification, central flow, CI or ECE, and so on, determined by two experienced ultrasound doctors) were analyzed. We compared these data and cervical lymph node metastasis between two groups.
Statistical analysis
Data were expressed as counts (percentage) or mean ± standard deviation (SD). A Chi-square test was used to compare the differences between two groups. Agreement between the two tests was determined by the kappa statistic. All statistical analyses were performed using the SPSS Statistics version 20.0 (SPSS Inc., Chicago, IL, USA). All tests were two-sided, and a P < 0.05 was considered statistically significant.
Results
The cohort of 166 patients consisted of 132 females (79.5%) and 34 males (20.5%), with a mean age of 46.0 ± 15.2 years. All 166 patients were diagnosed with PTCs, among whom 212 countable nodules were identified as carcinomas by postoperative pathology. Among these 212 countable nodules, 10 were excluded from the final analysis because of a lack of preoperative ultrasonic figures on nodule size (≤0.3 cm, 9 nodules; 0.6 cm, 1 nodule). Finally, 202 nodules which were confirmed by preoperative ultrasonography and postoperative pathology were analyzed. Among these 202 included nodules, 112 nodules involved the right lobe of the thyroid, and 90 involved the left lobe. The ultrasonographic assessment showed a mean size of 1.24 ± 1.07 cm in bilateral PTCs group and 1.25 ± 0.72 cm in unilateral PTCs group (P = 0.940). Positive ultrasound parameters include hypoechogenicity, margin irregularity, micro- or macro-calcifications, and internal flow [Table 1].
Table 1.
Ultrasonographic parameters of 202 nodules in this study
| Parameters | Values, n (%) |
|---|---|
| Echogenicity | |
| Hypoechogenicity | 201 (99.5) |
| Isoechogenicity | 1 (0.5) |
| Margins | |
| Irregular | 167 (82.7) |
| Regular | 35 (17.3) |
| Calcification (mm) | |
| Macrocalcification (≥2) | 55 (27.2) |
| Microcalcification (<2) | 35 (17.3) |
| Noncalcification | 112 (55.5) |
| Internal flow | |
| Rich flow | 17 (8.4) |
| Few flow | 97 (48.0) |
| Absent flow | 88 (43.6) |
In the present study, the disruption of thyroid capsules [Figure 1], invasion of surrounding muscles or fat [Figure 2], and contact with the adjacent capsule of papillary thyroid microcarcinoma (PTMC) [Figure 3] were the characteristics of CI or ECE, according to the preoperative ultrasonographic findings. The data of preoperative ultrasonography and postoperative pathology for the diagnoses of CI or ECE were compared, which showed that there was significant moderate agreement of two methods for diagnosing CI or ECE [κ= 0.622, P < 0.001; Table 2]. The diagnostic sensitivity was 92.0%, and specificity was 71.1%.
Figure 1.
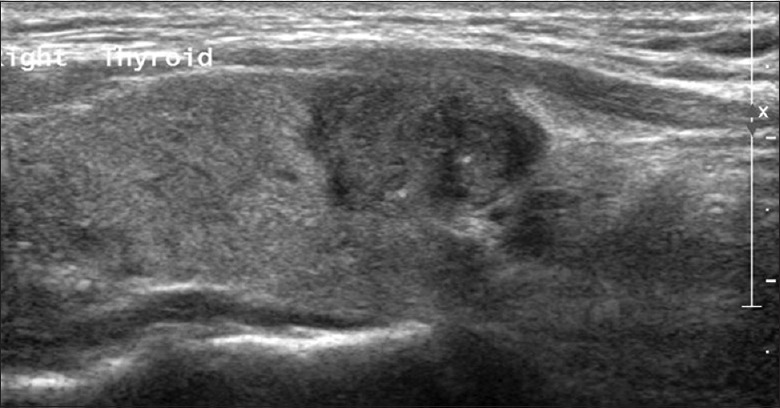
Longitudinal image of preoperative ultrasonography showed the disruption of part thyroid capsule.
Figure 2.
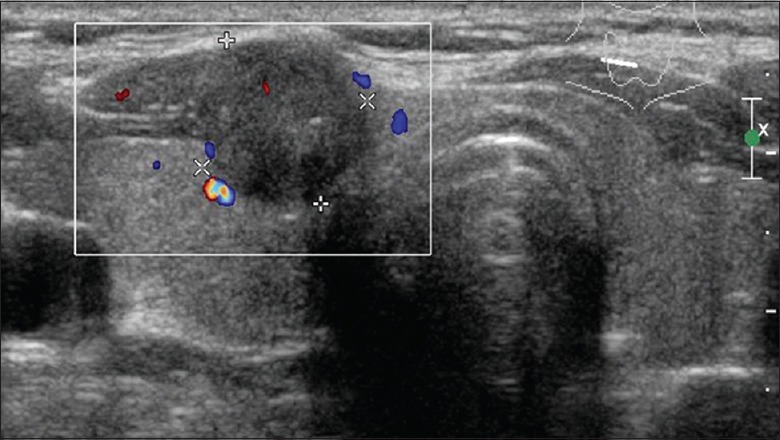
Transverse image of preoperative ultrasonography showed hypoechoic mass arising from the anterior surface of the thyroid. This mass invaded adjacent strap muscle.
Figure 3.
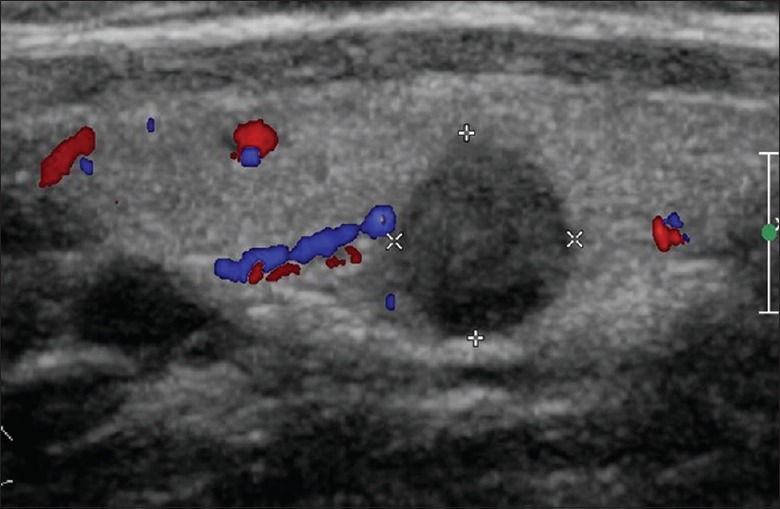
Longitudinal image of preoperative ultrasonography showed hypoechoic solid nodule and contact with the adjacent capsule of the thyroid.
Table 2.
Agreement between diagnosis of preoperative ultrasonography and postoperative pathology for CI or ECE (n)
| Postoperative pathology | Preoperative ultrasonography | κ | P | |
|---|---|---|---|---|
| Positive | Negative | |||
| Positive | 126 | 11 | 0.622 | <0.001 |
| Negative | 21 | 44 | ||
CI: Capsular invasion; ECE: Extracapsular extension.
This study also compared the CI or ECE with cervical lymph node metastasis between bilateral and unilateral PTCs groups. In bilateral PTCs group, 34 patients (81.0%) had CI or ECE, and 26 (61.9%) had cervical lymph node metastasis. In unilateral PTCs group, 95 patients (76.6%) had CI or ECE, and 72 (58.1%) had cervical lymph node metastasis. There were no significant differences in the incidence of CI or ECE (P = 0.559) and cervical lymph node metastasis (P = 0.662) between two groups [Table 3].
Table 3.
The incidences of CI or ECE with cervical LN metastasis between bilateral and unilateral PTCs groups (n)
| Parameters | Bilateral PTCs group | Unilateral PTCs group | Chi-square values | P |
|---|---|---|---|---|
| CI or ECE | ||||
| Yes | 34 | 95 | 0.341 | 0.559 |
| No | 8 | 29 | ||
| LN metastasis | ||||
| Yes | 26 | 72 | 0.191 | 0.662 |
| No | 16 | 52 |
CI: Capsular invasion; ECE: Extracapsular extension; LN: Lymph node; PTCs: Papillary thyroid carcinomas.
In this study, a significant weak agreement between CI or ECE and cervical lymph node metastasis could be found in unilateral PTCs group (κ= 0.145, P = 0.034; Table 4). However, the relationship between CI or ECE and cervical lymph node metastasis could not be evaluated efficiently in bilateral PTCs group, since bilateral, unilateral, or contralateral cervical lymph node metastasis might exist at the same time.
Table 4.
Agreement between diagnoses of CI or ECE and cervical LN metastasis in unilateral PTCs group
| LN metastasis | CI or ECE | Chi-square values | P | |
|---|---|---|---|---|
| Positive | Negative | |||
| Positive | 44 | 7 | 0.145 | 0.034 |
| Negative | 51 | 22 | ||
CI: Capsular invasion; ECE: Extracapsular extension; LN: Lymph node; PTCs: Papillary thyroid carcinomas.
Discussion
The results of this study confirmed the female predominance of PTCs. Papillary thyroid microcarcinoma (PTM) represents a subset PTCs measuring ≤10 mm in greatest dimension.[18,19]
Many studies have shown that most PTCs are hypoechoic and have irregular margins on ultrasound, with microcalcifications occurring in approximately 40% of cases.[20,21,22,23] Consistent with previous findings, this study verified hypoechogenicity and irregular margins as the strongest predictors of malignancy. However, the incidences of microcalcification (17.3%) and rich flow (8.4%) in this study were significantly lower than those of some previous studies.[20,23] Thus, future work remains in getting better understand of the incidences of microcalcification.
Some studies indicated that the preoperative ultrasonographic findings, such as the disruption of thyroid capsules, invasion of surrounding muscles or fat, and contact with the adjacent capsule of PTMC, were the characteristics of CI or ECE,[24,25,26,27] and the present study had similar results. In the present study, the incidence of CI or ECE and cervical lymph node metastasis were observed, but no significant differences in CI or ECE and cervical lymph node metastasis between the two groups was observed. However, a significant moderate agreement between preoperative ultrasonography parameters and postoperative pathology for the diagnosis of CI or ECE was observed. This study suggested that preoperative ultrasonography could provide a useful predictive information about PTCs.
Although ultrasonography was a good method for diagnoses of early intervention or ECE, the prognostic value of cervical lymph node metastasis in PTCs was controversial. Mazzaferri and Jhiang[16] reported that the extracapsular spread was highly predictive prognostic factors of either distant metastasis or locoregional. While Kwak et al.[25] reported that ECE in PTMC patients was not an independent factor of lateral lymph node metastasis recurrence. However, the present study showed that CI or ECE in unilateral PTCs implied the cervical lymph node metastasis. Therefore, ultrasonography examine for cervical lymph nodes should be meticulously performed, especially for central compartments of the neck.
There were several limitations of the current study. First, acoustic shadows on ultrasonic images might result in the misdiagnosis of CI or ECE. The possible solution methods are to operate the probe through multiple sections on nodules to avoid acoustic shadow interference. Second, although the present study was performed by appropriately trained ultrasound doctors, misleading figures could not be avoided. Third, the patients in this study were just from a single center which might not be representative of the general population. In the future, the large, multicenter clinical studies are required to further confirm the findings of this study.
In conclusion, ultrasonography was proved to be a valuable method for preoperative diagnosis of PTCs. Hypoechogenicity and irregular margins were strongly associated with PTCs. The CI or ECE in unilateral PTCs strongly implied the cervical lymph node metastasis. Therefore, the cervical lymph nodes should be carefully examined by ultrasonography in patients with PTCs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
References
- 1.Cisco RM, Shen WT, Gosnell JE. Extent of surgery for papillary thyroid cancer: Preoperative imaging and role of prophylactic and therapeutic neck dissection. Curr Treat Options Oncol. 2012;13:1–10. doi: 10.1007/s11864-011-0175-z. doi: 10.1007/s11864-011-0175-z. [DOI] [PubMed] [Google Scholar]
- 2.Li QS, Chen SH, Xiong HH, Xu XH, Li ZZ, Guo GQ. Papillary thyroid carcinoma on sonography. Clin Imaging. 2010;34:121–6. doi: 10.1016/j.clinimag.2009.03.003. doi: 10.1016/j.clinimag.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Iyer NG, Shaha AR. Management of thyroid nodules and surgery for differentiated thyroid cancer. Clin Oncol (R Coll Radiol) 2010;22:405–12. doi: 10.1016/j.clon.2010.03.009. doi: 10.1016/j.clon.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conzo G, Docimo G, Pasquali D, Mauriello C, Gambardella C, Esposito D, et al. Predictive value of nodal metastases on local recurrence in the management of differentiated thyroid cancer. Retrospective clinical study. BMC Surg. 2013;13(Suppl 2):S3. doi: 10.1186/1471-2482-13-S2-S3. doi: 10.1007/s003841-014-2099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coburn MC, Wanebo HJ. Prognostic factors and management considerations in patients with cervical metastases of thyroid cancer. Am J Surg. 1992;164:671–6. doi: 10.1016/s0002-9610(05)80732-9. doi: 10.1016/S0002-9610(05)80732-9. [DOI] [PubMed] [Google Scholar]
- 6.Noguchi S, Murakami N, Yamashita H, Toda M, Kawamoto H. Papillary thyroid carcinoma: Modified radical neck dissection improves prognosis. Arch Surg. 1998;133:276–80. doi: 10.1001/archsurg.133.3.276. doi: 10.1016/S0278-2391(98)90428-1. [DOI] [PubMed] [Google Scholar]
- 7.Ortiz S, Rodríguez JM, Soria T, Pérez-Flores D, Piñero A, Moreno J, et al. Extrathyroid spread in papillary carcinoma of the thyroid: Clinicopathological and prognostic study. Otolaryngol Head Neck Surg. 2001;124:261–5. doi: 10.1067/mhn.2001.113141. doi: 10.1067/mhn.2001.113141. [DOI] [PubMed] [Google Scholar]
- 8.Kim KE, Kim EK, Yoon JH, Han KH, Moon HJ, Kwak JY. Preoperative prediction of central lymph node metastasis in thyroid papillary microcarcinoma using clinicopathologic and sonographic features. World J Surg. 2013;37:385–91. doi: 10.1007/s00268-012-1826-3. doi: 10.1007/s00268-012-1826-3. [DOI] [PubMed] [Google Scholar]
- 9.Lew JI, Rodgers SE, Solorzano CC. Developments in the use of ultrasound for thyroid cancer. Curr Opin Oncol. 2010;22:11–6. doi: 10.1097/CCO.0b013e3283337f16. doi: 10.1097/MLG.0b013e318157465d. [DOI] [PubMed] [Google Scholar]
- 10.Moon HG, Jung EJ, Park ST, Ha WS, Choi SK, Hong SC, et al. Role of ultrasonography in predicting malignancy in patients with thyroid nodules. World J Surg. 2007;31:1410–6. doi: 10.1007/s00268-007-9013-7. doi: 10.1007/s00268-007-9013-7. [DOI] [PubMed] [Google Scholar]
- 11.Koike E, Noguchi S, Yamashita H, Murakami T, Ohshima A, Kawamoto H, et al. Ultrasonographic characteristics of thyroid nodules: Prediction of malignancy. Arch Surg. 2001;136:334–7. doi: 10.1001/archsurg.136.3.334. doi: 10.1007/s00330-015-3668-5. [DOI] [PubMed] [Google Scholar]
- 12.Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002;178:687–91. doi: 10.2214/ajr.178.3.1780687. doi: 10.1245/s10434-016-5572-x. [DOI] [PubMed] [Google Scholar]
- 13.Shimura H, Haraguchi K, Hiejima Y, Fukunari N, Fujimoto Y, Katagiri M, et al. Distinct diagnostic criteria for ultrasonographic examination of papillary thyroid carcinoma: A multicenter study. Thyroid. 2005;15:251–8. doi: 10.1089/thy.2005.15.251. doi: 10.1128/CVI.00132-08. [DOI] [PubMed] [Google Scholar]
- 14.Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of malignancy in nonpalpable thyroid nodules: Predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87:1941–6. doi: 10.1210/jcem.87.5.8504. doi: 10.1089/thy.2005.15.251. [DOI] [PubMed] [Google Scholar]
- 15.Choi YJ, Yun JS, Kook SH, Jung EC, Park YL. Clinical and imaging assessment of cervical lymph node metastasis in papillary thyroid carcinomas. World J Surg. 2010;34:1494–9. doi: 10.1007/s00268-010-0541-1. doi: 10.1007/s00268-010-0541-1. [DOI] [PubMed] [Google Scholar]
- 16.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–28. doi: 10.1016/0002-9343(94)90321-2. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 17.Ito Y, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, et al. Risk factors for recurrence to the lymph node in papillary thyroid carcinoma patients without preoperatively detectable lateral node metastasis: Validity of prophylactic modified radical neck dissection. World J Surg. 2007;31:2085–91. doi: 10.1007/s00268-007-9224-y. doi: 10.1007/s00268-007-9224-y. [DOI] [PubMed] [Google Scholar]
- 18.Shaha A. Treatment of thyroid cancer based on risk groups. J Surg Oncol. 2006;94:683–91. doi: 10.1002/jso.20697. doi: 10.1002/jso.20697. [DOI] [PubMed] [Google Scholar]
- 19.Hedinger C, Williams ED, Sobin LH. International Histological Classification of Tumours. No. 11. Geneva, Switzerland: World Health Organization; 1988. Histological typing of thyroid tumours; pp. 1–18. [Google Scholar]
- 20.Maia FF, Matos PS, Silva BP, Pallone AT, Pavin EJ, Vassallo J, et al. Role of ultrasound, clinical and scintigraphyc parameters to predict malignancy in thyroid nodule. Head Neck Oncol. 2011;3:17. doi: 10.1186/1758-3284-3-17. doi: 10.1186/1758-3284-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li RQ, Yuan GH, Chen M, Shao YM, Zhu SN, Zhang JQ, et al. Evaluation of diagnostic efficiency of ultrasound features on malignant thyroid nodules in Chinese patients. Chin Med J. 2016;129:1784–8. doi: 10.4103/0366-6999.186643. doi: 10.4103/0366-6999.186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu SL, Jiang YX, Yang X, Wu Q, Zhao RN, Li JC, et al. “Onion Skin-liked Sign” in thyroid ultrasonography: A characteristic feature of benign thyroid nodules. Chin Med J. 2016;129:1533–7. doi: 10.4103/0366-6999.184460. doi: 10.4103/0366-6999.184460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones R, Spendiff R, Fareedi S, Richard PS. The role of ultrasound in the management of nodular thyroid disease. Imaging. 2007;19:28–38. doi: 10.1259/imaging/49938227. [Google Scholar]
- 24.Kwak JY, Kim EK, Youk JH, Kim MJ, Son EJ, Choi SH, et al. Extrathyroid extension of well-differentiated papillary thyroid microcarcinoma on US. Thyroid. 2008;18:609–14. doi: 10.1089/thy.2007.0345. doi: 10.1089/thy.2007.0345. [DOI] [PubMed] [Google Scholar]
- 25.Kwak JY, Kim EK, Kim MJ, Son EJ, Chung WY, Park CS, et al. Papillary microcarcinoma of the thyroid: Predicting factors of lateral neck node metastasis. Ann Surg Oncol. 2009;16:1348–55. doi: 10.1245/s10434-009-0384-x. doi: 10.1245/s10434-009-0384-x. [DOI] [PubMed] [Google Scholar]
- 26.Bramley MD, H BJ. Papillary microcarcinoma of the thyroid gland. Br J Surg. 1996;83:1674–83. doi: 10.1002/bjs.1800831206. doi: 10.1002/bjs.1800831206. [DOI] [PubMed] [Google Scholar]
- 27.Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer. 1985;56:531–8. doi: 10.1002/1097-0142(19850801)56:3<531::aid-cncr2820560321>3.0.co;2-3. doi: 10.1245/s10434-009-0384-x. [DOI] [PubMed] [Google Scholar]


