Abstract
Depression prevention requires identifying key risk contributors. Prior studies have identified several factors related to late-life depression but have seldom addressed factors jointly or in dose-response fashion. This study aimed to examine a wide range of potential predisposing factors and to estimate individual and joint contributions to risk of late-life depression in women. A total of 21,728 women aged ≥65 years, without prior depression, in the Nurses’ Health Study conducted in the United States were followed from 2000–2010. Demographic, social, lifestyle/behavioral and health variables were selected a priori from the literature or previous findings in this cohort. Depression was defined as physician/clinician-diagnosed depression, regular antidepressant use, or the presence of severe depressive symptoms. During 10-year follow-up, 3,945 incident cases were identified. After simultaneous multivariable-adjustment, multiple factors in the domains of social stress (lower self-rated societal position and high volume of caregiving to disabled/ill relatives), unfavorable lifestyle (smoking, physical inactivity, heavy or binge drinking), and poor physical health (multiple comorbidity burden, excessive sleep, difficulty falling/staying asleep, bodily pain, and physical/functional limitation or disability) were significantly associated with higher depression risk; many featured dose-response relationships. Sensitivity analyses that excluded outcomes within 2 years yielded similar estimates. The total population attributable fraction for all factors was 55.5%. Physical/functional limitation accounted for one-quarter of population attributable fraction, followed by problematic sleep, inadequate exercise, and pain (combining for one-third of population attributable fraction). Efforts to remediate or prevent these factors may contribute to an efficient strategy for late-life depression prevention in women.
Keywords: Cohort, Depressive Disorders, Epidemiology, Geriatrics, Prevention, Risk Factors
Incident late-life depression (LLD) is defined as depression occurring for the first time typically after age 60 or 65, and is a common and life-impairing mental health problem in older people. LLD can be distinguished from early-life depression in both etiology and phenomology (1). Even with appropriate treatment, residual symptoms and dysfunction are common, underscoring the priority for prevention. A critical step in developing a rational prevention strategy is to determine major contributors to disease. Although the exact etiology is not fully understood, prior evidence points to key factors as potentially high-impact in LLD risk (2, 3), such as medical comorbidity burden and physical/functional limitations or disability. However, other potentially modifiable factors have been examined less comprehensively. In the literature in investigating LLD risk, potential limitations include: (1) risk factor information is often available once at baseline (4, 5); (2) association patterns (threshold/dose-response/plateau effects) are unclear due to lack of data (6); (3) since health and lifestyle behaviors are often correlated, studying one factor without adjustment for relevant confounders may bias results; and (4) although average daily alcohol intake has been examined in prospective studies (4, 7, 8), the specific relation of heavy or binge drinking to LLD risk has been relatively understudied.
To address the above challenges, we conducted prospective analyses in the Nurses’ Health Study (NHS), a well-characaterized cohort of women. We related potential risk factors to incident LLD, defined as onset among those aged ≥65 years, and aimed to investigate a comprehensive array of potential risk/protective factors simultaneously – with particular attention to potentially-modifiable factors. We applied the Institute of Medicine concept of selective prevention (addressing persons at heightened risk for a clinical outcome), by addressing demographic, social, lifestyle/behavioral and health/medical factors which may place older women at high risk for developing depression. With respect to health factors, we were specifically interested in addressing sleep issues because emerging evidence supports sleep difficulty as an independent risk factor for depression (9, 10) – rather than merely a manifestation of it.
METHODS
The Nurses’ Health Study
The NHS began in 1976 when 121,700 U.S. female nurses, aged 30–55 years, returned a mailed questionnaire regarding lifestyle and medical history. Participants have received questionnaires biennially since then, with >90% follow-up rate in each 2-year cycle.
Risk and protective factors
All of the potential risk/protective factors examined in this study were self-reported from NHS questionnaires. They were selected a priori from the literature or prior NHS findings (2, 3, 11) and were grouped into 4 categories:
Demographic: Age (continuous, in years); education (registered nurse/bachelor/advanced degree); and race/ethnicity (non-Hispanic whites/blacks/others).
Social: Social network, measured by the simplified Berkman-Syme Social Network Index (incorporating information of marital status, number of close contacts, church attendance, and participation in community organizations) (quintiles; higher quintile representing higher level of social network)(12); low subjective social status (measured using a 10-point visual analog scale of subjective feeling about standing in U.S. society) (high/medium-high/medium-low/low standing)(13); hours of regular caregiving to children/grandchildren and to disabled/ill relatives (no/some(1–20 hours/week)/a lot(>20 hours/week)).
Lifestyle/behavioral: Body mass index (BMI, in kg/m2) (<18.5/18.5–24.9/25.0–29.9/30.0–34.9/35.0+); alternate Mediterranean (aMed) diet score (quintiles; higher quintile representing better adherence to aMed diet)(14); cigarette smoking (never/past/current:1–14/15–24/25+ cigarettes/day); physical activity (measured as average hours/week engaging in moderate to vigorous exercise)(0/0.1–0.9/1.0–2.4/2.5–4.9/5.0+); largest number of alcoholic drinks in a single day of a typical month during the past year (none/1–2/3+; having ≥3 drinks is considered as heavy/binge drinking). Of note, although individual nutrients have been related to depression (15), we chose aMed diet to represent overall dietary pattern. Self-reported weight, physical activity, and dietary intake have been shown to be reliably and validly measured through NHS validation studies (16–20).
Health/medical: Medical comorbidity burden (≤1/2+)(21); daily hours of sleep (≤6/7–8/9/10+); difficulty falling/staying asleep (none/little/some/most or all of the time); total bodily pain (none/very mild or mild/moderate/severe or very severe); physical/functional limitations, defined as having any limitations in milder activities or more than moderate limitations in demanding activities (yes/no)(21). Questions on pain and physical/functional limitations came from 36-item Short-Form Health Status Survey (SF-36)(22).
For each variable, the category with hypothesized lower/lowest risk was the referent. The category with most individuals was the referent if the category of putatively lower/lowest risk was uncertain. For the factors that did not have straightforward cutoffs, the cutoffs were determined by the distribution of response options, their plausible expected associations, and their conceptual degrees of intensity or severity. For caregiving intensity, because our data could not distinguish between people providing no care and those who did not have specific family members to be cared for, both groups jointly served as the referent.
Assessment and measures of depression
Depression information included self-reported depressive symptoms, regular use of antidepressants, and physician/clinician diagnosis. Symptoms were assessed using the Mental Health Index-5 (MHI-5) subscale of the SF-36 in 1992, 1996, and 2000, the Center for Epidemiologic Studies Depression-10 (CESD-10) in 2004 and the Geriatric Depression Scale-15 (GDS-15) in 2008, all of which have validated cutpoints for clinical depression (23–25). Questions on antidepressant use and physician/clinician diagnosis of depression were assessed biennially since 1996 and 2000, respectively. Because 2000 was the earliest year in which we could classify women as ever having doctor-diagnosed depression, we designated this as the study baseline.
Because the NHS questionnaire was asked biennially, participants reported on their depressive symptoms, medications, or doctor diagnosis within each 2-year time window. We had no information on the number or duration of discrete depressive episodes within 2-year windows, so recurrent depression events could not be unambiguously determined; therefore, we only examined incidence in this study. The date of incident LLD onset was defined by the first occurrence of physician/clinician-diagnosed depression, regular antidepressant use, or severe depressive symptoms using published cutpoints during follow-up (24, 25). This ‘Boolean OR’ definition was applied, as preliminary data from an ongoing validation study support its optimal sensitivity and specificity. For antidepressants, we included selective serotonin reuptake inhibitors but not tricyclic antidepressants (TCAs), as we found elsewhere that TCAs would be more likely to be prescribed for other indications (26). Because we specifically aimed to examine the associations between sleep problems and depression risk, the item related to sleep in CESD-10 (“my sleep was restless”) was removed for scoring. To be conservative, we did not alter the cutoff score of CESD-10 for probable depression after excluding the sleep item, so that a participant’s CESD-10 score was not influenced by her sleep symptoms but by the severity of the remaining depressive symptoms. As expected, the observed LLD incidence was lower when using only 9 items compared to using 10 items (21.9 and 26.4 per 1000 person-years, respectively), although both estimates were in the range of LLD incidence estimates among women in prior studies that featured clinical evaluations of depression (27–29).
Sample for analysis
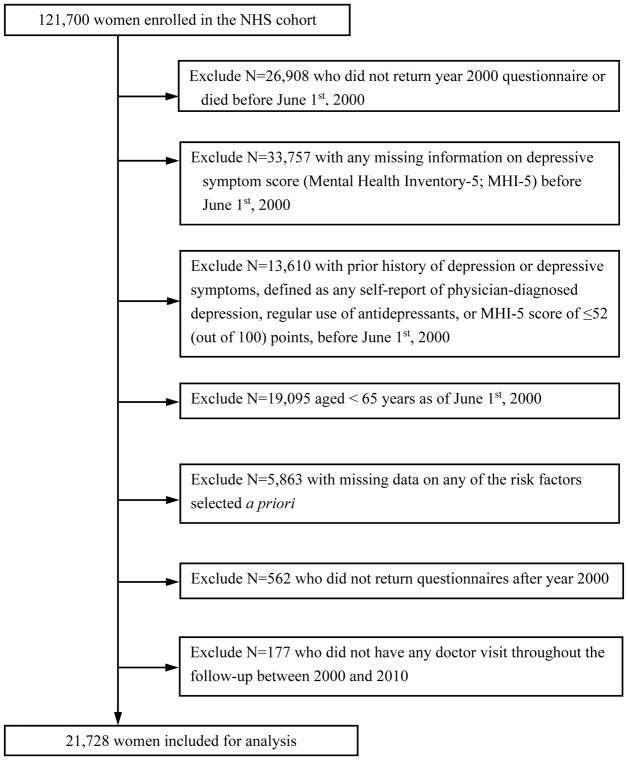
After excluding women who died before 2000 or did not return the 2000 questionnaire (n=26,908), whose history of depression could not be determined (n=33,757), who had prior indication of depression assessed by MHI-5 score, physician/clinician diagnosis or antidepressant medication (n=13,610), who aged under 65 years (n=19,095), who did not provide information on all risk factors selected a priori (n=5,863), who stopped returning questionnaires after 2000 (n=562) or had no health examination during follow-up (so there is no opportunity for depression detection)(n=177), 21,728 women were included for analysis (Figure 1). The institutional review board at Brigham and Women’s Hospital approved the study protocol.
Figure 1.
Study Flow Diagram Illustrating the Nurses’ Health Study Cohort Exclusions at Study Baseline in 2000
Statistical methods
Estimating depression incidence
Since the study baseline, 4 biennial follow-up questionnaire cycles were completed (i.e., 2002–04/2004–06/2006–08/2008–10). Individuals contributed person-years from the baseline questionnaire return date to the date of incident LLD, death, end of follow-up (6/1/2010), or last returned questionnaire, whichever occurred first. Age- and multivariable-adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) of developing LLD were estimated from Cox proportional hazards models. Breslow approximation (30) was used to address ties. Exposures were first entered into the models as indicator variables and were next examined for possible dose-response relationships. Model 1 only adjusted for age (16 separate models, one for each exposure of interest). Model 2 simultaneously included all 16 exposures but only with baseline values. Model 3 (final model) included the same set of covariates as Model 2, but exposures were updated in a time-varying fashion where possible (including social network, all lifestyle behaviors, comorbidity, and physical/functional limitations). We carried forward risk/protective factor information in the prior questionnaire cycle if missing during follow-up (6.4% of data). The collinearity diagnostics results suggested that the multicollinearity was not a major concern; the variance inflation factors of all variables included in the model were 1.01–2.60. The proportional hazards assumptions were not violated.
To scrutinize robustness of results, we conducted three sensitivity analyses: (1) excluding cases in the first 2 years of follow-up and adding a 2-year outcome lag to address potential reverse causation (e.g., incipient depression may lead to changes in sleep or physical activity), and further performing a 6-year lagged analysis for additional scrutiny of reverse causation; (2) applying alternative definitions of depression – either a stricter definition of clinical depression, utilized previously, based only on diagnosis or treatment (31) or a less strict definition of diagnosis, symptoms, or antidepressant use including TCAs; and (3) censoring person-years once participants failed to provide information on all exposures of interest anytime during follow-up.
Estimating contributions to total risk of late-life depression
To estimate the proportion of LLD attributable to different factors, we calculated population attributable fractions (PAF) and corresponding 95% CIs using methods detailed elsewhere (32). We interpreted the PAF as the estimated percentage of new LLD cases occurring in this population that could have been prevented if all women had been in the low-risk group. We dichotomized each factor in PAF calculation for simplicity and increased statistical efficiency. We chose the binary cutoffs which may optimally reflect the most relevant contrasts and reasonable counterfactual referent in the older population. This dichotomization was also guided by the primary analysis results (Model 3, Table 2). The estimated PAF calculated for each risk factor was adjusted for other significant exposures in the primary analysis. Because physical/functional limitation was the most prevalent risk factor with the largest single PAF (see under Results), yet was more difficult to modify, we further performed PAF analyses stratified by this factor to investigate the contributions of different risk factors to LLD risk among those with and without physical/functional limitations.
Table 2.
Distribution and Relative Risk of Late-Life Depression among 21,728 Elderly Women in the Nurses’ Health Study, 2000–2010
| Variable | No. of cases/person-years | Incident rate per 1000 person-years | Model 1a
|
Model 2b
|
Model 3c
|
||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | (95% CI) | P for trend | |||
| Education level | |||||||||
| Registered nurse degree | 2973/129700 | 22.92 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 0.26 |
| Bachelor degree | 687/34277 | 20.04 | 0.92 | 0.85, 1.00 | 0.98 | 0.90, 1.07 | 0.99 | 0.91, 1.08 | |
| Advanced degree | 285/16163 | 17.63 | 0.81 | 0.71, 0.91 | 0.92 | 0.82, 1.03 | 0.92 | 0.81, 1.04 | |
| Race/ethnicity | |||||||||
| Non-Hispanic White | 3841/175121 | 21.93 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | N/A |
| Black | 33/1673 | 19.72 | 0.88 | 0.63, 1.24 | 0.88 | 0.63, 1.25 | 0.88 | 0.62, 1.25 | |
| Others | 71/3346 | 21.22 | 1.02 | 0.81, 1.29 | 1.13 | 0.89, 1.43 | 1.11 | 0.88, 1.40 | |
| Social network (quintiles) | |||||||||
| 5th quintile (most social integration) | 480/24739 | 19.40 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 0.99 |
| 4th quintile | 1249/59475 | 21.00 | 1.06 | 0.95, 1.18 | 1.02 | 0.92, 1.14 | 1.03 | 0.92, 1.14 | |
| 3rd quintile | 762/32244 | 23.63 | 1.20 | 1.07, 1.35 | 1.16 | 1.03, 1.30 | 1.14 | 1.01, 1.28 | |
| 2nd quintile | 574/24833 | 23.11 | 1.17 | 1.04, 1.32 | 1.07 | 0.95, 1.21 | 1.09 | 0.96, 1.23 | |
| 1st quintile (least social integration) | 880/38850 | 22.65 | 1.15 | 1.03, 1.29 | 1.05 | 0.94, 1.18 | 1.05 | 0.94, 1.18 | |
| Subjective social status | |||||||||
| High | 458/25590 | 17.90 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 0.002 |
| Medium-high | 2042/97251 | 21.00 | 1.17 | 1.06, 1.30 | 1.10 | 1.00, 1.22 | 1.09 | 0.98, 1.21 | |
| Medium-low | 1338/54431 | 24.58 | 1.32 | 1.19, 1.47 | 1.16 | 1.04, 1.30 | 1.14 | 1.02, 1.27 | |
| Low | 107/2869 | 37.29 | 1.93 | 1.56, 2.38 | 1.50 | 1.21, 1.85 | 1.45 | 1.17, 1.80 | |
| Regular caregiving to children/grandchildren | |||||||||
| No | 2820/125249 | 22.52 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 0.02 |
| Some (1–20 hrs/wk) | 9451/46027 | 20.53 | 0.97 | 0.90, 1.04 | 0.94 | 0.87, 1.01 | 0.94 | 0.87, 1.01 | |
| A lot (>20 hrs/wk) | 180/8865 | 20.30 | 0.95 | 0.82, 1.11 | 0.87 | 0.75, 1.02 | 0.87 | 0.74, 1.01 | |
| Regular caregiving to disabled or ill relatives | |||||||||
| No | 2981/141438 | 21.08 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | <0.0001 |
| Some (1–40 hrs/wk) | 724/30837 | 23.48 | 1.13 | 1.04, 1.23 | 1.15 | 1.06, 1.25 | 1.15 | 1.06, 1.25 | |
| A lot (>40 hrs/wk) | 240/7866 | 30.51 | 1.40 | 1.23, 1.60 | 1.34 | 1.18, 1.54 | 1.33 | 1.17, 1.52 | |
| Body mass index (BMI) | |||||||||
| <18.5 | 118/4798 | 24.59 | 1.19 | 0.99, 1.44 | 1.13 | 0.90, 1.41 | 1.13 | 0.94, 1.36 | 0.006 |
| 18.5–24.9 | 1752/84579 | 20.71 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |
| 25.0–29.9 | 1342/59976 | 22.38 | 1.08 | 1.01, 1.16 | 1.02 | 0.95, 1.09 | 0.97 | 0.90, 1.04 | |
| 30.0–34.9 | 531/22618 | 23.48 | 1.16 | 1.05, 1.27 | 0.96 | 0.87, 1.06 | 0.92 | 0.83, 1.01 | |
| ≥35.0 | 202/8170 | 24.73 | 1.23 | 1.06, 1.42 | 0.95 | 0.83, 1.10 | 0.86 | 0.73, 1.00 | |
| Alternate Mediterranean diet score (quintiles)d | |||||||||
| 5th quintile (most adherence) | 659/34621 | 19.03 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 0.12 |
| 4th quintile | 889/44447 | 20.00 | 1.14 | 1.03, 1.26 | 1.04 | 0.93, 1.15 | 1.04 | 0.95, 1.15 | |
| 3rd quintile | 751/33701 | 22.28 | 1.19 | 1.07, 1.32 | 1.03 | 0.92, 1.16 | 1.03 | 0.93, 1.15 | |
| 2nd quintile | 819/34454 | 23.77 | 1.28 | 1.15, 1.43 | 1.03 | 0.91, 1.16 | 1.08 | 0.96, 1.28 | |
| 1st quintile (least adherence) | 827/32917 | 25.10 | 1.36 | 1.21, 1.51 | 1.04 | 0.91, 1.18 | 1.09 | 0.98, 1.22 | |
| Cigarette smoking | |||||||||
| Never smoker | 1695/85031 | 19.93 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | <0.0001 |
| Past smoker | 2015/86449 | 23.31 | 1.21 | 1.13, 1.29 | 1.15 | 1.07, 1.23 | 1.16 | 1.08, 1.24 | |
| Current smoker, 1–14 cig/d | 114/5018 | 22.72 | 1.16 | 0.96, 1.41 | 1.31 | 1.10, 1.55 | 1.11 | 0.91, 1.34 | |
| Current smoker,15–24 cig/d | 97/2966 | 32.71 | 1.68 | 1.37, 2.06 | 1.53 | 1.26, 1.86 | 1.44 | 1.17, 1.78 | |
| Current smoker, ≥25 cig/d | 24/676 | 32.71 | 1.81 | 1.20, 2.71 | 1.81 | 1.28, 2.55 | 1.54 | 1.02, 2.32 | |
| Moderate to vigorous activity | |||||||||
| ≥5.0 hours/week (hrs/wk) | 334/24206 | 13.80 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | <0.0001 |
| 2.5–4.99 hrs/wk | 435/24678 | 17.63 | 1.11 | 0.96, 1.28 | 1.12 | 0.98, 1.28 | 1.03 | 0.89, 1.19 | |
| 1.0–2.49 hrs/wk | 475/23689 | 20.05 | 1.23 | 1.07, 1.42 | 1.18 | 1.03, 1.35 | 1.08 | 0.93, 1.24 | |
| 0.1–0.99 hrs/wk | 780/29928 | 26.06 | 1.47 | 1.29, 1.67 | 1.23 | 1.09, 1.40 | 1.20 | 1.05, 1.37 | |
| 0 hr/wk | 1921/77639 | 24.74 | 1.60 | 1.42, 1.80 | 1.19 | 1.06, 1.33 | 1.23 | 1.09, 1.39 | |
| Largest number of drinks in a single day | |||||||||
| none | 1695/73540 | 23.05 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 0.07 |
| 1–2 drinks | 1838/88951 | 20.66 | 0.93 | 0.87, 1.00 | 1.00 | 0.94, 1.08 | 1.00 | 0.94, 1.08 | |
| ≥3 drinks | 412/17650 | 23.34 | 1.13 | 1.01, 1.26 | 1.16 | 1.03, 1.30 | 1.15 | 1.03, 1.29 | |
| Comorbidity | |||||||||
| ≤1 | 3248/159538 | 20.36 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | N/A |
| ≥2 | 697/20603 | 33.83 | 1.64 | 1.51, 1.78 | 1.29 | 1.16, 1.43 | 1.40 | 1.28, 1.52 | |
| Hours of actual sleep per day | |||||||||
| ≤6 hrs | 1101/44445 | 24.77 | 1.23 | 1.15, 1.32 | 0.98 | 0.91, 1.06 | 1.03 | 0.95, 1.11 | 0.0006 |
| 7–8 hrs | 2442/121415 | 20.11 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |
| 9 hrs | 322/12470 | 25.82 | 1.28 | 1.14, 1.44 | 1.18 | 1.04, 1.34 | 1.21 | 1.08, 1.36 | |
| ≥10 hrs | 80/1810 | 44.19 | 2.26 | 1.80, 2.82 | 1.51 | 1.18, 1.93 | 1.96 | 1.56, 2.46 | |
| Difficulty falling asleep or staying asleep | |||||||||
| None of the time | 1034/63460 | 16.29 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | <0.0001 |
| A little of the time | 1221/59950 | 20.37 | 1.25 | 1.15, 1.36 | 1.21 | 1.11, 1.31 | 1.21 | 1.11, 1.31 | |
| Some of the time | 1437/50739 | 28.32 | 1.73 | 1.59, 1.87 | 1.55 | 1.43, 1.69 | 1.53 | 1.41, 1.67 | |
| Most or all of the time | 253/5991 | 42.23 | 2.52 | 2.19, 2.89 | 2.10 | 1.81, 2.44 | 2.04 | 1.77, 2.36 | |
| Bodily pain | |||||||||
| None | 467/34210 | 13.65 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | <0.0001 |
| Very mild/mild | 2125/105754 | 20.09 | 1.48 | 1.34, 1.64 | 1.29 | 1.17, 1.43 | 1.29 | 1.17, 1.43 | |
| Moderate | 1129/35440 | 31.86 | 2.24 | 2.01, 2.49 | 1.67 | 1.48, 1.87 | 1.65 | 1.47, 1.85 | |
| Severe/very severe | 224/4737 | 47.29 | 3.21 | 2.74, 3.77 | 2.23 | 1.89, 2.63 | 2.22 | 1.88, 2.62 | |
| Physical/functional limitation | |||||||||
| No | 793/55594 | 14.26 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | N/A |
| Yes | 3152/124547 | 25.31 | 1.86 | 1.72, 2.02 | 1.37 | 1.26, 1.48 | 1.42 | 1.30, 1.55 | |
Abbreviations: HR, hazard ratio; CI, confidence interval; N/A, not applicable; hrs/wk, hours per week
Model 1 adjusted for age (years) only (predictors are time-varying when applicable).
Model 2 simultaneously adjusted for age (years) and baseline covariates listed in the table.
Model 3 simultaneously adjusted for age (years) and time-varying covariates (except for subjective social status, caregiving frequency, difficulty sleeping, and bodily pain which were only available at baseline) listed in the table.
Age-adjusted and multivariable-adjusted effect of alternate Mediterranean diet score was also adjusted for energy intake (quintiles).
Statistical analyses were conducted using SAS v. 9.3 (SAS Institute Inc., Cary, NC). All P-values were 2-sided (P < 0.05).
RESULTS
The distributions of baseline characteristics were shown in Table 1. We documented 3,945 incident LLD cases during 10-year follow-up; the overall incidence was 21.9 per 1000 person-years, consistent with age- and gender-specific depression incidence observed previously (27, 28). The following factors were significantly associated with higher LLD risk in age-adjusted models, listed here by category: social factors (lower social network; lower subjective social status; high caregiving burden to disabled/ill relatives), lifestyle/behavioral factors (overweight/obesity; low aMed diet score; cigarette smoking; physical inactivity; heavy/binge drinking), and health/medical conditions (medical comorbidity; ≤6 or ≥9 hours/day of sleep; difficulty falling/staying asleep; bodily pain; physical/functional limitations); higher education level was associated with lower LLD risk (Table 2). Multivariable-adjusted models including baseline versus time-varying exposures yielded generally consistent findings (Models 2 and 3). Most factors significantly associated with LLD in age-adjusted models remained significant in the final model (Model 3), with evidence of dosage effects. Exceptions were education, social network, and low aMed diet; none of the top category remained significant. Higher BMI was significantly associated with lower LLD risk in Model 3 (Ptrend=0.006). With regard to protective factors, there was a suggestive association between higher levels of caregiving to children/grandchildren and a lower LLD risk in Model 3 (Ptrend=0.02). The categories with the largest effect magnitudes for higher LLD risk were: severe/very severe bodily pain [HR (95% CI), 2.22 (1.88, 2.62)], difficulty sleeping most/all the time [2.04 (1.77, 2.36)], and daily sleep of ≥10 hours [1.96 (1.56, 2.46)]. The most prevalent risk factor, physical/functional limitations, was associated with 42% increased risk (95% CI: 30%, 55%).
Table 1.
| Variable | Mean (SD) or % |
|---|---|
| Age | 71.4 (4.1) |
| Body Mass Index, kg/m2 | 26.1 (4.9) |
| Alternate Mediterranean diet score | 4.5 (1.8) |
| Moderate to vigorous activity per week (hours) | 1.6 (2.8) |
| Education level | |
| Registered nurse degree, % | 72.5 |
| Bachelor degree, % | 18.8 |
| Advanced degree, % | 8.7 |
| Self-reported race/ethnicity | |
| Non-Hispanic White, % | 97.2 |
| Black, % | 0.9 |
| Others, % | 1.8 |
| Social network (in quintiles) | |
| 1st quintile (least social integration), % | 19.6 |
| 2nd quintile, % | 13.5 |
| 3rd quintile, % | 17.9 |
| 4th quintile, % | 34.7 |
| 5th quintile (most social integration), % | 14.2 |
| Subjective self-rated societal position | |
| High, % | 14.0 |
| Medium-high, % | 53.7 |
| Medium-low, % | 30.6 |
| Low, % | 1.7 |
| Regular caregiving to children/grandchildren | |
| No, % | 69.9 |
| Some, % | 25.2 |
| High, % | 4.8 |
| Regular caregiving to ill relatives | |
| No, % | 78.4 |
| Some, % | 17.1 |
| High, % | 4.6 |
| Current smokers, % | 6.2 |
| Largest number of drinks in a single day | |
| None, % | 41.1 |
| 1–2 drinks, % | 49.2 |
| ≥3, % | 9.7 |
| Comorbidity | |
| ≤1, % | 91.9 |
| ≥2, % | 8.1 |
| Hours of actual sleep per day | |
| ≤6, % | 25.8 |
| 7–8, % | 66.9 |
| 9, % | 6.2 |
| ≥10, % | 1.2 |
| Difficulty falling or staying asleep | |
| None of the time, % | 34.5 |
| A little of the time, % | 32.9 |
| Some of the time, % | 29.0 |
| Most or all of the time, % | 3.7 |
| Total bodily pain | |
| None, % | 18.3 |
| Very mild/mild, % | 57.9 |
| Moderate, % | 20.8 |
| Severe/very severe, % | 3.1 |
| Physical/functional limitation | |
| No, % | 35.5 |
| Yes, % | 64.5 |
Abbreviation: SD, standard deviation
Values are expressed as means (standard deviation) or percentages.
All variables were age-adjusted except for age variable
Three sensitivity analyses showed similar results. (1) exposure-LLD associations remained largely unchanged when we imposed a 2-year lag between exposure assessment and each follow-up period (Supplemental Table 1). Notably, there was no significant inverse association between BMI and LLD risk in the lagged analysis, suggesting that the observed significance in primary analyses may be attributable to weight change as an early manifestation of depression. The positive association between difficulty sleeping and LLD risk remained significant both in the 2- and 6-year lagged analyses. 6-year lagged analysis showed that compared to women without any sleep difficulty, those with difficulty falling/staying asleep most/all the time had a HR of 1.56 (95% CI: 1.15, 2.10; data not shown in table). (2) When depression was alternatively defined by diagnosis or treatment, the estimated LLD incidence was 12.5 per 1,000 person-years, given lower case sensitivity of this definition. However, findings were mostly consistent with those from primary analyses. Exceptions were heavy/binge drinking, subjective social status, and smoking: point estimates were similar but no longer statistically significant, likely due to substantial reductions in category numbers in these already small groups (data not shown). When the definition of depression included TCAs use (adding additional 5% of cases), the findings were mostly consistent with those in the primary analyses, except the suggestive association between the lowest aMed diet score and LLD [HR(95% CI): 1.10 (0.99, 1.23)]. (3) Finally, the LLD incidence was identical in analyses with and without carrying-forward missing information on risk factors during follow-up. Effect estimates were highly similar using either approach (data not shown).
When relating risk factors to population impact from the prevention perspective, the factor with the largest PAF was physical/functional limitation (26.4%). Other factors with PAF values of ≥10% included: sleep difficulty some to all of the time, no/very little exercise, and moderate to very severe bodily pain, together accounted for 31.6% of cases. Considering all significant risk factors in this study, the total PAF was 55.5% (95% CI: 42.1%, 66.5%). When grouping related factors, the estimated PAFs due to health problems, unfavorable lifestyle behaviors, and social factors were 46.1%, 12.5%, and 6.4%, respectively. After stratifying by the presence of physical/functional limitations, problematic sleep, low exercise, and bodily pain remained the top risk factors, by PAF, in both subgroups; however, they jointly accounted for almost double the LLD cases among women with limitations compared to those without limitations. Heavy/binge drinking accounted for 1.7% of new cases overall but explained 4.3% among those without limitations (Table 4). Overall, behavioral factors appeared to contribute approximately equally to LLD among women with and without physical/functional limitations; however, health factors had much larger contributions to risk among women with limitations.
DISCUSSION
To out knowledge, this is the largest study to date that comprehensively and simultaneously examined a wide array of exposures for LLD in women. In this prospective cohort of U.S. women, we observed that poor physical health, unhealthy behaviors and social stressors were significantly associated with increased LLD risk in a dose-response fashion. Sleep difficulty was significantly associated with subsequent LLD risk, and heavy/binge drinking may be an important risk factor for LLD in women. Furthermore, the PAF analysis findings have public health implications. First, physical/functional limitation was the top risk factor in explaining new LLD cases. Our results point to the importance of early interventions to prevent onset of such limitations with respect to depression risk. Second, because we investigated risk factors separately among women with versus without physical/functional limitations, our data can preliminarily inform how depression prevention strategies may need to differ between these groups and also highlight the need for additional research in this area. In addition, the risk factors examined in this study combined to play a larger role, in PAF, among women with physical/functional limitations compared to those without, suggesting that other risk factors remain to be elucidated among women without limitations. Finally, our data suggest that one-third of all incident LLD may be explained by sleep, exercise and pain; optimizing primary care approaches to intervene early or to prevent sleep problems and pain before reaching clinical manifestations and to promote exercise appears a logical “next-step” for LLD prevention.
In placing these findings in the context of the existing literature, several points can be highlighted. First, several of our key findings (e.g., regarding physical/functional limitation, medical comorbidity and sleep) were consistent with prior reports (2, 3, 9, 33, 34). Similarly in keeping with prior work (4, 5, 8, 35–38), we observed that physical inactivity and smoking were independently related to LLD risk. Second, in an expansion upon the existing literature, we were able to demonstrate dosage effects for most exposures; prior studies typically lacked adequate sample sizes or exposure detail to do so (4, 8, 35, 37, 38). Third, our findings regarding caregiving reinforced the importance of distinguishing types of caregiving activities: in our study, high volume of caregiving to disabled/ill persons was related to higher LLD risk, in line with a cross-sectional study (39), while decreased LLD risk was found for high volume of caregiving to children/grandchildren. Indeed, Tsai et al. reported that interactions with children/grandchildren may benefit older adults’ psychological well-being (40). Fourth, although significant positive associations have been reported elsewhere (33, 38, 41), significant associations between low aMed diet and short sleep duration and LLD risk were only observed in age- but not multivariable-adjusted analyses in our study. These underscore the importance of confounder adjustment when examining such associations (e.g., in our study, the estimated risk associated with sleeping ≤6 hours/day was confounded by having difficulty falling/staying asleep). Furthermore, opposite association directions between overweight/obesity and LLD risk were observed in age- and multivariable-adjusted models; yet, overweight/obesity was not significantly associated with LLD risk in 2-year lagged analyses, highlighting the complex relationships between BMI and depression. Finally, our results raised awareness of relatively understudied factors: e.g., heavy/binge drinking was a risk factor for LLD, particularly among women without physical/functional limitations; further work is warranted to confirm these findings.
The strengths of the study include prospective design, large sample size, lengthy follow-up, a comprehensive set of risk factors, and repeated assessments of health and behavioral variables. An advantage of the current study, compared to prior investigations with smaller sample sizes, was the ability to consider a broad a range of predictors simultaneously and to use finer categorizations of exposures to explore intensity/dose-response relations. The use of time-varying data also allowed better handling of potential confounding. Finally, the large number of incident cases facilitated addressing contributions to total risk, including within subgroups.
Limitations also warrant discussion. First, outcome misclassification is anticipated when self-reported physican/clinician diagnosis, antidepressant use, or depressive symptoms were used to define depression. Clinicians may incorrectly consider some depressive symptoms as part of normal aging, leading to under-diagnosis of depression. However, the LLD incidence in our study is consistent with estimates from prior studies featuring clinical evaluations to define depression (27, 28). Furthermore, prior NHS publications have illustrated the ability to use our depression definitions to predict other outcomes (31) or to relate individual factors to depression risk (42). Although the outcome we used may capture both major and minor depressions, these different endpoints have equal health burden in older persons including medical costs or functional outcomes (43) and, thus, have public health importance. Although lack of diagnositic interview remains a limitation, we observe consistent findings in multiple sensitivity analyses applying alternate outcome definitions. Second, because all exposures were self-reported from the questionniares, misclassification is unevitable. However, many have been validated in the NHS (16–20); for example, self-reported and measured weights were highly correlated (γ = 0.97). Third, although sleep difficulty was significantly associated with high LLD risk in both 2- and 6-year lagged analyses, reverse causation cannot be completely ruled out if sleep disturbance manifests very early in the process. Fourth, PAF estimates assume causal links, which cannot be tested in observational studies including our study. However, a long-term randomizaed trial has inherent challenges; for example, the necessity of a large sample size, lengthy follow-up, participant adherence to assigned lifestyle prescriptions are difficult to achieve. Furthermore, the assignment to specific health conditions has ethnic problems to implement. Therefore, carefully-conducted observational studies provide a reasonable approach for evaluating the study aims. We also estimated the PAF from multivariable-adjusted models to minimize confounding; yet, residual confounding remained likely. Furthermore, we had to dichotomize exposures to increase interpretability and statistical efficiency in PAF analyses, although many showed strong dose-response relations. Finally, our findings from this all-female cohort may not be directly generalizable to men. Further research directly conducted in men and diverse race/ethnic groups would be valuable.
In conclusion, we identified several major risk factors in the social, lifestyle/behavioral and health/medical domains with trend effects. Together, model predictors accounted for almost 60% of all new LLD cases in this population, and physical/functional limitation is the largest single contributor to total risk. A substantial proportion of LLD cases may be preventable by increasing exercise and intervening or preventing sleep difficulties and pain. These results may translate to public health opportunities in reducing depression burden.
Supplementary Material
Table 3.
Overall and Physical/Functional Limitation-Stratified Population Attributable Fraction of Incident Late-Life Depression in 21,728 Elderly Women a
| Risk indicator | Overall analysis | Without physical/functional limitation | With physical/functional limitation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| HR | 95% CI | PAF | 95% CI | HR | 95% CI | PAF | 95% CI | HR | 95% CI | PAF | 95% CI | |
| Social network | ||||||||||||
| High (top 40%) | 1 | Referent | 1 | Referent | 1 | Referent | ||||||
| otherwise | 1.08 | 1.02, 1.16 | 4.4 | 0.9, 7.9 | 1.10 | 0.96, 1.27 | 4.6 | −2.2, 11.5 | 1.08 | 1.01, 1.16 | 4.3 | 0.3, 8.3 |
| Subjective social status | ||||||||||||
| High | 1 | Referent | 1 | Referent | 1 | Referent | ||||||
| otherwise | 1.36 | 1.12, 1.65 | 0.7 | 0.2, 1.3 | 1.40 | 0.79, 2.49 | 0.4 | −0.5, 1.4 | 1.35 | 1.10, 1.65 | 0.8 | 0.1, 1.4 |
| Regular caregiving to ill relatives | ||||||||||||
| no or some | 1 | Referent | 1 | Referent | 1 | Referent | ||||||
| a lot | 1.30 | 1.14, 1.48 | 1.4 | 0.6, 2.2 | 1.52 | 1.13, 2.04 | 2.0 | 0.3, 3.7 | 1.25 | 1.08, 1.44 | 1.2 | 0.3, 2.1 |
| Cigarette smoking | ||||||||||||
| non-current smoker | 1 | Referent | 1 | Referent | 1 | Referent | ||||||
| current smoker | 1.18 | 1.03, 1.35 | 0.9 | 0.1, 1.7 | 1.22 | 0.90, 1.65 | 1.0 | −0.7, 2.6 | 1.18 | 1.02, 1.37 | 0.9 | 0, 1.8 |
| Moderate to vigorous activity | ||||||||||||
| yes | 1 | Referent | 1 | Referent | 1 | Referent | ||||||
| no or very little (<1 hour/week) | 1.18 | 1.10, 1.26 | 10.2 | 5.7, 14.6 | 1.16 | 1.00, 1.33 | 6.0 | 0, 11.9 | 1.19 | 1.09, 1.29 | 11.5 | 6.1, 16.8 |
| Heavy/binge drinking | ||||||||||||
| No (<2 drinks per day) | 1 | Referent | 1 | Referent | 1 | Referent | ||||||
| Yes (3+ drinks per day) | 1.20 | 1.08, 1.33 | 1.7 | 0.7, 2.7 | 1.40 | 1.15, 1.71 | 4.3 | 1.6, 7.0 | 1.13 | 1.01, 1.28 | 1.1 | 0, 2.2 |
| Comorbidity | ||||||||||||
| ≤1 | 1 | Referent | 1 | Referent | 1 | Referent | ||||||
| ≥2 | 1.43 | 1.31, 1.55 | 5.2 | 3.7, 6.8 | 1.63 | 1.25, 2.11 | 3.0 | 0.8, 5.1 | 1.40 | 1.28, 1.53 | 5.7 | 4.0, 7.5 |
| Hours of actual sleep per day | ||||||||||||
| <9 hrs | 1 | Referent | 1 | Referent | 1 | Referent | ||||||
| 9+ hrs | 1.28 | 1.15, 1.42 | 2.2 | 1.2, 3.3 | 1.10 | 0.83, 1.47 | 0.6 | −1.2, 2.4 | 1.31 | 1.17, 1.46 | 2.6 | 1.4, 3.9 |
| Difficulty falling or staying asleep | ||||||||||||
| none to a little of the time | 1 | Referent | 1 | Referent | 1 | Referent | ||||||
| some to all of the time | 1.48 | 1.39, 1.58 | 13.9 | 11.4, 16.5 | 1.32 | 1.14, 1.54 | 7.8 | 3.3, 12.3 | 1.52 | 1.41, 1.63 | 15.6 | 12.7, 18.4 |
| Bodily pain | ||||||||||||
| none to mild pain | 1 | Referent | 1 | Referent | 1 | Referent | ||||||
| moderate to very severe pain | 1.41 | 1.32, 1.51 | 10.0 | 7.5, 12.4 | 1.61 | 1.29, 2.02 | 4.0 | 1.7, 6.4 | 1.40 | 1.30, 1.50 | 11.3 | 8.6, 14.0 |
| Physical/functional disability | ||||||||||||
| no | 1 | Referent | – | – | – | – | ||||||
| yes | 1.49 | 1.37, 1.62 | 26.4 | 20.9, 31.8 | ||||||||
|
| ||||||||||||
| PAF due to social factors b | 6.4 | 0.6, 12.1 | 7.0 | −4.9, 18.7 | 6.2 | −0.4, 12.7 | ||||||
| PAF due to lifestyle/behaviors c | 12.5 | 6.5, 18.4 | 10.9 | 1.1, 20.4 | 13.2 | 6.1, 20.2 | ||||||
| PAF due to health/medical factors d | 46.1 | 37.3, 54.1 | 14.5 | 4.3, 24.5 | 30.9 | 24.3, 37.2 | ||||||
| PAF due to all 3 domains of risk factors combined | 55.5 | 42.1, 66.5 | 29.1 | 2.4, 51.9 | 43.6 | 29.6, 55.8 | ||||||
| PAF due to lack of exercise, sleep problems, and bodily pain | 31.6 | 23.3, 39.4 | 17.3 | 2.4, 31.4 | 35.2 | 26.1, 43.6 | ||||||
Abbreviations: HR, hazard ratio; PAF, population attributable fraction; CI, confidence interval
Models adjusted for all the covariates listed in the table simultaneously
Social factors include low social network, low subjective social status, and regular care to ill relatives
Behavioral factors include cigarette smoking, infrequent moderate to vigorous activity, and heavy/binge drinking
Health factors include physical/functional limitation, comorbidity, difficulty falling or staying asleep, long sleep hours, and bodily pain. Physical/functional limitation was not included in the stratified analyses
Acknowledgments
This work was supported by National Institutes of Health research grants UM1 CA186107 and R01 MH091448. Dr. Okereke also received funding support from the Harvard Medical School Office for Diversity Inclusion and Community Partnership Faculty Fellowship. Dr. Chang is also supported by a NARSAD Young Investigator Award from the Brain & Behavior Research Foundation. The sponsors of this study had no role in the design of study or in the collection, management, analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript. Drs. Shun-Chiao Chang and Olivia I. Okereke had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations
- LLD
late-life depression
- NHS
Nurses’ Health Study
- PAF
population attributable fraction
- BMI
body mass index
- MHI-5
5-item Mental Health Index
- CESD-10
10-item version of the Center for Epidemiologic Studies Depression
- GDS-15
15-item version of the Geriatric Depression Scale
- TCAs
tricyclic antidepressants
- SD
standard deviation
- HR
hazard ratio
- CI
confidence interval
- N/A
not applicable
Footnotes
Conflict of Interest: The authors have no conflicts of interest.
References
- 1.Sheline YI, Pieper CF, Barch DM, Welsh-Bohmer K, McKinstry RC, MacFall JR, D’Angelo G, Garcia KS, Gersing K, Wilkins C, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67(3):277–85. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyness JM, Yu Q, Tang W, Tu X, Conwell Y. Risks for depression onset in primary care elderly patients: potential targets for preventive interventions. Am J Psychiatry. 2009;166(12):1375–83. doi: 10.1176/appi.ajp.2009.08101489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoevers RA, Smit F, Deeg DJ, Cuijpers P, Dekker J, van Tilburg W, Beekman AT. Prevention of late-life depression in primary care: do we know where to begin? Am J Psychiatry. 2006;163(9):1611–21. doi: 10.1176/appi.ajp.163.9.1611. [DOI] [PubMed] [Google Scholar]
- 4.Park JE, Lee JY, Kim BS, Kim KW, Chae SH, Cho MJ. Above-moderate physical activity reduces both incident and persistent late-life depression in rural Koreans. Int J Geriatr Psychiatry. 2014 doi: 10.1002/gps.4244.. [DOI] [PubMed] [Google Scholar]
- 5.Luijendijk HJ, Stricker BH, Hofman A, Witteman JC, Tiemeier H. Cerebrovascular risk factors and incident depression in community-dwelling elderly. Acta Psychiatr Scand. 2008;118(2):139–48. doi: 10.1111/j.1600-0447.2008.01189.x. [DOI] [PubMed] [Google Scholar]
- 6.Almeida OP, Hankey GJ, Yeap BB, Golledge J, McCaul K, Flicker L. A risk table to assist health practitioners assess and prevent the onset of depression in later life. Prev Med. 2013;57(6):878–82. doi: 10.1016/j.ypmed.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Tait RJ, French DJ, Burns R, Anstey KJ. Alcohol use and depression from middle age to the oldest old: gender is more important than age. Int Psychogeriatr. 2012;24(8):1275–83. doi: 10.1017/S1041610212000087. [DOI] [PubMed] [Google Scholar]
- 8.Weyerer S, Eifflaender-Gorfer S, Wiese B, Luppa M, Pentzek M, Bickel H, Bachmann C, Scherer M, Maier W, Riedel-Heller SG. Incidence and predictors of depression in non-demented primary care attenders aged 75 years and older: results from a 3-year follow-up study. Age Ageing. 2013;42(2):173–80. doi: 10.1093/ageing/afs184. [DOI] [PubMed] [Google Scholar]
- 9.Almeida OP, Alfonso H, Yeap BB, Hankey G, Flicker L. Complaints of difficulty to fall asleep increase the risk of depression in later life: the health in men study. J Affect Disord. 2011;134(1–3):208–16. doi: 10.1016/j.jad.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 10.Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Lombardo C, Riemann D. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1–3):10–9. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Brown WJ, Ford JH, Burton NW, Marshall AL, Dobson AJ. Prospective study of physical activity and depressive symptoms in middle-aged women. Am J Prev Med. 2005;29(4):265–72. doi: 10.1016/j.amepre.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109(2):186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 13.Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol. 2000;19(6):586–92. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- 14.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119(8):1093–100. doi: 10.1161/CIRCULATIONAHA.108.816736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucas M, Mirzaei F, O’Reilly EJ, Pan A, Willett WC, Kawachi I, Koenen K, Ascherio A. Dietary intake of n−3 and n−6 fatty acids and the risk of clinical depression in women: a 10-y prospective follow-up study. Am J Clin Nutr. 2011;93(6):1337–43. doi: 10.3945/ajcn.111.011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. International journal of epidemiology. 1989;18(4):858–67. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 17.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. American journal of epidemiology. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 18.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466–73. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–9. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 20.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123(5):894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 21.Sun Q, Townsend MK, Okereke OI, Rimm EB, Hu FB, Stampfer MJ, Grodstein F. Alcohol consumption at midlife and successful ageing in women: a prospective cohort analysis in the nurses’ health study. PLoS Med. 2011;8(9):e1001090. doi: 10.1371/journal.pmed.1001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 23.Yamazaki S, Fukuhara S, Green J. Usefulness of five-item and three-item Mental Health Inventories to screen for depressive symptoms in the general population of Japan. Health and quality of life outcomes. 2005;3:48. doi: 10.1186/1477-7525-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 25.Friedman B, Heisel MJ, Delavan RL. Psychometric properties of the 15-item geriatric depression scale in functionally impaired, cognitively intact, community-dwelling elderly primary care patients. J Am Geriatr Soc. 2005;53(9):1570–6. doi: 10.1111/j.1532-5415.2005.53461.x. [DOI] [PubMed] [Google Scholar]
- 26.Okereke OI, Cook NR, Albert CM, Van Denburgh M, Buring JE, Manson JE. Effect of long-term supplementation with folic acid and B-vitamins on risk of depression in older women. Br J Psychiatry. 2015;206(4):324–31. doi: 10.1192/bjp.bp.114.148361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luijendijk HJ, van den Berg JF, Dekker MJ, van Tuijl HR, Otte W, Smit F, Hofman A, Stricker BH, Tiemeier H. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394–401. doi: 10.1001/archpsyc.65.12.1394. [DOI] [PubMed] [Google Scholar]
- 28.Norton MC, Skoog I, Toone L, Corcoran C, Tschanz JT, Lisota RD, Hart AD, Zandi PP, Breitner JC, Welsh-Bohmer KA, et al. Three-year incidence of first-onset depressive syndrome in a population sample of older adults: the Cache County study. Am J Geriatr Psychiatry. 2006;14(3):237–45. doi: 10.1097/01.JGP.0000196626.34881.42. [DOI] [PubMed] [Google Scholar]
- 29.Palsson SP, Ostling S, Skoog I. The incidence of first-onset depression in a population followed from the age of 70 to 85. Psychological medicine. 2001;31(7):1159–68. doi: 10.1017/s0033291701004524. [DOI] [PubMed] [Google Scholar]
- 30.Breslow N. Covariance analysis of censored survival data. Biometrics. 1974;30(1):89–99. [PubMed] [Google Scholar]
- 31.Pan A, Okereke OI, Sun Q, Logroscino G, Manson JE, Willett WC, Ascherio A, Hu FB, Rexrode KM. Depression and incident stroke in women. Stroke. 2011;42(10):2770–5. doi: 10.1161/STROKEAHA.111.617043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18(5):571–9. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 33.Szklo-Coxe M, Young T, Peppard PE, Finn LA, Benca RM. Prospective associations of insomnia markers and symptoms with depression. Am J Epidemiol. 2010;171(6):709–20. doi: 10.1093/aje/kwp454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaussent I, Bouyer J, Ancelin ML, Akbaraly T, Peres K, Ritchie K, Besset A, Dauvilliers Y. Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep. 2011;34(8):1103–10. doi: 10.5665/SLEEP.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Litwin H. Physical activity, social network type, and depressive symptoms in late life: an analysis of data from the National Social Life, Health and Aging Project. Aging Ment Health. 2012;16(5):608–16. doi: 10.1080/13607863.2011.644264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epstein JF, Induni M, Wilson T. Patterns of clinically significant symptoms of depression among heavy users of alcohol and cigarettes. Prev Chronic Dis. 2009;6(1):A09. [PMC free article] [PubMed] [Google Scholar]
- 37.Bots S, Tijhuis M, Giampaoli S, Kromhout D, Nissinen A. Lifestyle- and diet-related factors in late-life depression--a 5-year follow-up of elderly European men: the FINE study. Int J Geriatr Psychiatry. 2008;23(5):478–84. doi: 10.1002/gps.1919. [DOI] [PubMed] [Google Scholar]
- 38.Byers AL, Vittinghoff E, Lui LY, Hoang T, Blazer DG, Covinsky KE, Ensrud KE, Cauley JA, Hillier TA, Fredman L, et al. Twenty-year depressive trajectories among older women. Arch Gen Psychiatry. 2012;69(10):1073–9. doi: 10.1001/archgenpsychiatry.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards B, Higgins DJ. Is caring a health hazard? The mental health and vitality of carers of a person with a disability in Australia. Med J Aust. 2009;190(7 Suppl):S61–5. doi: 10.5694/j.1326-5377.2009.tb02472.x. [DOI] [PubMed] [Google Scholar]
- 40.Tsai FJ, Motamed S, Rougemont A. The protective effect of taking care of grandchildren on elders’ mental health? Associations between changing patterns of intergenerational exchanges and the reduction of elders’ loneliness and depression between 1993 and 2007 in Taiwan. BMC Public Health. 2013;13:567. doi: 10.1186/1471-2458-13-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rienks J, Dobson AJ, Mishra GD. Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid-aged women: results from a large community-based prospective study. Eur J Clin Nutr. 2013;67(1):75–82. doi: 10.1038/ejcn.2012.193. [DOI] [PubMed] [Google Scholar]
- 42.Mekary RA, Lucas M, Pan A, Okereke OI, Willett WC, Hu FB, Ding EL. Isotemporal substitution analysis for physical activity, television watching, and risk of depression. Am J Epidemiol. 2013;178(3):474–83. doi: 10.1093/aje/kws590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katon WJ, Lin E, Russo J, Unutzer J. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psychiatry. 2003;60(9):897–903. doi: 10.1001/archpsyc.60.9.897. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.