Abstract
Costimulatory molecules, such as the programmed death ligand (PD-L1), might exert differential effects on T-cell function, depending on the clinical setting and/or immunological environment. Given the impact of T cells on bronchiolitis obliterans (BO) in lung transplantation, we used an established tracheal transplant model inducing BO-like lesions to investigate the impact of PD-L1 on alloimmune responses and histopathological outcome in BO. In contrast to other transplant models in which PD-L1 generally shows protective functions, we demonstrated that PD-L1 has divergent effects depending on its location in donor versus recipient tissue. Although PD-L1 deficiency in donor tissue worsened histopathological outcome, and increased systemic inflammatory response, recipient PD-L1 deficiency induced opposite effects. Mechanistic studies revealed PD-L1–deficient recipients were hyporesponsive toward alloantigen, despite increased numbers of CD8+ effector T cells. The function of PD-L1 on T cells after unspecific stimulation was dependent on both cell type and strength of stimulation. This novel function of recipient PD-L1 may result from the high degree of T-cell activation within the highly immunogenic milieu of the transplanted tissue. In this model, both decreased T-cell alloimmune responses and the reduction of BO in PD-L1–deficient recipients suggest a potential therapeutic role of selectively blocking PD-L1 in the recipient. Further investigation is warranted to determine the impact of this finding embedded in the complex pathophysiological context of BO.
T-cell–mediated alloimmune responses limit allograft and patient survival after solid organ transplantation.1, 2 Manipulating the complex process of T-cell activation in the setting of solid organ transplantation is a promising approach to limit T-cell–mediated alloimmune reactions and subsequent allograft tissue damage. Optimal activation of naïve T cells to acquire an effector phenotype requires two types of signals. The first signal is provided by the interaction of the antigen-specific T-cell receptor with the antigen presented in conjunction with the major histocompatibility complex on antigen-presenting cells (signal one), but this signal alone is not sufficient to elucidate T-cell activation by itself. A second signal, mediated by costimulatory molecules, is needed to achieve optimal T-cell activation. As these costimulatory pathways can be either activating (positive) or regulating (negative) in nature, the net effect of costimulatory signals determines the outcome of the immune response.3 An important costimulatory pathway is the programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) system. PD-L1 (B7-H1; CD274) and its receptor PD-1 (CD279) belong to the CD28 family of coreceptors that are involved in T-cell activation and tolerance signals.4, 5, 6 In many studies, the interaction of PD-1 with PD-L1 has been shown to decrease T-cell proliferation and survival of T cells and is generally thought to exert inhibitory functions in experimental models of autoimmune diseases, chronic viral infections, response to tumors, and tissue transplantation.3, 7, 8, 9, 10 In contrast, some authors suggest that PD-L1 enhances T-cell activation and proliferation.11
Despite the important role of PD-L1 in T-cell biology and increasing knowledge of the function of PD-L1 in solid organ transplantation, its role in lung transplantation is unknown. Lung transplantation is the only definitive treatment available for patients with end-stage lung diseases, such as chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, cystic fibrosis, α1-antitrypsin disease, and primary pulmonary hypertension.12, 13 Major improvements in surgical techniques, novel immunosuppressive agents, and control of infections have improved 1-year survival after lung transplantation to 70% to 80%, but long-term survival is still limited because of persistent immune injury resulting in chronic rejection processes that manifest as bronchiolitis obliterans (BO).13, 14 BO is clinically characterized by the progressive loss of lung function due to airflow obstruction, characteristic of bronchiolitis obliterans syndrome and eventually resulting in respiratory failure and death. BO-related mortality remains alarmingly high, with only 40% to 50% patient survival 5 years after the onset of BO. The lung has the highest rejection rates among all solid organ transplants, probably as the result of epithelial immunological vulnerability and injury because of its constant exposure to airborne antigens, pathogens, and pollutants. Consequently, even more than three decades after the first lung transplant, BO remains a daunting challenge, with no effective therapies.12, 14, 15 Etiology and pathophysiology of BO are poorly understood; it comprises loss of airway epithelium, peribronchial inflammation, immune injury, and subsequent airway fibrosis, leading to obliterative airway disease.12 Repair mechanisms during BO are insufficient because of the complex nature of immune injury. During the past years, humoral immunity, including both human leukocyte antigen and non–human leukocyte antigen antibodies, as well as autoimmunity and complement activation have been identified as important mechanisms that contribute to the outcome after lung transplantation,16 and the fact that BO is resistant toward currently used immunosuppressive strategies suggests a complexity in the immune pathogenesis that requires further elucidation.13 Effector T cells, however, appear to be important mediators of immune injury leading to BO, as adoptive transfer of effector T cells into mice lacking T, B, and natural killer cells causes BO.17 It has also been shown that CD8+ T cells activated by major histocompatibility complex class I molecules can cause obliterative airway disease.18 It is therefore essential to identify the mechanisms by which activated effector T cells are recruited and contribute to the pathogenesis of BO.
In this study, we investigated the function of PD-L1 in a heterotopic tracheal transplant model. Although this model does not reflect all pathophysiological components involved in BO, it does provide the opportunity to analyze histopathological features of BO, as like human BO it is alloimmune mediated and presents with luminal obliteration and loss of airway epithelium. This allows us to investigate the systemic alloimmune response in a highly immunogenic environment, particularly T-cell–mediated effects, which play a major role in this model and influence the histopathological outcome.
The presented data suggest a divergent role of PD-L1 in the donor tissue versus the immune system of the graft recipient, which has impact for the preservation of the respiratory epithelium and the alloimmune responses after transplantation, particularly for the development of effector T cells.
Materials and Methods
Mice
Wild-type (WT) C57BL/6 (H-2b, B6) and BALB/c (H-2d) mice were purchased from Taconic (Germantown, NY). PD-L1– and PD-1–deficient (PD-L1−/−, PD-1−/−) mice on the B6 background were obtained from I.G. and M.H.S. and maintained as a breeding colony in our animal facility. All mice were used at 8 to 12 weeks of age and all animal protocols were approved by the Boston Children's Hospital Animal Care and Use Committe.
Heterotopic Airway Transplant Model
All studies were performed according to institutional guidelines for animal use and care. We used an established model of obliterative airway disease involving heterotopic tracheal transplant with major histocompatibility complex–mismatched donor and recipient combinations of C57BL/6 (H-2b) and BALB/c (H-2d) mice, as described previously.19, 20, 21 Tracheas from donor mice were transplanted s.c. into the backs of recipient mice. Grafts are not primarily vascularized, but blood supply occurs via neovascularization by the recipient's blood vessels. The role of PD-L1 in donor versus recipient was investigated using PD-L1−/− mice on the B6 background as donors or recipients, and WT BALB/c mice as the respective counterparts. In some transplant series, mice were additionally treated with a blocking monoclonal antibody against PD-1 (clone J43; BioXcell, West Lebanon, NH; 500 μg on day 0; 250 μg on day 2, 4, 6, 8, 10, and 12 after transplantation) or appropriate isotype control, respectively. Moreover PD-1−/− mice (B6 background) were used as recipients. Isogeneic transplants were performed analogously, using WT and PD-L1−/− mice on the same background as donors and recipients. Mice were sacrificed 2, 7, or 14 days after transplantation, and grafts and spleens were harvested for further analysis.
Histology
Tracheas were fixed in formalin-free zinc fixative (BD Biosciences, San Jose, CA), cut in half, and paraffin embedded. Cross sections of tracheal tissues (5 μm thick) were stained with hematoxylin and eosin. Sections were graded by two independent investigators (K.S.-N. and M.Su.) who were blinded for the groups. The samples were scored using the following criteria: A luminal obliteration was scored as follows: 0 indicates no change; 1, ≤25% obliteration; 2, 25% to 50% obliteration; 3, 51% to 75% obliteration; and 4, ≥75% obliteration. Airway lining epithelial loss was scored as follows: 0 indicates no change; 1, ≤25% circumference loss; 2, 25% to 50% circumference loss; 3, 51% to 75% circumference loss; and 4, ≥75% circumference loss.
Immunohistochemistry staining for PD-L1 in naïve and allogeneic trachea transplantation was performed as described by Guleria et al.22 Briefly, tracheas were embedded in Tissue Tek OCT compound (Sakura Finetek, Alphen aan den Rijn, the Netherlands) and frozen in liquid nitrogen. Immunohistochemistry was performed on frozen tissue sections (5 μm thick) with PD-L1 antibody (MIH6) using the avidin-biotin technique (Vector Laboratories, Burlingame, CA).
Immunohistochemistry staining for polymorphonuclear neutrophils and macrophages was performed on paraffin sections. Briefly, sections were deparaffinized with xylene and rehydrated through graded alcohols to water. Polymorphonuclear neutrophils were stained with a rat anti-mouse antibody to granulocyte marker Ly-6C/6G (Gr-1; BD Biosciences). Macrophages were stained with rat anti-mouse Mac-3 (M3/84; BD Biosciences). CD4 and CD8 staining was performed on frozen sections using antibodies to mouse CD4 and CD8 (CD4: GK1.5; CD8: 53-6.7; Abcam, Cambridge, MA). A standard ABC vector kit (Vector Laboratories, Burlingame, CA) and a diaminobenzidine reagent were used. Specificity of the staining was confirmed by using isotype control antibody. Polymorphonuclear neutrophils and macrophages were counted manually in the entire section (outside and inside of the tracheal lumen) of the allograft. CD4- and CD8-positive cells were counted in five random high-power fields under a microscope. Immunofluorescence studies for IgG, IgM, and C4d were performed with standard techniques23 using frozen sections (4 μm thick) of trachea and fluoresceinated monoclonal antibodies to mouse IgG and IgM (eBioscience, San Diego, CA) and mouse C4d (Abcam). Coded sections were scored using an epiillumination Nikon microscope (Nikon Instruments Inc., Melville, NY) and photographed with a Spot digital camera (Diagnostic Instruments Inc., Sterling Heights, MI). Evidence was sought for deposition in vessels, epithelium, and connective tissue.
Flow Cytometry
Splenocytes from transplanted animals were stained for CD4+ and CD8+ effector T cells (CD44highCD62Llow phenotype) and regulatory T cells (CD4+CD25+FoxP3+), using fluorochrome-labeled monoclonal antibodies against CD4 (RM4-5), CD8 (53-6.7), CD44 (IM7), CD62L (MEL-14), CD25 (PC61), and FoxP3 (FJK-16s). Intracellular FoxP3 staining was performed after overnight permeabilization, using a FixPerm Kit (eBioscience). Frequencies of effector and regulatory T cells are given as percentage of the total CD4+ or CD8+ population.
Flow cytometry analysis of tracheal tissue was performed. Briefly, tracheal grafts were removed from recipients and rinsed with RPMI 1640 medium plus 5% fetal bovine serum. Three tracheas were then minced using a scalpel in a digestion medium of 3 mL RPMI 1640 medium containing 0.1% collagenase type 4 (Worthington Biochemical, Lakewood, NJ), 0.1% soybean trypsin inhibitor (Sigma-Aldrich, St. Louis, MO), 50 U/mL DNase (Roche, Basel, Switzerland), 10% fetal bovine serum, 10 mol/L HEPES (Life Technologies, Carlsbad, CA), and 1% penicillin-streptomycin (Life Technologies). The suspension was placed on an agitator at 37°C for 30 minutes, and the digestion mixture was passed through a 70-μm nylon cell strainer (BD Biosciences) to remove undigested tissue and debris. Cell suspension was then centrifuged at 151 × g for 5 minutes and the pellet suspended in staining buffer. For flow cytometry, antibodies against CD326 (EpCAM, G8.8) and CD45 (104) were used. Cells were measured on a MoFlo Flow Cytometer (Cytomation, Fort Collins, CO) equipped with Spectra Physics 277-GA01 laser, and data were analyzed using Summit software version 4.3 (MoFlow). Epithelial cells were identified as CD45−CD326+.
ELISPOT Assays
ELISPOT assays were used to assess the frequency of alloreactive interferon (IFN)-γ–, IL-4–, IL-6–, and granzyme B–producing cells, according to manufacturer's instructions (ELISPOT Kits for IFN-γ, IL-6, and IL-4 from BD Biosciences; ELISPOT Development Module for Granzyme B from R&D, Minneapolis, MN). Usually, 0.5 × 106 recipient-derived splenocytes were stimulated with 0.5 × 106 irradiated donor-type stimulator cells for 8 hours (granzyme B) or 24 hours (IFN-γ, IL-4, IL-6) at 37°C and 5% CO2. The mitogen concanavalin A was used as stimulus for positive controls. The resulting red spots were counted on a computer-assisted enzyme-linked immunospot image analyzer (Cellular Technology, Kennesaw, GA), and frequencies were usually expressed as the number of spots per 0.5 × 106 responder cells. In some experiments, 1 × 104 flow-sorted CD4+ and CD8+ effector cells (CD44highCD62Llow phenotype) from transplanted mice were used as responder cells instead of unselected splenocytes.
T-Cell Activation Assay
CD4+ and CD8+ splenocytes from naïve WT B6 or PD-L1−/− B6 mice were separately isolated using magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany; purity, >93%), suspended in supplemented RPMI 1640 medium (10% newborn calf serum, 1% penicillin/streptomycin, 1% l-glutamine), and stimulated with plate-bound anti-CD3 and soluble anti-CD28 (BD Biosciences) at either a low level (both antibodies at 0.5 μg/mL) or at a high level of T-cell stimulation (both antibodies at 4 μg/mL). After 48 hours' incubation at standard conditions (37°C, 5% CO2), cell culture supernatants were harvested and analyzed for various cytokines, using LUMINEX technology.
LUMINEX Assay
Cell-free supernatants of T-cell cultures after unspecific or allostimulation were removed after 48 hours of incubation and analyzed by a cytokine bead–based immunoassay using a mouse cytokine multiplex detection kit (Millipore, Billerica, MA), according to the manufacturer's instructions. All samples were tested in triplicate wells.
In all in vitro assays mentioned in the previous two paragraphs, the conditions were optimized with respect to numbers of responder and stimulator cells and kinetics.
Statistical Analysis
A t-test was used for comparison of means between two groups, and P < 0.05 was considered significant. Data were expressed as means ± SEM.
Results
PD-L1 Is Up-Regulated in Airway Epithelial Cells on Transplantation
To assess the expression pattern of PD-L1 on transplantation, immunohistochemical staining for PD-L1 in airway epithelial cells was performed in naïve BALB/c tracheas and in BALB/c tracheas 2 weeks after transplantation into B6 recipients. PD-L1 expression was low in the epithelium of naïve tracheas but was significantly up-regulated in allografts, as assessed by both immunohistochemistry (Figure 1A) and flow cytometry (Figure 1B). This suggests a functional role of PD-L1 expression in the airway of tracheal transplants.
Figure 1.
PD-L1 is up-regulated in airway epithelial cells of tracheal allografts. A: Frozen sections of naïve tracheas and tracheal allografts at day 14 after transplantation were stained with an antibody to PD-L1 (immunohistochemistry). PD-L1 expression is low in the epithelium of naive BALB/c tracheas but up-regulated in BALB/c tracheal allografts 14 days after transplantation into B6 recipients. Boxed areas indicate the tracheal epithelium and are shown at higher magnification in the insets. B: Flow cytometry was performed on tracheal tissue and CD45−CD326+ cells were identified as epithelial cells. PD-L1 expression in epithelial cells was measured as fluorescence intensity. Tracheal allografts show up-regulation of PD-L1 on epithelial cells on day 14 after transplantation compared to naïve tracheas. Original magnification, ×10 (A). FMO, fluorescence minus one control.
Donor PD-L1 Expression Protects Tracheal Allografts from Injury
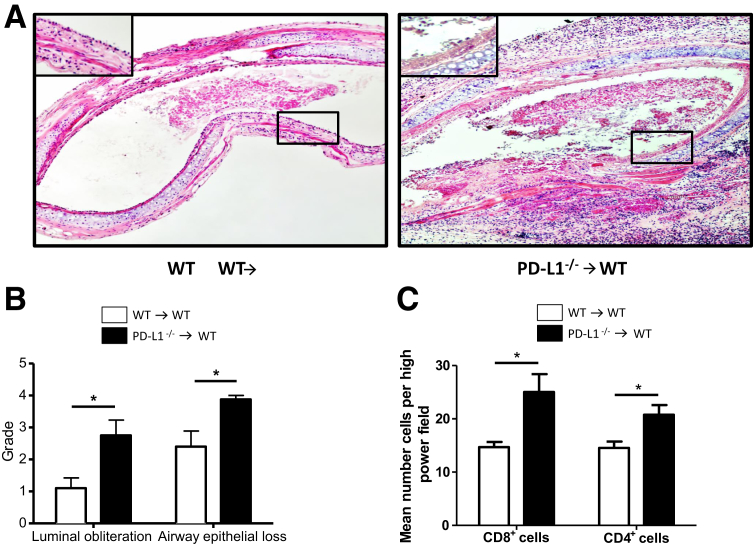
To determine the role of PD-L1 in the donor tissue, tracheas from WT or PD-L1−/− B6 mice were transplanted into WT BALB/c mice. A histopathological examination of hematoxylin and eosin–stained trachea sections revealed significantly worse outcome when donor trachea was PD-L1−/− as compared to WT donors, as assessed by the degree of luminal obliteration and airway epithelial loss 14 days after transplantation (Figure 2, A and B). Accordingly, PD-L1−/− allografts showed increased infiltration with CD4+ and CD8+ T cells (Figure 2C). These data emphasize a protective function of intragraft PD-L1 expression in obliteration of the airways.
Figure 2.
PD-L1–deficient donor grafts are more susceptible to BO development. Tracheas from WT and PD-L1−/− B6 donors were transplanted into WT BALB/c recipients and harvested 14 days after transplantation. A: Cross sections of paraffin-embedded tracheal tissues (5 μm thick) were stained with hematoxylin and eosin to assess the degree of luminal obliteration and epithelial loss. Boxed areas indicate the tracheal epithelium and are shown at higher magnification in the insets. B: Sections were graded by two independent investigators (K.S.-N., M.Su.) who were blinded for the groups. PD-L1−/− donor tracheas show more severe luminal obliteration and airway epithelial loss compared to WT grafts. C: Immunohistochemistry analysis of allograft infiltrating CD4+ and CD8+ T cells was performed on frozen tracheal sections and reveals significantly higher numbers in PD-L1−/− allografts when compared to WT allografts. Cells were counted in five random high-power fields. Data are representative of three transplant series. n = 4 animals per group (A–C). ∗P < 0.05. Original magnification, ×10 (A).
PD-L1 Deficiency in the Recipient Provides Protection against Allograft Injury
To test the role of PD-L1 in the graft recipient, tracheas from WT BALB/c donor mice were grafted into WT or PD-L1−/− B6 recipients. Surprisingly, PD-L1−/− recipients showed significantly less luminal obliteration and airway epithelium loss compared to WT recipients, which showed pronounced signs of allograft injury (Figure 3, A and B). Despite the lower degree of histopathological damage, allografts in PD-L1−/− recipients showed higher infiltration with CD4+ and CD8+ T cells (Figure 3C). These data reveal an unexpected role of PD-L1 in tracheal allograft recipients, given the detrimental effects of PD-L1 deficiency in tracheal allografts.
Figure 3.
PD-L1–deficient recipients are protected from BO. Tracheas from WT BALB/c donors were transplanted into WT and PD-L1−/− B6 recipients and harvested 14 days after transplantation. A: Cross sections of paraffin-embedded tracheal tissues (5 μm thick) were stained with hematoxylin and eosin to assess the degree of luminal obliteration and epithelial loss. Boxed areas indicate the tracheal epithelium and are shown at higher magnification in the insets. B: Sections were graded by two independent investigators (K.S.-N., M.Su.) who were blinded for the groups. PD-L1−/− recipients show significantly decreased degrees of luminal obliteration and loss of airway epithelium. C: Immunohistochemical analysis of allograft infiltrating CD4+ and CD8+ T cells was performed on frozen tracheal sections and reveals significantly higher numbers in PD-L1−/− recipients, when compared to WT recipients. Cells were counted in five random high-power fields. Data are representative of four transplant series. n = 4 animals at least per group (A–C). ∗P < 0.05, ∗∗∗P < 0.001. Original magnification, ×10 (A).
PD-1 Deficiency in the Recipient Provides Protection against Allograft Injury
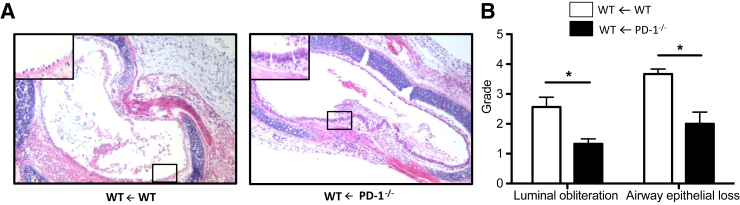
As it is the action of PD-L1 on PD-1 that normally constrains the immune response, we also used PD-1−/− B6 mice as recipients of BALB/c grafts. Similar to PD-L1−/− recipients, PD-1−/− recipients were protected from luminal obliteration and airway epithelial loss (Figure 4) when compared to WT recipients. The data suggest that complete interruption of PD-1/PD-L1 signaling mediates graft-protecting effects.
Figure 4.
PD-1–deficient recipients are protected from BO. Tracheas from WT BALB/c donors were transplanted into WT and PD-1−/− B6 recipients and harvested 14 days after transplantation. A: Cross sections of paraffin-embedded tracheal tissues (5 μm thick) were stained with hematoxylin and eosin to assess the degree of luminal obliteration and epithelial loss. Boxed areas indicate the tracheal epithelium and are shown at higher magnification in the insets. B: Sections were graded by two independent investigators (K.S.-N., M.Su.) who were blinded for the groups. Histopathological grading reveals significantly reduced degrees of luminal obliteration and loss of airway epithelium in PD-1−/− B6 recipients compared to WT B6 recipients. Data are representative of four transplant series. n = 4 animals at least per group (A and B). ∗P < 0.05. Original magnification, ×10 (A).
T Cellular Overexpression of PD-1 Is Not a Prerequisite for Graft Protection in PD-L1–Deficient Recipients
PD-L1−/− recipients showed increased expression of PD-1 on CD4+ and CD8+ effector T cells when compared to WT animals (Figure 5A). To test whether graft protection in PD-L1−/− recipients of WT tracheas results from increased interaction between PD-1 on recipient immune cells and PD-L1 on graft tissue, blocking anti–PD-1 monocloncal antibody was administered to PD-L1−/− recipients of WT tracheas. Interruption of the interaction between tissue PD-L1 and PD-1 on recipient T cells did not abrogate tissue protection (Figure 5B), indicating that this interaction is not essential for the observed beneficial effects in PD-L1–deficient graft recipients, and supporting the conclusion from the observation in PD-1–deficient recipients mentioned in the previous paragraph.
Figure 5.
Overexpression of PD-1 on CD4+ and CD8+ effector T cells is not required for graft protection in PD-L1–deficient recipients. A: Tracheas from WT BALB/c donors were transplanted into WT and PD-L1−/− B6 recipients, and spleens were harvested 7 days after transplantation. PD-1 expression on T-effector cells was analyzed by flow cytometry. The percentage of PD-1–positive CD4+ and CD8+ effector cells is significantly higher in PD-L1−/− recipients. Data are representative of four different experiments. B: To test whether the enhanced PD-1 expression and a subsequent increased interaction between PD-1 on PD-L1−/− immune cells and PD-L1 within the graft tissue mediates the protective effects observed in PD-L1−/− recipients, PD-1 was blocked in these mice by administration of a monoclonal antibody against PD-1. Cross sections of paraffin-embedded tracheal tissues (5 μm thick) were stained with hematoxylin and eosin to assess the degree of luminal obliteration and epithelial loss. Sections were graded by two independent investigators (K.S.-N., M.Su.) who were blinded for the groups. Histopathological scoring did not reveal any significant effects of PD-1 blockade in PD-L1−/− recipients when compared to isotype-treated controls 14 days after transplantation. Data are representative of three different experiments. n = 4 animals per group (A and B). ∗P < 0.05, ∗∗∗P < 0.001.
Given the histopathological changes, the data suggest a possible relation between PD-1/PD-L1 signaling within the recipient's immune system and the susceptibility of allografts to injury. Hypothesizing that one possible explanation for the observed results would be differences in the effector functions between WT and PD-L1/PD-1–deficient recipients prompted us to further elucidate the systemic alloimmune responses in vitro.
Tracheal Allografts Lacking PD-L1 Elicit Increased Systemic Alloimmune Responses in the Graft Recipient
To assess the systemic alloimmune response in BALB/c WT recipients of WT B6 or PD-L1−/− B6 grafts, we determined the percentages of splenic effector and regulatory T cells by flow cytometry at day 7 after transplantation. Both CD4+ and CD8+ effector T cells were increased in WT recipients of PD-L1−/− tracheas, when compared to WT recipients of WT tracheas, whereas there was no difference in the frequencies of T regulatory cells (Figure 6A). Next, we assessed the frequencies of alloreactive, cytokine-producing splenocytes after restimulation with alloantigen, using ELISPOT assays. WT recipients of PD-L1−/− tracheas showed increased frequencies of IFN-γ–, IL-6–, IL-4–, and granzyme B–producing splenocytes (Figure 6B). These data were confirmed by LUMINEX assays, which were performed to assess the cytokine and chemokine production of recipient T cells after restimulation with irradiated donor-type splenocytes (data not shown). These data support a protective function of PD-L1 expression within the donor tissue.
Figure 6.
Recipients of PD-L1–deficient tracheal allografts show increased systemic alloimmune responses. Tracheas from WT and PD-L1−/− B6 donors were transplanted into WT BALB/c recipients. To assess systemic alloimmune response in the recipient, spleens were harvested 7 days after transplantation. A: Flow cytometry analysis of CD4+ and CD8+ effector cells (CD44highCD62Llow phenotype) of spleens from WT recipients of PD-L1−/− tracheas show increased percentages of CD4+ and CD8+ effector cells when compared to WT recipients of WT tracheas 7 days after transplantation. Frequencies of effector and regulatory T cells are given as percentage of the total CD4+ or CD8+ population. B: Similarly, the frequencies of cytokine-producing splenocytes, as analyzed by ELISPOT assays, increase in WT recipients of PD-L1−/− tracheas 7 days after transplantation. Frequencies are expressed as the number of spots per 0.5 × 106 responder cells. ELISPOT experiments were performed in triplicate. Data are representative of three independent experiments. n = 5 animals at least per group (A and B). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
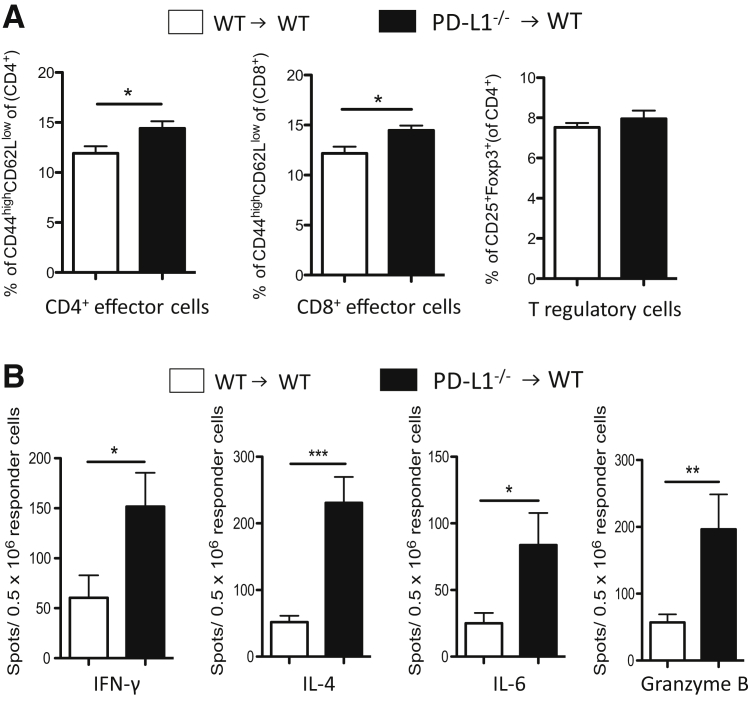
PD-L1–Deficient Graft Recipients Show Increased Effector T Cells but Decreased Systemic Alloreactivity after Trachea Transplantation
PD-L1−/− B6 recipients of WT BALB/c tracheas showed significantly increased percentages of splenic CD4+ and CD8+ effector T cells and CD4+ T regulatory cells, when compared to WT B6 recipients (Figure 7A). However, despite the increased numbers of effector T cells, the frequencies of IFN-γ–, IL-6–, IL-4–, and granzyme B–producing splenocytes were significantly reduced in PD-L1−/− recipients compared to WT recipients, as assessed by ELISPOT assays (Figure 7B), and these data were also confirmed by LUMINEX assays testing the cytokine production of splenocytes after restimulation with alloantigen (data not shown). In summary, these data confirm the novel dual role of PD-L1 in donor versus recipient.
Figure 7.
PD-L1–deficient recipients show decreased systemic alloimmune responses. Tracheas from WT BALB/c donors were transplanted into WT and PD-L1−/− B6 recipients. To assess the systemic alloimmune response in the recipient, spleens were harvested 7 days after transplantation. A: Flow cytometry analysis of CD4+ and CD8+ effector cells (CD44highCD62Llow phenotype) and CD4+ T regulatory cells (Tregs; CD4+CD25+FoxP3+) of spleens from PD-L1−/− recipients shows increased percentages of CD4+ and CD8+ effector cells and CD4+ Tregs when compared to WT recipients 7 days after transplantation. Frequencies of effector and regulatory T cells are given as percentage of the total CD4+ or CD8+ population. B: Despite the increased numbers of effector T cells, PD-L1−/− recipients show decreased frequencies of cytokine-producing splenocytes 7 days after transplantation compared to WT recipients, as assessed by ELSIPOT assays. Frequencies are expressed as the number of spots per 0.5 × 106 responder cells. ELISPOT experiments were performed in triplicate. Data are representative of four independent experiments. n = 4 animals per group (A and B). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
PD-1–Deficient Graft Recipients Show Decreased Systemic Alloreactivity after Trachea Transplantation
Similar to PD-L1−/− recipients, PD-1−/− recipients showed decreased frequencies of cytokine-producing cells (Figure 8A), indicating a protective effect of interrupted PD-1/PD-L1 pathway within the immune system of tracheal allograft recipients per se. Blockade of PD-1 by administration of anti–PD-1 antibody in PD-L1−/− recipients did not abrogate the observed decrease in alloreactivity (Figure 8B) (data shown for IFN-γ only), which further underlines that the protective effect in PD-L1−/− mice does not depend on increased PD-1 expression on PD-L1−/− immune cells and subsequently increased interaction with tissue PD-L1, but is because of interrupted PD-1/PD-L1 signaling among immune cells of the recipient.
Figure 8.
PD-1–deficient recipients show decreased systemic alloimmune responses. Tracheas from WT BALB/c donors were transplanted into WT and PD-1−/− B6 recipients or PD-L1−/−B6 recipients treated with an anti–PD-1 antibody. To assess the systemic alloimmune response in the recipient, spleens were harvested 7 days after transplantation. A: ELISPOT analysis shows significantly decreased frequencies of cytokine-producing splenocytes in PD-1−/− B6 recipients of BALB/c tracheas 7 days after transplantation. B: PD-1 blockade in PD-L1−/− recipients does not affect systemic alloimmune response. ELISPOT assays show similar frequencies of cytokine-producing splenocytes with and without PD-1 blockade (shown for IFN-γ only). Frequencies are expressed as the number of spots per 0.5 × 106 responder cells. ELISPOT experiments were performed in triplicate. Data are representative of four different experiments. n = 4 animals per group (A and B). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
PD-L1–Deficient CD8+ Effector T Cells Are Hyporesponsive toward Alloantigen
Given the discrepancy between the increased T effector cell numbers but decreased alloreactivity in PD-L1−/− B6 recipients, we determined the function of CD4+ and CD8+ effector T cells in WT and PD-L1−/− B6 recipients of WT BALB/c tracheal allografts. CD4+ and CD8+ T effector cells (CD44highCD62Llow splenocytes) from transplanted mice were flow sorted and restimulated with donor-type splenocytes in an IFN-γ ELISPOT assay. Although CD4+ effector T cells from both WT and PD-L1−/− recipients generally did not show pronounced alloreactivity, WT CD8+ effector T cells showed marked IFN-γ production on restimulation with alloantigen. In contrast, PD-L1−/− CD8+ effector T cells were hyporesponsive toward alloantigen (Figure 9A). Similar results were also obtained with PD-1–deficient effector T cells (data not shown). These results possibly explain why PD-L1−/− recipients show a decreased alloimmune response even though CD8+ effector cells are increased in numbers. The data highlight the important role of CD8+ T-cell function in this model, and that blockade of PD-1/PD-L1 interaction promotes hyporesponsiveness of these cells.
Figure 9.
The function of PD-L1 depends on the cell type and the degree of T-cell stimulation. A: Tracheas from WT BALB/c donors were transplanted into WT and PD-1−/− B6 recipients. Flow-sorted CD4+ and CD8+ effector T cells (CD44highCD62Llow phenotype) from WT or PD-L1−/−recipients at day 7 after transplantation were restimulated with donor antigen in an IFN-γ ELISPOT assay. CD4+ T-cell response is low generally in both WT and PD-L1−/− recipients, whereas CD8+ effector cells from WT recipients show notably increased IFN-γ production on restimulation with alloantigen. In contrast, CD8+ effector T cells from PD-L1−/− recipients are hyporesponsive toward alloantigen. Frequencies are expressed as the number of spots per 0.5 × 106 responder cells. B: CD4+ cells from naïve WT and PD-L1−/− B6 animals were isolated by magnetic cell separation and stimulated with low and high concentrations of plate-bound anti-CD3 and soluble anti-CD28. Measurements of IFN-γ production by Luminex assays shows that the regulatory effect of PD-L1 on the activation of CD4+ T cells is restricted to low levels of T-cell activation, whereas it is obscured at high levels of T-cell activation. C: CD8+ cells from naïve WT and PD-L1−/− B6 animals were isolated by magnetic cell separation and stimulated with low and high concentrations of plate-bound anti-CD3 and soluble anti-CD28. In PD-L1−/− CD8+ T cells, IFN-γ production is generally inhibited, regardless of the degree of T-cell stimulation (Luminex). ELISPOT and Luminex experiments were performed in triplicate. Data are representative of three different experiments. n = 4 animals per group (A–C). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Function of PD-L1 in Activated T Cells Depends on Both Cell Type and Strength of Stimulation
To test the role of PD-L1 in T cells after unspecific stimulation, CD4+ and CD8+ T cells from naïve B6 mice were isolated by magnetic cell separation and stimulated in vitro with plate-bound anti-CD3 and soluble anti-CD28, using different degrees of stimulation, as it has been suggested that the function of the PD-1/PD-L1 system depends on the level of T-cell stimulation. IFN-γ levels were measured in the cell culture supernatants. IFN-γ production of CD4+ T cells was dependent on the strength of stimulation; however, PD-L1−/− CD4+ T cells produced more IFN-γ than their WT counterparts at a low degree of T-cell stimulation, and this difference was obscured at a high degree of T-cell stimulation (Figure 9B). In contrast, CD8+ T cells lacking PD-L1 generally showed decreased production of IFN-γ when compared to PD-L1–proficient CD8+ T cells, regardless of the degree of T-cell stimulation (Figure 9C). These data show that the effect of PD-L1 expression on activated T cells is dependent on the respective cell type and the degree of T-cell activation.
Effects of PD-L1 in Tracheal Transplantation Are Predominantly Mediated by T Cellular Alloimmune Responses
To investigate a potential effect of PD-L1 on early graft damage (eg, by ischemia reperfusion injury), all grafts were stained for polymorphonuclear neutrophils and macrophages at day 2 after transplantation. Numbers of polymorphonuclear neutrophils and macrophages were similar between all groups (data not shown). Moreover, IgG, IgM, and C4d, indicating autoimmunity or alloantibody-mediated rejection, were not expected and not detectable in any of the grafts at days 2, 7, and 14. In addition, WT and PD-L1−/− recipients of isogeneic grafts similarly showed a mild degree of tissue injury, indicating that predominantly alloimmune reactions rather than any other causes of graft damage are responsible for the observed differences between WT and PD-L1−/− recipients in the heterotopic tracheal transplant model.
Discussion
The costimulatory molecule PD-L1 is involved in several immunological settings, including autoimmunity, alloimmunity, and tumor immunity, and has been studied in a broad variety of experimental disease models. We tested the effects of PD-L1 on both histopathology and systemic alloimmune responses in a murine model of tracheal transplantation, as PD-L1 has not been studied in lung transplantation and obliterative airway disease.3
The lung has the highest rate of rejection among all solid organ transplants, likely because of its constant exposure to atmospheric elements and pathogens, making it particularly vulnerable to immunological and inflammatory injury.13 Treatment with current immunosuppressive agents has not ameliorated obliterative airway disease, suggesting that immune injury mechanisms remain to be defined in depth.
Using a murine airway transplant model of trachea transplantation, we first showed that PD-L1 is up-regulated in the epithelium of tracheas grafted into fully major histocompatibility complex–disparate recipients compared to naive tracheas. This observation suggests a functional role of PD-L1 in the airway of tracheal transplants. We then showed that PD-L1 within the donor tissue provides allograft protection, as PD-L1−/− donor tissue showed significantly increased luminal obliteration and airway epithelial loss. This observation is in accordance with other transplant studies showing a protective role of tissue PD-L1 expression for allograft survival.8 A similar protective function of PD-L1 was also shown in another model of epithelial injury, in which CD8+ T-cell–mediated injury to the epithelium in a renal immune injury model was abrogated when PD-L1 was expressed by the epithelium.24 Similarly, tumor cells expressing PD-L1 have been shown to escape anti-tumor immune reactions.25
More notably, we show that PD-L1 has dichotomous effects, depending on its location, as WT tracheas transplanted into PD-L1−/− recipients were protected from tissue injury. So far, this has not been reported in other transplant models and points to a novel function of PD-L1 in the context of a highly immunogenic model.
Although PD-L1 has been characterized as an inhibitory molecule providing T-cell regulation on ligation to its receptor PD-1 in the vast majority of the experimental and clinical studies, this is not completely unequivocal. Several authors report that the regulatory function of PD-L1 is only present at low levels of T-cell activation,26, 27 and Subudhi et al11 showed that overexpression of PD-L1 on pancreatic islet B cells causes accelerated rejection of transplanted allogenic PD-L1–expressing cells and increased CD8+ T-cell proliferation.
The prevention from allograft injury in PD-1−/− recipients points to a significant effect of the lacking interaction between PD-L1 and PD-1 per se, or to a potential yet unidentified receptor providing a positive costimulatory signal,28 within the recipient's immune system. As we and others29 observed increased PD-1 expression on effector and regulatory T cells from PD-L1−/− mice, we tested whether overexpression of PD-1 mediates graft protection in PD-L1−/− mice via interaction with tissue PD-L1. As a blocking monoclonal antibody against PD-1 did not worsen graft histology and systemic alloimmune response in PD-L1−/− recipients of WT tracheas, overexpression of PD-1 and subsequently increased interaction with tissue PD-L1 in these mice is not the dominant mechanism of graft protection. The histological results in PD-L1−/− recipients and even more compelling in PD-1−/− and anti–PD-1–treated PD-L1−/− recipients suggest a connection between the PD-1/PD-L1 interaction in the recipients' immune system and susceptibility of grafts to injury. Therefore, we further analyzed the systemic immune responses in these animals.
The frequencies of cytokine-producing cells were in accordance with the respective histopathological outcome. Although WT recipients of PD-L1–deficient donor tissue exhibited an increased alloreactivity toward donor antigen, PD-L1–deficient recipients, although displaying an increased number of T cells both in the spleen and the graft, showed a decreased alloimmune response. The observation that PD-1−/− and PD-L1−/− recipients similarly showed reduced graft pathology and systemic alloimmune responses when compared to WT recipients suggests that the interrupted PD-1/PD-L1 pathway is limiting immune responses within the recipient.
In subsequent experiments, we demonstrated that particularly CD8+ effector cells, which showed strong IFN-γ production after restimulation with alloantigen in WT recipients, were hyporesponsive in PD-L1−/− mice, whereas IFN-γ production by CD4+ T cells was weak in both WT and PD-L1−/− recipients. As previously demonstrated by Freeman et al,27 the regulatory effect of PD-L1 on the activation of CD4+ T cells during stimulation with different concentrations of anti-CD3 and anti-CD28 was restricted to low levels of T-cell activation, whereas it was obscured at high levels of T-cell activation. We were not only able to confirm these results, but also further demonstrated that PD-L1−/− CD8+ T cells generally showed decreased IFN-γ production, regardless of the degree of T-cell activation. Taken together, our data suggest that in solid organ transplantation the net effect of the PD-1/PD-L1 pathway is not uniformly regulatory, but rather depends on both the effector cells and the degree of T-cell activation, and might be organ specific. In our experiments, CD8+ T cells proved to be highly powerful effectors, confirming the particular pathophysiological relevance of CD8+ T cells in this model, which has been shown by several authors.18, 30, 31
However, further investigation is needed to determine whether the changes in T-cell responses observed in this study can directly explain the apparent decrease in tracheal allograft injury, and to understand the underlying mechanisms in detail.
PD-L1−/− recipients showed increased percentages of CD4+CD25+FoxP3+ regulatory T cells when compared to WT recipients, whereas IL-10 production by splenocytes after restimulation with allogeneic cells was low and did not differ between WT and PD-L1−/− recipients (data not shown). Further compensatory immunoregulative reactions might contribute to the observed phenotype, and the underlying pathophysiology remains to be fully clarified in future studies.
Other mechanisms, such as ischemia-reperfusion injury, antibody-mediated rejection, and autoimmunity, can also contribute to the development of BO syndrome,32 and it cannot be excluded that the mutant strains of mice might be less or more susceptible to such injury. However, early damage by ischemia-induced injury does not differ between both groups, as histology and infiltration of neutrophils and macrophages were similar 2 days after transplantation, and the lack of any IgG, IgM, or C4d deposits in the graft tissues of WT and PD-L1−/− recipients indicates that autoimmunity and alloantibody-mediated rejection do not play a relevant role in this model.
Furthermore, the nonsterile nature of lung transplants and the resulting high immunogenicity might form the basis for the unexpected functions of PD-L1 in the trachea transplantation model. Indeed, most experimental solid organ transplant studies showing a protective effect of the PD-1/PD-L1 pathway, as recently reviewed by Riella et al,33 used transplant models with limited T-cell reactivity because of concomitant immunosuppression, additional blockade of CD28/B7 costimulation, or limited major histocompatibility complex mismatch. Although it is now generally considered that the regulatory functions of the PD-1/PD-L1 pathway might not be effective in models with strong T-cell activation, the observed adverse effects of PD-L1 in the BO model remain intriguing and highlight the biological complexity of T-cell costimulatory or coinhibitory pathways, which can have divergent functions in different tissues and under varying immunological settings. This should particularly be considered in future clinical studies targeting the PD-1/PD-L1 system by means of blocking and agonistic antibodies and fusion proteins.
Acknowledgment
We thank Dr. Robert B. Colvin (Massachusetts General Hospital) for the IgG, IgM, and C4d immunofluorescence studies and their quantification.
Footnotes
Supported by NIH grant KO8 HL084242-01 and Boston Children's Hospital institutional support (M.S.).
K.S.-N., O.B., and H.H. contributed equally to this work.
Disclosures: None declared.
Current address of O.B., Department of Intensive Care Medicine, University Hospital Hamburg-Eppendorf, Hamburg, Germany.
References
- 1.Li X.C., Rothstein D.M., Sayegh M.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. Immunol Rev. 2009;229:271–293. [Google Scholar]
- 2.Alegre M.L., Najafian N. Costimulatory molecules as targets for the induction of transplantation tolerance. Curr Mol Med. 2006;6:843–857. doi: 10.2174/156652406779010812. [DOI] [PubMed] [Google Scholar]
- 3.Francisco L.M., Salinas V.H., Brown K.E., Vanguri V.K., Freeman G.J., Kuchroo V.K., Sharpe A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenwald R.J., Freeman G.J., Sharpe A.H. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 5.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 6.Odorizzi P.M., Wherry E.J. Inhibitory receptors on lymphocytes: insights from infections. J Immunol. 2012;188:2957–2965. doi: 10.4049/jimmunol.1100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J., Popoola J., Khandwala S., Vadivel N., Vanguri V., Yuan X., Dada S., Guleria I., Tian C., Ansari M.J., Shin T., Yagita H., Azuma M., Sayegh M.H., Chandraker A. Critical role of donor tissue expression of programmed death ligand-1 in regulating cardiac allograft rejection and vasculopathy. Circulation. 2008;117:660–669. doi: 10.1161/CIRCULATIONAHA.107.741025. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka K., Albin M.J., Yuan X., Yamaura K., Habicht A., Murayama T., Grimm M., Waaga A.M., Ueno T., Padera R.F., Yagita H., Azuma M., Shin T., Blazar B.R., Rothstein D.M., Sayegh M.H., Najafian N. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol. 2007;179:5204–5210. doi: 10.4049/jimmunol.179.8.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boenisch O., Sayegh M.H., Najafian N. Negative T-cell costimulatory pathways: their role in regulating alloimmune responses. Curr Opin Organ Transplant. 2008;13:373–378. doi: 10.1097/MOT.0b013e328306117f. [DOI] [PubMed] [Google Scholar]
- 10.Pilat N., Sayegh M.H., Wekerle T. Costimulatory pathways in transplantation. Semin Immunol. 2011;23:293–303. doi: 10.1016/j.smim.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subudhi S.K., Zhou P., Yerian L.M., Chin R.K., Lo J.C., Anders R.A., Sun Y., Chen L., Wang Y., Alegre M.L., Fu Y.X. Local expression of B7-H1 promotes organ-specific autoimmunity and transplant rejection. J Clin Invest. 2004;113:694–700. doi: 10.1172/JCI19210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belperio J.A., Lake K., Tazelaar H., Keane M.P., Strieter R.M., Lynch J.P., III Bronchiolitis obliterans syndrome complicating lung or heart-lung transplantation. Semin Respir Crit Care Med. 2003;24:499–530. doi: 10.1055/s-2004-815601. [DOI] [PubMed] [Google Scholar]
- 13.Boehler A., Estenne M. Post-transplant bronchiolitis obliterans. Eur Respir J. 2003;22:1007–1018. doi: 10.1183/09031936.03.00039103. [DOI] [PubMed] [Google Scholar]
- 14.Belperio J.A., Weigt S.S., Fishbein M.C., Lynch J.P., III Chronic lung allograft rejection: mechanisms and therapy. Proc Am Thorac Soc. 2009;6:108–121. doi: 10.1513/pats.200807-073GO. [DOI] [PubMed] [Google Scholar]
- 15.Shilling R.A., Wilkes D.S. Immunobiology of chronic lung allograft dysfunction: new insights from the bench and beyond. Am J Transplant. 2009;9:1714–1718. doi: 10.1111/j.1600-6143.2009.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Budding K., van de Graaf E.A., Otten H.G. Humoral immunity and complement effector mechanisms after lung transplantation. Transpl Immunol. 2014;31:260–265. doi: 10.1016/j.trim.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Xue J., Zhu X., George M.P., Myerburg M.M., Stoner M.W., Pilewski J.W., Duncan S.R. A human-mouse chimeric model of obliterative bronchiolitis after lung transplantation. Am J Pathol. 2011;179:745–753. doi: 10.1016/j.ajpath.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richards D.M., Dalheimer S.L., Hertz M.I., Mueller D.L. Trachea allograft class I molecules directly activate and retain CD8+ T cells that cause obliterative airways disease. J Immunol. 2003;171:6919–6928. doi: 10.4049/jimmunol.171.12.6919. [DOI] [PubMed] [Google Scholar]
- 19.Casey A., Dirks F., Liang O.D., Harrach H., Schuette-Nuetgen K., Leeman K., Kim C.F., Gerard C., Subramaniam M. Bone marrow-derived multipotent stromal cells attenuate inflammation in obliterative airway disease in mouse tracheal allografts. Stem Cells Int. 2014;2014:468927. doi: 10.1155/2014/468927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang O.D., Kleibrink B.E., Schuette-Nuetgen K., Khatwa U.U., Mfarrej B., Subramaniam M. Green tea epigallo-catechin-galleate ameliorates the development of obliterative airway disease. Exp Lung Res. 2011;37:435–444. doi: 10.3109/01902148.2011.584359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hele D.J., Yacoub M.H., Belvisi M.G. The heterotopic tracheal allograft as an animal model of obliterative bronchiolitis. Respir Res. 2001;2:169–183. doi: 10.1186/rr55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guleria I., Khosroshahi A., Ansari M.J., Habicht A., Azuma M., Yagita H., Noelle R.J., Coyle A., Mellor A.L., Khoury S.J., Sayegh M.H. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med. 2005;202:231–237. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyajima M., Chase C.M., Alessandrini A., Farkash E.A., Della Pelle P., Benichou G., Graham J.A., Madsen J.C., Russell P.S., Colvin R.B. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol. 2011;178:1635–1645. doi: 10.1016/j.ajpath.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waeckerle-Men Y., Starke A., Wuthrich R.P. PD-L1 partially protects renal tubular epithelial cells from the attack of CD8+ cytotoxic T cells. Nephrol Dial Transplant. 2007;22:1527–1536. doi: 10.1093/ndt/gfl818. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L., Gajewski T.F., Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114:1545–1552. doi: 10.1182/blood-2009-03-206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Latchman Y.E., Liang S.C., Wu Y., Chernova T., Sobel R.A., Klemm M., Kuchroo V.K., Freeman G.J., Sharpe A.H. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freeman G.J., Long A.J., Iwai Y., Bourque K., Chernova T., Nishimura H., Fitz L.J., Malenkovich N., Okazaki T., Byrne M.C., Horton H.F., Fouser L., Carter L., Ling V., Bowman M.R., Carreno B.M., Collins M., Wood C.R., Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S., Bajorath J., Flies D.B., Dong H., Honjo T., Chen L. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med. 2003;197:1083–1091. doi: 10.1084/jem.20021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller S.N., Vanguri V.K., Ha S.J., West E.E., Keir M.E., Glickman J.N., Sharpe A.H., Ahmed R. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J Clin Invest. 2010;120:2508–2515. doi: 10.1172/JCI40040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.West E.E., Lavoie T.L., Orens J.B., Chen E.S., Ye S.Q., Finkelman F.D., Garcia J.G.N., McDyer J.F. Pluripotent allospecific CD8+ effector T cells traffic to lung in murine obliterative airway disease. Am J Respir Cell Mol Biol. 2006;34:108–118. doi: 10.1165/rcmb.2005-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah P.D., West E.E., Whitlock A.B., Orens J.B., McDyer J.F. CD154 deficiency uncouples allograft CD8+ T-cell effector function from proliferation and inhibits murine airway obliteration. Am J Transplant. 2009;9:2697–2706. doi: 10.1111/j.1600-6143.2009.02805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer K.C., Raghu G., Verleden G.M., Corris P.A., Aurora P., Wilson K.C., Brozek J., Glanville A.R. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J. 2014;44:1479–1503. doi: 10.1183/09031936.00107514. [DOI] [PubMed] [Google Scholar]
- 33.Riella L.V., Watanabe T., Sage P.T., Yang J., Yeung M., Azzi J., Vanguri V., Chandraker A., Sharpe A.H., Sayegh M.H., Najafian N. Essential role of PDL1 expression on nonhematopoietic donor cells in acquired tolerance to vascularized cardiac allografts. Am J Transplant. 2011;11:832–840. doi: 10.1111/j.1600-6143.2011.03451.x. [DOI] [PubMed] [Google Scholar]