Abstract
Background:
Collapsin response mediator protein-2 (CRMP2) has been shown to be involved in ischemia/hypoxia (IH) injury. We determined whether CRMP2 modulates ischemic injury in the retinal of Ocular ischemic syndrome (OIS). This study was to explore the molecular mechanisms underlying OIS in a novel mice model.
Methods:
Experiments were performed on adult male C57/BL6 mice that received bilateral internal carotid arteries ligation for 1, 2, or 4 weeks. The mice received injection of calpeptin group before occlusion for 4 weeks or not. The expression of CRMP2 in the retinal was examined by western blotting (WB) analysis and immunohistochemical analysis (IHC). The effects of ischemic injury on retinal were evaluated by fundus examination, fundus fluorescein angiography, electroretinogram, cell counting of retinal ganglion cell (RGC), and measurement of the thickness of the retina.
Results:
The veins dilated after chronic ischemia. In the electroretinography, the amplitudes of a- and b-waves kept diminishing in an ischemia time-dependent manner. Moreover, the tail vein-retinal circulation time prolonged in the 1- and 2-week group. In comparison, thickness of the retina decreased gradually with the ischemia time elapsed. WB analysis showed the CRMP2 and p-CRMP2 levels decreased in the 2- and 4-week groups. The results of IHC analysis were compatible with our results of WB. The loss of RGCs, decrease of the total reaction time and reduction of CRMP2 was alleviated by intravitreal injection of calpeptin.
Conclusions:
These results revealed that bilateral ligation of the internal carotid artery causes retinal ischemia in mice. Moreover, CRMP2 might play a pivotal role during the ischemic injury in the retina and inhibit the cleavage of CRMP2 can ameliorate the IH injury.
Keywords: Calpeptin, Collapsin Response Mediator Protein 2, Ischemia Injury, Ocular Ischemic Syndrome, Phosphorylation
Introduction
Ocular ischemic syndrome (OIS) is a sight-threatening disease which is caused by ocular hypoperfusion as the result of occlusion or stenosis of the common or internal carotid arteries.[1,2] At a 1-year follow-up, the visual acuity of 58% of patients deteriorated to counting fingers or worse.[3] One major barrier to the prevention of this poor vision prognosis is the lack of understanding of the mechanism of chronic retinal ischemia. One of the most pivotal steps to investigate the mechanism of the disease is to develop a suitable animal model of chronic retinal ischemia. Although a number of animal models have been made to study retinal ischemia, there have been few if any satisfying models that closely simulated the clinical situation of OIS in humans.[4] In our previous experiment,[5,6] one ocular ischemic model of bilateral common carotid arteries (CCAs) occlusion (BCCAO) was established, but in this model, the degree of ischemia is far more severe than that in most patients. In this experiment, we established a novel chronic retinal ischemia model for OIS. In this model, the blood flow insufficiency is milder than that after BCCAO, and the degree of ischemia is closer to the clinical situation of OIS. The collapsin response mediator protein (CRMP) family is composed of five cytosolic phosphoproteins (CRMP1 to CRMP5) that are highly expressed in the developing nervous system.[7,8] CRMP2 is the first member of this family to be discovered and has been studied most frequently. It was identified as a cytosolic protein that plays a critical role in the transduction pathway of the extracellular semaphorin 3A signal. Recent studies[9] indicated CRMP2 has been shown to be involved in ischemia/hypoxia (IH) injury. Hypophosphorylation and cleavage of CRMP2 were observed after ischemic/hypoxic exposure in the nervous system.[10,11] It was suggested that CRMP2 could be activated in the ischemic condition, and the hypophosphorylation is the active form of CRMP2. According to the existing research, an important function of CRMP2 is axonal guidance and regeneration.[12,13] We hypothesized that CRMP2 would play an important role in axonal regeneration after ischemic injury, but there has been little necessary research on retinal ischemia. Therefore, we explored the functions of CRMP2 in our OIS model.
A number of studies have found that total CRMP2 was decreased due to calpain cleaved in ischemic/hypoxic injury.[1,14,15] To explore the role of CRMP2 in ischemic injury, we used the selective inhibitor of calpain (calpeptin) to inhibit the cleavage of CRMP2 in the IH injury process. Accordingly, we used the novel OIS model to test the expression and role of CRMP2 in the chronic ischemic retina in the present study.
Methods
Animals
Experiments were performed on adult male C57/BL6 mice, aged 8–10 weeks and weighing 20–25 g, which were purchased from the Experimental Animal Center of Chinese Academy of Medical Sciences, China. The mice were kept under controlled lighting conditions (12 h: 12 h light/dark). All experiments were performed in accordance with the guidelines set by the Animal Care and Use Committee of Capital Medical University and Animal Research: Reporting In vivo Experiments, and are consistent with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23).
Ocular ischemic model in mice
The mice were assigned into six groups: naive, sham surgery, 1 week (ischemia lasting for 1 week), 2 weeks (ischemia lasting for 2 weeks), 4 weeks (ischemia lasting for 4 weeks), and calpeptin (intravitreal injection of calpeptin group before occlusion for 4 weeks). Anesthesia was induced with 4.0–5.0% chloral hydrate (400 mg/kg, i.p.). The surgery was done under a stereomicroscope (OLYMPUS, SZ61). A cervical midline incision was made, and the bilateral CCAs and their bifurcation were exposed and separated from the carotid sheath and vagus nerve. Then, the proximal internal carotid arteries were ligated bilaterally with 10–0 silk sutures, and the neck incision was closed. The sham surgery animals were submitted to the same procedure without occlusion of the arteries.
Intravitreal injection
The mice went through binocular intravitreal injection before arteries occlusion. The mice were intravitreally injected with the selective inhibitor of calpain (calpeptin, 1 μl) after being anesthetized. We used a 35-G needle on a Hamilton microsyringe (Hamilton, Reno, NV, USA) for the injection. Broad-spectrum antibiotic (levofloxacin) ophthalmic ointment was applied after injection. No change in pupil size or reactivity to light was observed in injected eyes.
Electroretinogram recording
Scotopic electroretinogram (ERG) was used to estimate retinal function in the animals. Scotopic ERG was recorded for each group. The a-wave represented the function of the photoreceptor, whereas the b-wave represented the function of Müller cells, bipolar cells, or both. The oscillatory potentials (OPs) were also recorded. They are derived from inner retinal neurons including ganglion cells and amacrine. In our preliminary experiment, it was observed that there was no difference in the clarity of the cornea or lens between bilateral internal carotid artery (ICA) ligation mice and sham surgery mice. The mice were kept in a completely dark room for 24 h and anesthetized intraperitoneally with a mixture of ketamine (120 mg/kg) and xylazine (6 mg/kg). The pupils were dilated with 2.5% phenylephrine and 1% tropicamide (Santen Pharmaceutical Co., Ltd.). Flash ERG was recorded in the left eye. A golden ring electrode (Mayo, Aichi, Japan) was placed in contact with the cornea and a reference electrode (Nihon Kohden, Tokyo, Japan) was placed through the tongue. A neutral electrode was inserted near the tail subcutaneously. All procedures were conducted in dim red light carefully, and mice were kept warm during the procedure. We measured the amplitude of the a-wave from the baseline to the maximum a-wave peak, and the b-wave was from the maximum a-wave peak to the maximum b-wave peak. OP amplitudes were measured in the time domain between the a-wave peak and the major b-wave peak.
Fundus examination
Fundus photographs were taken after the ERG recordings. The binocular fundus of each of the mice were photographed with a fundus camera (Kowa, Nagoya, Japan). To evaluate the changes in retinal vessel diameters after ischemia, we binarized fundus images and set the threshold for defining the outline of vessel walls. Then, we chose three major arterial and venous vessels, respectively, from each binarized image (1024×768 pixels), and measured the vessel diameter at the point of 100 pixels from the optic nerve. Measurement of vessel diameter was performed using the straight tool of ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html). These measurements were conducted in a masked fashion by a researcher. In each group, we took the fundus images from three or five mice, and data were averaged from them.
Fluorescein fundus angiography
To assess the effect of BICAO on the blood flow velocity, fundus fluorescein angiography was conducted after fundus photographs were taken. In brief, C57/BL6J mice were anesthetized with a combination of ketamine and xylazine, as described above. Sodium fluorescein (Fluorescite; Alcon, Fort Worth, TX, USA) of 0.04 ml was injected into the tail vein at once, and the processes of blood flow were observed with a scanning laser ophthalmoscope (Rodenstock Instrument, Munich, Germany) in the 40° field. We recorded from the start of fluorescein infusion at the video rate of 30 frames/s.
The blood flow is assessed in the fluorescein angiography video along with a pixel-by-pixel analysis to return values for the tail vein-retinal circulation time (the mean time from injection of sodium fluorescein to the artery fill), the arterial phase (the mean time from the first artery fill to the first vein fill), and the venous phase (the mean time of all the vein fills) within the large blood vessels. The mean time of each phase was measured and compared in each group.
Histology
Mice were euthanized and the left eyes were enucleated and kept immersed in a fixative solution containing 4% paraformaldehyde at least 24 h at 4°C. Six paraffin-embedded sections (thickness 7 μm) were cut parallel with the maximum circle of the eyeball through the optic disc. When we extracted the eyeball, we used the optic axis as the landmark and checked the top of the dorsal point. Moreover, hematoxylin and eosin were used to stain it. Four sections from each eyeball were used for the histological analysis. Light microscope (Olympus) images were photographed, and the cell counts in the ganglion cell layer (GCL) between 375 and 62 μm from the optic disc were measured. The thickness of the inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), and total thickness was measured at three points per section in a masked fashion by a single observer. The standard area for measurement was at the central retina, approximately 200 μm from the optic disc. Data from four sections were averaged for each eye and were used to evaluate the cell count in the GCL and the thickness of the IPL, the INL, the OPL, and the ONL, as well as the retinal total thickness.
Sample preparation and western blot analysis
Mice were euthanized and their eyeballs were rapidly removed, and the retinas were carefully separated from the eyeballs and quickly frozen in dry ice. For protein extraction, the tissue was homogenized at 4°C in Buffr C (50 mmol/L Tris-Cl, pH 7.5) containing 2 mmol/L DTT, 2 mmol/L EGTA, 2 mmol/L EDTA, 50 mmol/L 4-[2-aminoethyl]-benzenesulfonylfloride hydrochloride; 5 mg/ml each of leupeptin, aprotinin, pepstatin A, and chymostatin; and 50 mmol/L KF, 5 mmol/L sodium pyrophosphate, 50 mmol/L okadaic acid, and 2% SDS, and sonicated to dissolve the retinal tissue completely. Protein concentration was measured with a BCA Protein Assay Kit (Thermo Scientifi, Pittsburgh, PA, USA).
SDS-polyacrylamide gel electrophoresis (PAGE) and Western blot analysis were performed as in a previous study.[16] In brief, each lane was loaded 60 μg of total protein in each sample for a 10% SDS-PAGE gel. Then, polyvinylidene difloride membrane (PVDF, GE Healthcare, USA) was used to electrophoresis and transfer of proteins onto at 4°C. After that, the PVDF membrane was dip into 10% nonfat milk in TTBS (20 mmol/L Tris-Cl, pH 7.5, containing 0.15 mol/L NaCl and 0.05% Tween 20) for 1 h. The blocked PVDF membrane was incubated with primary rabbit polyclonal antibody against pCRMP2 (T514) (1:1000, Abcam Technology, Cambridge, MA, USA) for 2 h or for 3 h in CRMP2 (1:1000, Cell Signaling Technology, #9393, USA). The same PVDF membrane was reprobed with primary mouse monoclonal antibody against β-actin (1:4000, Sigma-Aldrich Corp. St. Louis, MO 63103, USA) for 1 h to ensure uniform loading of protein. We used the anti-mouse IgG (1:5,000; Stressgen Biotechnologies Corporation, Victoria BC, Canada) or horseradish peroxidase-conjugated goat anti-rabbit as second antibodies and incubated the membrane for 1 h. An enhanced chemiluminescence kit (PerkinElmer Life Science, USA) was used to identify the signals on radiographic film. The sequence to detect the target protein is CRMP2, pCRMP2 (T514), β-actin.
Immunohistochemistry
Immunohistochemistry was performed using the avidin-biotin (“ABC”) technique. Xylene was used to remove the paraffin. And then, the sections were rehydrated with a graded series of alcohol and rinsed with phosphate-buffered saline (PBS). Slides were placed in 200 ml of target retrieval solution (DAKO, Carpinteria, CA, USA) and heated in a microwave oven (Kenmore, maximal power 800 W; Sears, Hoffman Estates, IL, USA) to antigen retrieval. We preheated the buffer for 5 min at 60% power setting, until it boiled for 5 min. And then put the sections at room temperature to cool for 20 min. Sections were incubated in 0.3% H2O2 for 15 min to inactivate endogenous peroxidase after rinsing with PBS. Then, sections were blocked with 10% normal serum from the species of secondary antibody, Avidin D solution (Avidin/Biotin Blocking Kit, Vector, Burlingame, CA, USA) and 0.25% Triton X-100 for 30 min. Sections were washed with 0.25% Triton X-100 in PBS. After these steps, sections were incubated with the primary antibody for 1 h at room temperature. Moreover, the primary antibody was diluted in PBS containing biotin solution (Avidin/Biotin Blocking Kit, Vector), 0.25% of Triton X-100, and 1% normal serum from the species of the secondary antibody. After washed with 0.25% Triton X-100 in PBS, they were reacted with the secondary antibodies (1:250; Vector) and streptavidin-HRP (K0377; DAKO). A rat adsorbed horse anti-mouse IgG secondary antibody (BA-2001, Vector) was used to avoid cross-reactions. Sections were reacted with the peroxidase block and overlaid with HRP-conjugated lectin from Bandeiraea simplicifolia BS-1 (1:50; L 5391, SIGMA; Streit, 1990) for identifying microglial cells. Then, we used 0.05% diaminobenzidine including 0.003% H2O2 to make the peroxidase activity visible. The omission of the primary antibodies served as naive, and no immunoreactivity was obtained. Sections were finally dehydrated and coverslipped with DePeX (Aldrich, Milwaukee, WI, USA).
Each immunohistochemical (IHC) marker showed different features of the retina and specific changes during BCCAO; therefore, a specific evaluation was needed. In general, results from three sections per retina immunostained on the same slide were averaged, and groups of animals were available for different time points of BCCAO.
Statistical analysis
Statistical analysis was conducted using one-way analysis of variance followed by all pairwise multiple comparison procedures using Bonferroni test using SPSS Statistics 20.0 (IBM, Armonk, NY, USA) software. A value of P < 0.05 was considered statistically significant.
Results
Ocular fundus photography of ischemic retina
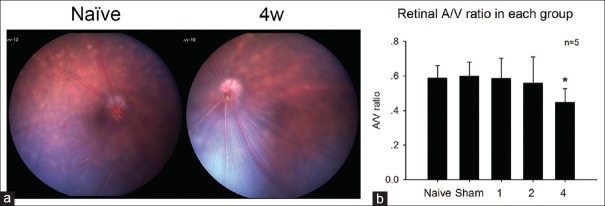
After the internal carotid arteries were bilaterally ligated for one (A/V = 0.584 ± 0.118) or two (A/V = 0.558 ± 0.152) weeks, retinal blood vessels had no difference with the normal mice (A/V = 0.587 ± 0.072). However, after 4 weeks (A/V = 0.446 ± 0.080) of ischemia, the veins significantly dilated compared to those of normal mice [Figure 1] (P < 0.05).
Figure 1.
(a) The typical fundus color photography of the normal group and the four weeks one. (b) After four weeks of ischemia, the veins significantly dilated comparing to those of normal mice. *P < 0.05 compared with naive group.
Functional analysis of the retinal function after ligation using electroretinography
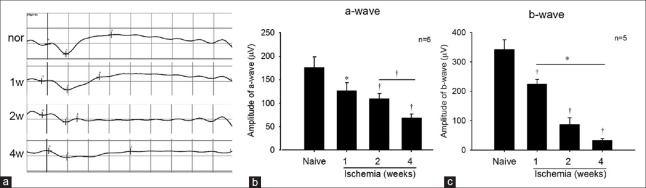
In the ischemia group, the amplitudes of both the a- and b-waves significantly decreased after ligation for 1 week (P < 0.05), and the amplitudes kept diminishing in an ischemia time-dependent manner [Figure 2a]. The a-wave amplitude of naive, 1-week, 2-week and 4-week groups were, 176.6 ± 22.1 μV, 126.4 ± 17.1 μV, 109.4 ± 10.6 μV and 69.1 ± 7.5 μV, respectively [Figure 2b]. Moreover, the b-wave amplitude of each group were 341.8 ± 33.2 μV, 224.3 ± 16.0 μV, 86.6 ± 23.2 μV and 32.8 ± 6.9 μV, respectively [Figure 2c].
Figure 2.
(a) The typical result of electroretinogram for each group. (b and c) The amplitudes of both the a- and b-waves were reduced gradually with the ischemia time going on. *P < 0.05, †P < 0.01 compared with naive group.
Fluorescein fundus angiography
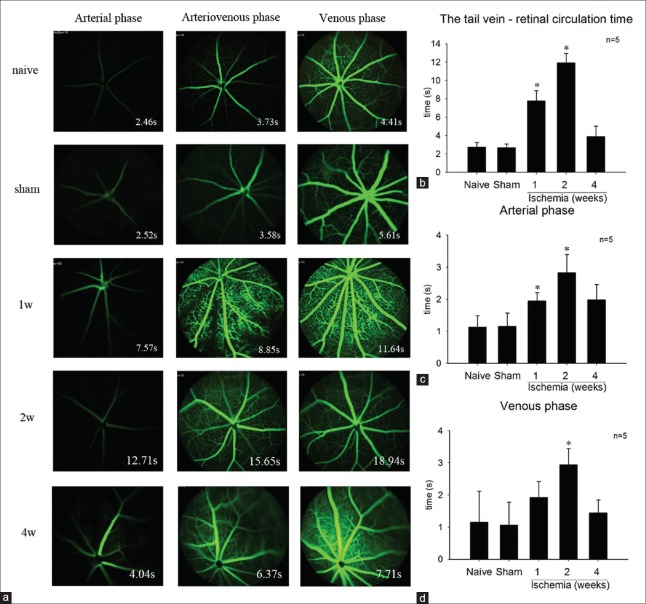
We assessed blood flow in each group (n = 5) using video fluorescein angiography [Figure 3a]. The tail vein-retinal circulation time [Figure 3b] significantly prolonged in the 1-week (7.778 ± 1.122 s) and 2-week (11.922 ± 1.029 s) group versus the sham (2.692 ± 0.403 s) or naive group (2.743 ± 0.504 s) (P < 0.05). However, it turned normal (P > 0.05) in the 4-week group (3.904 ± 1.134 s), which may be because of the establishment of collateral circulation after the chronic ischemia. The arterial phase [Figure 3c] showed the seam tendency as the tail vein-retinal circulation time. The arterial phase for each group was 1.136 ± 0.357 s (naive group), 1.166 ± 0.408 s (sham group), 1.952 ± 0.255 s (1-week group), 2.834 ± 0.568 s (2-week group) and 1.995 ± 0.473 s (4-week group). As for the venous phase, the 2-week group (2.938 ± 0.506) was significantly prolonged compared with that of the sham (1.070 ± 0.707 s) or naive group (1.159 ± 0.963 s) (P < 0.05). While the 1-week group (1.921 ± 0.497 s) and the 4-week group (1.445 ± 0.404 s) showed no significant difference versus the control groups (P > 0.05). (Arterial phase: the time from arterial begin filling to venous start filling. Arteriovenous phase: the time from arterial finish filling to venous start filling. Venous phase: the time when venous fill.)
Figure 3.
(a) The fundus fluorescein angiography of each group at different phase. (b) The tail vein-retinal circulation time was significant prolonged in 1- and 2-week group. However, it was no significant difference between the 4-week group and the sham or naive group. (c) The arterial phase was significant prolonged in the 1- and 2-week group compared with that of the sham or naive group. And, there was no significant difference between the 4-week group and the control group. (d) The venous phase significant prolonged in the 2-week group. *P < 0. 01 compared with naive group.
Histological damage in the ischemic retina
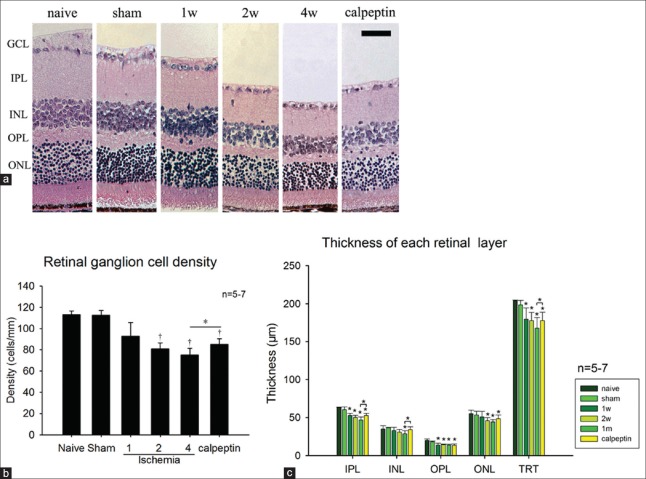
There was no significant difference between the sham and the naive group in the thickness of each retinal layer or the retinal ganglion cells (RGCs) number (P > 0.05) [Figure 4a and 4b]. After 1 week (179.8 ± 14.7 μm) of ischemia, thickness of the total retina (total reaction time [TRT]) (P < 0.01) [Figure 4c] was significantly diminished compared with that of the sham (198.4 ± 6.0 μm) or naive group (203.9 ± 2.0 μm), but there were no evident differences in the other layer. After ischemia for at least 2 weeks, RGCs in the GCL were lost (80.8 ± 5.5 cells/mm) (P < 0.01) [Figure 4c] and thickness of the IPL (50.1 ± 2.8 μm) [Figure 4c], ONL (45.9 ± 4.0 μm) [Figure 4c], and OPL (14.3 ± 0.8 μm) [Figure 4c] decreased (P < 0.05). After ischemia for 4 weeks, thickness of the INL (28.8 ± 3.8 μm) showed a significant decrease (P < 0.05) [Figure 4c]. However, the loss of RGCs and decrease of the TRT in the calpeptin group (84.9 ± 5.4 cells/mm and 177.9 ± 11.0 μm) was alleviated compared with the 4-week group (75.0 ± 6.4 cells/mm and 168.1 ± 13.8 μm) [Figure 4a–4c].
Figure 4.
(a) The H and E stain of retina of each group. Thickness of the retina decreased gradually with the ischemia time going on. The loss of RGC (b) and the thickness decrease of each retinal layer (c) after ischemia were shown. And the lost of RGCs and decrease of the IPL, INL and TRT in the calpeptin group alleviated compared with the 4-week group. *P < 0.05 compared with naive group, †P < 0.01 compared with naive group. Scale bar = 50 μm. RGC: Retinal ganglion cell; TRT: Total reaction time; IPL: Inner plexiform layer; INL: Inner nuclear layer.
Western blog analysis of the collapsin response mediator protein-2 expression and phosphorylation level in the chronic ischemic retina
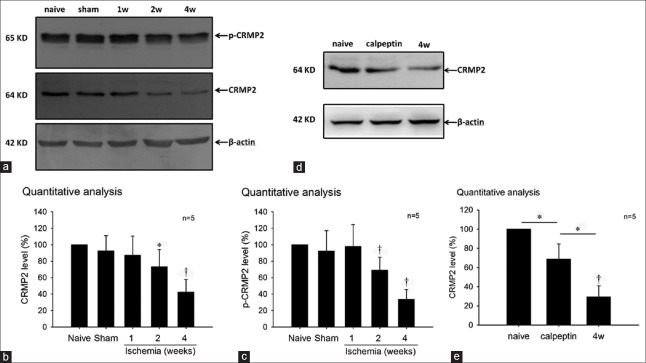
To detect the changes of CRMP2 expression and phosphorylation level in the chronic ischemic retina, we used Western blot to evaluate the CRMP2 and p-CRMP2 (Thr514) levels in each group. In our results [Figure 5], levels of CRMP2 [Figure 5b] and p-CRMP2 (Thr514) [Figure 5c] were markedly decreased in the 2- and 4-week ischemia groups versus the naive group, whereas there were no significant changes in the 1-week or the sham group. In the calpeptin group, the reduction of CRMP2 was alleviated compared with the 4-week group [Figure 5d and 5e]. The results suggested that CRMP2 proteolysis and hypophosphorylation at Thr514 were involved in the chronic ischemic retina, and the calpeptin prevented the CRMP2 from a decrease in the ischemic retina.
Figure 5.
(a) Western blot results of the CRMP2 expression and phosphorylation level in the chronic ischemic retina. Quantitative analysis showed CRMP2 (b) and p-CRMP2 (c) level decreased in the 2 and 4 weeks ischemia groups versus the naive group. (d and e) The reduction of CRMP2 was alleviated in the calpeptin group comparing with the 4 week group. *P < 0.05 compared with naive group. †P < 0. 01 compared with naive group. CRMP2: Collapsin response mediator protein-2.
Immunohistochemistry analysis of the collapsin response mediator protein-2 expression and phosphorylation level in the chronic ischemic retina
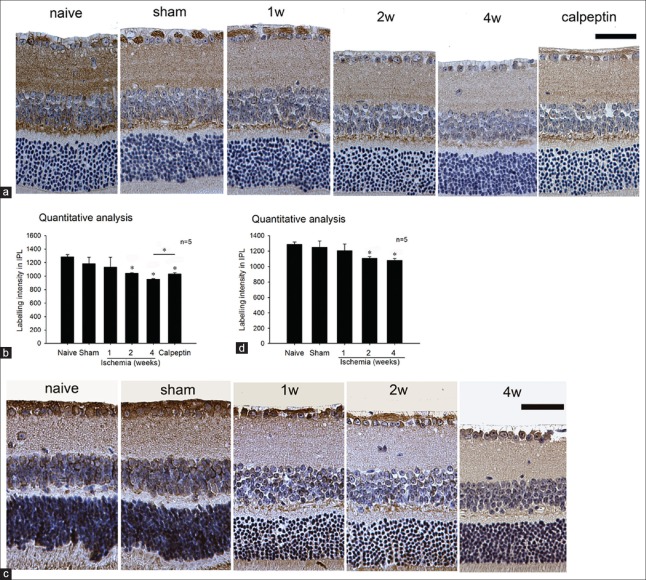
CRMP2 is contained in the cell body and synapses of the adult mice's RGC. Semi-quantitative analysis of the CRMP2 expression level and p-CRMP2 at Thr514 in the IPL of the ischemic retina was compatible with our results of Western blot analysis. Levels of CRMP2 and p-CRMP2 (Thr514) were markedly decreased versus the naive group in the 2- and 4-week ischemia groups [Figure 5b and 5d]. In the calpeptin group, the reduction of CRMP2 in the IPL was alleviated compared with the 4-week group [Figure 6b], whereas there were no significant changes in the other groups.
Figure 6.
Semi-quantitative analysis showed levels of CRMP2 (a and b) and p-CRMP2 (Thr514) (c and d) were markedly decreased in the 2 and 4 weeks ischemia groups versus the naive group. *P < 0.05 compared with naive group. Scale bar = 50 μm. CRMP2: Collapsin response mediator protein-2.
Discussion
In this study, we characterized histological changes and functional impairment in the OIS animal model induced by permanent occlusion of bilateral internal carotid arteries. The expression and phosphorylation level changes of CRMP2 in the retina at different time points following ischemia were also characterized for the first time. We shall outline differences between our OIS model and other existing animal models, analyze the histological and functional impairment in the model, and discuss the role of CRMP2 in the retinal ischemic injury.
A number of mammalian models have been established to study retinal ischemia. High intraocular pressure (IOP)-induced ocular ischemia is a common method of research retinal ischemia and has been repeated in a number of studies.[17,18,19] The blood flow of the ocular was obstructed completely due to the increased IOP that was higher than the ocular perfusion pressure. However, a high IOP model is not suitable to provide data for the research of OIS, because both the high IOP and ischemia are responsible for the retinal damage, which does not correspond with the typical clinical situation of OIS. Vascular ligation is another frequently used model for retinal ischemia research. The simplest form of it is bilateral occlusion of common carotid artery (BCCAO/2-vessel occlusion),[20,21,22] and a more technically demanding method of vascular ligation is bilateral occlusion of both vertebral and CCAs (4-vessel occlusion).[23] Although these models are more semblable with severe carotid insufficiency in the OIS, the blood flow insufficiency is much more severe when compared with general clinical patients.
The ICA arises from the CCA and provides blood supply to the pterygopalatine artery (PPA), from which the ophthalmic artery originates. As we already know, the ophthalmic artery is in charge of the blood supply of the eyes.[24,25] Hence, the ocular blood flow shall be obstructed when the ICA is ligated. Whereas it is found that latent anastomosis existed between the PPA and the ECA,[26] the PPA is ready to supply blood to the ICA immediately after the ICA occlusion, which results in reduction rather than obstruction of the blood flow of the eyes after the ICA ligation. In the bilateral ICA occlusion model, the blood flow insufficiency is milder than that after the BCCAO, which is closer to the clinical situation of OIS. Even so, the surgical mortality is as high as 15%.
Retinal pathology is an important consequence of OIS in humans presenting with venous dilation, retinal hemorrhage, and microaneurysms. Moreover, serious ischemic changes include retinal arteriolar narrowing, retinal capillary nonperfusion, macular edema, and optic disk and retinal neovascularization.[10,11] In our experiment, the retinal A/V ratio significantly increased after 4 weeks of ischemia [Figure 1a]. This result was consistent with the clinical situation of OIS patients.
ERG recording is an international and objective method to evaluate the function of the inner and outer retina, with the a-wave providing information about the function of the photoreceptors and the b-wave providing information about the functions of bipolar cells and Müller cells. According to various reports, the amplitude of both a- and b-waves in the ERG are reduced after ischemia.[27,28] After bilateral ligation of the ICA for 1 week [Figure 2], the amplitudes of both the a- and b-waves were reduced, which was consistent with aforementioned reports. The present result suggested the dysfunction of bipolar and/or Müller cells and photoreceptors in the hypoperfusional retina. Interestingly, except the decrease of the amplitudes, we also observed the change in the latency of b-wave. However, there is difficult to understand the varying pattern of it. We will try to explore the relation between retinal ischemia and the latency of b-wave in the next experiment.
Fluorescein angiography is a commonly used test to assess the blood flow of the retinal circulation. In the OIS, the prolonged arm-to-retina circulation time is a frequent sign. Irregular and/or prolonged retinal filing time is present in approximately 60% of OIS patients. This is the most specific, but not the most sensitive, fluorescein angiography sign of OIS. The prolonged retinal arteriovenous time is present in up to 95% of cases, which is the most sensitive angiographic sign of OIS.[3,4,8] In animal experiments, the tail vein–retinal circulation time is to be equivalent to the arm-to-retina circulation time in humans. Our results [Figure 3b] showed the tail vein–retinal circulation time to be prolonged after ischemia for 1 and 2 weeks. However, it turned normal after ischemia for 4 weeks. We suppose that this may be because of the establishment of collateral circulation after the chronic ischemia. The prolonged arterial phase and venous phase in our model corresponded with the recognized angiographic sign of OIS patients [Figure 2c and 2d].
In the histological assessment [Figure 4], TRT showed a decrease after 1 week of ischemia with no other significant changes. According to existing studies,[26] the RGCs tend to be more sensitive to ischemic injury. Hence, our results imply that the decrease of TRT may be due to the diminishing of the nerve fiber layer, which includes the axons of the RGCs. After ischemia for at least 2 weeks, RGCs at lost and more severe retina thickness decrease is demonstrated [Figure 4b and 4c]. The histological results showed that the retinal damage in our model was mild compared with other ischemic models, such as high IOP[17,18,19] and 4-vessel occlusion.[23,29] Taken together, the present findings indicate that bilateral ligation of the ICA-induced mild retinal histological and functional impairments similar to the OIS caused by carotid artery stenosis.
CRMP2 is a phosphoprotein that can be phosphorylated at multiple sites by several kinases to regulate its activity. In the IH process, hypophosphorylation of CRMP2 at Thr514 occurred. This has been reported by several studies in the neurology field. In the hypoxia-ischemia-induced periventricular leukomalacia (PVL) rat model,[1] hypophosphorylation of CRMP2 at Thr514 was observed after 48 h post-HI in the PVL brain. In another hypoxia-ischemia model, hypophosphorylation of CRMP2 at Thr514 also occurred in the neonatal brain after both unilateral ligation of CCA and hypoxic gas exposure.[30] In our study, we demonstrated hypophosphorylation of CRMP2 at Thr514 in the retina after bilateral ligation of the ICA for at least 2 weeks, which corresponded to other researches [Figure 5c]. On the contrary, Hou et al.[31] detected hyperphosphorylation at Thr555 during ischemic injury in the ischemic mature brain. Although the causes of the discrepancy in the phosphorylation level of CRMP2 at different states remain unclear, it might be associated with the regulation of activity after ischemic injury. Phosphorylation of CRMP2 at Thr514 mainly relates to axonal elongation and down-regulates its activity.[1,2] Yin et al.[2] have reported the formation of multiple axons induced by the expression of the nonphosphorylated CRMP2 in hippocampal neurons. This indicates that phosphorylation will inactivate CRMP2 while hypo- or non-phosphorylation will activate it. In summary, our results suggest that CRMP2 could be activated in the chronic ischemic retina. However, the role of activation in this condition remains to be elucidated.
The cleavage of CRMP2 is another ischemic/hypoxic injury marker besides hypophosphorylation. A number of studies[1,14,15] have found that total CRMP2 was decreased due to calpain cleaved in ischemic/hypoxic injury. Moreover, the cleavage of CRMP2 is proved to be deleterious for the neurons. Studies[2,32] have showed that Tat-CRMP2, which may decrease the cleavage of CRMP2 by calpain, may reduce ischemic injury and increase the survival of neurons. In this study, we detected the reduction of the total CRMP2 in the ischemic retina [Figure 5b]. When we used the inhibitor of calpain (calpeptin) to inhibit the cleavage of CRMP2, the decrease of CRMP2 was alleviated in the ischemic retina [Figure 5e]. HE staining showed the calpeptin prevented a decrease in the thickness of ischemic retina. It indicated that inhibiting the cleavage of CRMP2 would alleviate the injury of the ischemic retina. However, the cleaved band of CRMP2 which has been found in the early stage of the IH process[1,2] has not been discovered in our experiment. That may be because the cleaved band had been cleaved and resolved by calpain in the early time period (such as 72 h) after ligation. This hypothesis remains to be confirmed by further experiment.
From these findings, we draw the conclusion that both hypophosphorylation of CRMP2 at Thr514 and reduction of total CRMP2 occurred in chronic ischemic retina induced by bilateral ligation of the ICA, and inhibiting the cleavage of CRMP2 would alleviate the degree of retinal ischemia injury.
CRMP2 is highly expressed in the developing vertebrate nervous system and in adult brain structures that still retain the capacity of differentiation. Christie et al.[33] found that CRMP2 is expressed in the RGC layer in the eye of zebrafish embryos. In our IHC results [Figure 6], we demonstrated that CRMP2 is also expressed in the cell body and synapses of the adult mice's RGC, which implies the capacity of plasticity and differentiation of the RGC. Semi-quantitative analysis [Figure 6b and 6d] of CRMP2 expression level and p-CRMP2 at Thr514 in the IPL of the ischemic retina revealed that both expression level and phosphorylation of CRMP2 significantly decreased in the chronic ischemic retina, which was compatible with our results of Western blot analysis.
Taken together, our findings revealed that bilateral ligation of the ICA causes retinal ischemia in mice comparable to OIS in humans. Therefore, our experiment represents a novel model that may have potential use in exploring the special mechanisms of OIS. Moreover, CRMP2 might play a key role during the ischemic/hypoxic injury in the retina and inhibiting the cleavage of CRMP2 can ameliorate the IH injury. Future treatment strategies of OIS might target CRMP2 as a crucial factor, and more research needs to be done to uncover the mechanism of chronic ischemic injury in the retina.
Financial support and sponsorship
This study was supported by a grant from National Science Foundation of China (No. 81173412).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
References
- 1.Sato Y, Ishida-Nakajima W, Kawamura M, Miura S, Oguma R, Arai H, et al. Hypoxia-ischemia induces hypo-phosphorylation of collapsin response mediator protein 2 in a neonatal rat model of periventricular leukomalacia. Brain Res. 2011;1386:165–74. doi: 10.1016/j.brainres.2011.02.027. doi: 10.1016/j.brainres.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 2.Yin Y, Wang Y, Chen L, Han S, Zhao L, Luo Y, et al. Tat-collapsin response mediator protein 2 (CRMP2) increases the survival of neurons after NMDA excitotoxity by reducing the cleavage of CRMP2. Neurochem Res. 2013;38:2095–104. doi: 10.1007/s11064-013-1118-9. doi: 10.1007/s11064-013-1118-9. [DOI] [PubMed] [Google Scholar]
- 3.Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome – A systematic review. Med Sci Monit. 2012;18:RA138–44. doi: 10.12659/MSM.883260. doi: 10.12659/MSM.883260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Du R, Wang JL, Wang YL. Role of RhoA/MERK1/ERK1/2/iNOS signaling in ocular ischemic syndrome. Graefes Arch Clin Exp Ophthalmol. 2016;254:2217–26. doi: 10.1007/s00417-016-3456-1. doi: 10.1007/s00417-016-3456-1. [DOI] [PubMed] [Google Scholar]
- 6.Xie GL, Li JF, Wang Y, Jonas JB, Li HY, Wang YL. MicroRNA-126 regulates angiogenic growth factors through targeting Spred-1 in a model of chronic ocular ischemia. J Biomater Tissue Eng. 2016;6:122–33. doi: 10.1166/jbt.2016.1426. [Google Scholar]
- 7.Deo RC, Schmidt EF, Elhabazi A, Togashi H, Burley SK, Strittmatter SM. Structural bases for CRMP function in plexin-dependent semaphorin3A signaling. EMBO J. 2004;23:9–22. doi: 10.1038/sj.emboj.7600021. doi: 10.1038/sj.emboj.7600021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charrier E, Reibel S, Rogemond V, Aguera M, Thomasset N, Honnorat J. Collapsin response mediator proteins (CRMPs): involvement in nervous system development and adult neurodegenerative disorders. Mol Neurobiol. 2003;28:51–64. doi: 10.1385/MN:28:1:51. doi: 10.1385/MN: 28: 1: 51. [DOI] [PubMed] [Google Scholar]
- 9.Yu Y, Jian MY, Wang YZ, Han RQ. Propofol ameliorates calpain-induced collapsin response mediator protein-2 proteolysis in traumatic brain injury in rats. Chin Med J. 2015;128:919–27. doi: 10.4103/0366-6999.154298. doi: 4103/0366-6999.154298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt EF, Strittmatter SM. The CRMP family of proteins and their role in Sema3A signaling. Adv Exp Med Biol. 2007;600:1–11. doi: 10.1007/978-0-387-70956-7_1. doi: 10.1007/978-0-387-70956-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchida Y, Ohshima T, Yamashita N, Ogawara M, Sasaki Y, Nakamura F, et al. Semaphorin3A signaling mediated by Fyn-dependent tyrosine phosphorylation of collapsin response mediator protein 2 at tyrosine 32. J Biol Chem. 2009;284:27393–401. doi: 10.1074/jbc.M109.000240. doi: 10.1074/jbc.M109.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole AR, Knebel A, Morrice NA, Robertson LA, Irving AJ, Connolly CN, et al. GSK-3 phosphorylation of the Alzheimer epitope within collapsin response mediator proteins regulates axon elongation in primary neurons. J Biol Chem. 2004;279:50176–80. doi: 10.1074/jbc.C400412200. doi: 10.1074/jbc.C400412200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fei T, Xia K, Li Z, Zhou B, Zhu S, Chen H, et al. Genome-wide mapping of SMAD target genes reveals the role of BMP signaling in embryonic stem cell fate determination. Genome Res. 2010;20:36–44. doi: 10.1101/gr.092114.109. doi: 10.1101/gr.092114.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nada SE, Tulsulkar J, Raghavan A, Hensley K, Shah ZA. A derivative of the CRMP2 binding compound lanthionine ketimine provides neuroprotection in a mouse model of cerebral ischemia. Neurochem Int. 2012;61:1357–63. doi: 10.1016/j.neuint.2012.09.013. doi: 10.1016/j.neuint.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang SX, Kappler J, Zurakowski B, Desbois A, Aylsworth A, Hou ST. Calpain cleavage of collapsin response mediator proteins in ischemic mouse brain. Eur J Neurosci. 2007;26:801–9. doi: 10.1111/j.1460-9568.2007.05715.x. doi: 10.1111/j.1460-9568.2007.05715.x. [DOI] [PubMed] [Google Scholar]
- 16.Kitaoka Y, Munemasa Y, Hayashi Y, Kuribayashi J, Koseki N, Kojima K, et al. Axonal protection by 17ß-estradiol through thioredoxin-1 in tumor necrosis factor-induced optic neuropathy. Endocrinology. 2011;152:2775–85. doi: 10.1210/en.2011-0046. doi: 10.1210/en.2011-0046. [DOI] [PubMed] [Google Scholar]
- 17.Russo R, Cavaliere F, Varano GP, Milanese M, Adornetto A, Nucci C, et al. Impairment of neuronal glutamate uptake and modulation of the glutamate transporter GLT-1 induced by retinal ischemia. PLoS One. 2013;8:e69250. doi: 10.1371/journal.pone.0069250. doi: 10.1371/journal.pone.0069250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu XQ, Wu BJ, Pan WH, Zhang XM, Liu JH, Chen MM, et al. Resveratrol mitigates rat retinal ischemic injury: The roles of matrix metalloproteinase-9, inducible nitric oxide, and heme oxygenase-1. J Ocul Pharmacol Ther. 2013;29:33–40. doi: 10.1089/jop.2012.0141. doi: 10.1089/jop.2012.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SY, Shim MS, Kim KY, Weinreb RN, Wheeler LA, Ju WK. Inhibition of cyclophilin D by cyclosporin A promotes retinal ganglion cell survival by preventing mitochondrial alteration in ischemic injury. Cell Death Dis. 2014;5:e1105. doi: 10.1038/cddis.2014.80. doi: 10.1038/cddis.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavinsky D, Arterni NS, Achaval M, Netto CA. Chronic bilateral common carotid artery occlusion: A model for ocular ischemic syndrome in the rat. Graefes Arch Clin Exp Ophthalmol. 2006;244:199–204. doi: 10.1007/s00417-005-0006-7. doi: 10.1007/s00417-005-0006-7. [DOI] [PubMed] [Google Scholar]
- 21.Werling D, Reglodi D, Kiss P, Toth G, Szabadfi K, Tamas A, et al. Investigation of PACAP fragments and related peptides in chronic retinal hypoperfusion. J Ophthalmol 2014. 2014 doi: 10.1155/2014/563812. 563812. doi: 10.1155/2014/563812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo XJ, Tian XS, Ruan Z, Chen YT, Wu L, Gong Q, et al. Dysregulation of neurotrophic and inflammatory systems accompanied by decreased CREB signaling in ischemic rat retina. Exp Eye Res. 2014;125:156–63. doi: 10.1016/j.exer.2014.06.003. doi: 10.1016/j.exer.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Yu B, Xiang YH, Han XJ, Xu Y, So KF, et al. Changes in retinal morphology, electroretinogram and visual behavior after transient global ischemia in adult rats. PLoS One. 2013;8:e65555. doi: 10.1371/journal.pone.0065555. doi: 10.1371/journal.pone.0065555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kidoguchi K, Tamaki M, Mizobe T, Koyama J, Kondoh T, Kohmura E, et al. In vivo X-ray angiography in the mouse brain using synchrotron radiation. Stroke. 2006;37:1856–61. doi: 10.1161/01.STR.0000226904.96059.a6. doi: 10.1161/01.STR.0000226904.96059.a6. [DOI] [PubMed] [Google Scholar]
- 25.Steele EC, Jr, Guo Q, Namura S. Filamentous middle cerebral artery occlusion causes ischemic damage to the retina in mice. Stroke. 2008;39:2099–104. doi: 10.1161/STROKEAHA.107.504357. doi: 10.1161/STROKEAHA.107.504357. [DOI] [PubMed] [Google Scholar]
- 26.Tamaki M, Kidoguchi K, Mizobe T, Koyama J, Kondoh T, Sakurai T, et al. Carotid artery occlusion and collateral circulation in C57Black/6J mice detected by synchrotron radiation microangiography. Kobe J Med Sci. 2006;52:111–8. [PubMed] [Google Scholar]
- 27.Grozdanic SD, Sakaguchi DS, Kwon YH, Kardon RH, Sonea IM. Functional characterization of retina and optic nerve after acute ocular ischemia in rats. Invest Ophthalmol Vis Sci. 2003;44:2597–605. doi: 10.1167/iovs.02-0600. doi: 10.1167/iovs.02-0600. [DOI] [PubMed] [Google Scholar]
- 28.Ogishima H, Nakamura S, Nakanishi T, Imai S, Kakino M, Ishizuka F, et al. Ligation of the pterygopalatine and external carotid arteries induces ischemic damage in the murine retina. Invest Ophthalmol Vis Sci. 2011;52:9710–20. doi: 10.1167/iovs.11-8160. doi: 10.1167/iovs.11-8160. [DOI] [PubMed] [Google Scholar]
- 29.Barros CA, Ekuni R, Moro MA, Pereira FM, Dos Santos Pereira MA, Milani H. The cognitive and histopathological effects of chronic 4-vessel occlusion in rats depend on the set of vessels occluded and the age of the animals. Behav Brain Res. 2009;197:378–87. doi: 10.1016/j.bbr.2008.10.023. doi: 10.1016/j.bbr.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Bhatia I, Cai Z, He QY, Cheung PT, Chiu JF. Proteomic analysis of neonatal mouse brain: Evidence for hypoxia- and ischemia-induced dephosphorylation of collapsin response mediator proteins. J Proteome Res. 2008;7:2507–15. doi: 10.1021/pr800108k. doi: 10.1021/pr800108k. [DOI] [PubMed] [Google Scholar]
- 31.Hou ST, Jiang SX, Aylsworth A, Ferguson G, Slinn J, Hu H, et al. CaMKII phosphorylates collapsin response mediator protein 2 and modulates axonal damage during glutamate excitotoxicity. J Neurochem. 2009;111:870–81. doi: 10.1111/j.1471-4159.2009.06375.x. doi: 10.1111/j.1471-4159.2009.06375.x. [DOI] [PubMed] [Google Scholar]
- 32.Bu X, Zhang N, Yang X, Liu Y, Du J, Liang J, et al. Proteomic analysis of cPKCßII-interacting proteins involved in HPC-induced neuroprotection against cerebral ischemia of mice. J Neurochem. 2011;117:346–56. doi: 10.1111/j.1471-4159.2011.07209.x. doi: 10.1111/j.1471-4159.2011.07209.x. [DOI] [PubMed] [Google Scholar]
- 33.Christie TL, Starovic-Subota O, Childs S. Zebrafish collapsin response mediator protein (CRMP)-2 is expressed in developing neurons. Gene Expr Patterns. 2006;6:193–200. doi: 10.1016/j.modgep.2005.06.004. doi: 10.1016/j.modgep.2005.06.004. [DOI] [PubMed] [Google Scholar]