Abstract
Recent studies implicate the Cyr61, CTGF, Nov (CCN) matricellular signaling protein family as emerging players in vascular biology, with NOV (alias CCN3) as an important regulator of vascular homeostasis. Herein, we examined the role of CCN3 in the pathogenesis of atherosclerosis. In response to a 15-week high-fat diet feeding, CCN3-deficient mice on the atherosclerosis-prone Apoe−/− background developed increased aortic lipid-rich plaques compared to control Apoe−/− mice, a result that was observed in the absence of alterations in plasma lipid content. To address the cellular contributor(s) responsible for the atherosclerotic phenotype, we performed bone marrow transplantation experiments. Transplantation of Apoe; Ccn3 double-knockout bone marrow into Apoe−/− mice resulted in an increase of atherosclerotic plaque burden, whereas transplantation of Apoe−/− marrow to Apoe; Ccn3 double-knockout mice caused a reduction of atherosclerosis. These results indicate that CCN3 deficiency, specifically in the bone marrow, plays a major role in the development of atherosclerosis. Mechanistically, cell-based studies in isolated peritoneal macrophages demonstrated that CCN3 deficiency leads to an increase of lipid uptake and foam cell formation, an effect potentially attributed to the increased expression of scavenger receptors CD36 and SRA1, key factors involved in lipoprotein uptake. These results suggest that bone marrow–derived CCN3 is an essential regulator of atherosclerosis and point to a novel role of CCN3 in modulating lipid accumulation within macrophages.
Studies during the past several decades have established that atherosclerosis is a chronic inflammatory disease of the arterial wall.1 The pathogenesis of atherosclerosis represents dynamic interactions between circulating inflammatory factors and vascular cells, including endothelial cells, smooth muscle cells, monocytes, and lymphocytes. Experimental and clinical observations support a central role for monocytes/macrophages in atherogenesis.2 However, despite tremendous efforts, the molecular regulation of macrophage function within the vasculature remains incompletely understood.
Several groups have produced convincing research that the CCN (Cyr61, CTGF, Nov) matricellular family of proteins plays important biological roles within the vasculature.3 These studies have provided functional insights into the biological effects of CCN family members, including regulation of angiogenesis, inflammation, reactive oxygen species production, and vascular remodeling.4, 5 Most of these studies, however, focused on the contributions of vascular resident cells, such as smooth muscle cells, endothelial cells, and fibroblasts. Lesser known is the role of CCN proteins in bone marrow–derived immune cells, a group of cells that are critical regulators of vascular function. Recently, studies have shed light on key contributions of CCN1 in promoting macrophage inflammation and neutrophil efferocytosis,6, 7 and the role of CCN3-deficient myeloid cells on the progression of abdominal aortic aneurysm.5 However, outside of these reports, the function of CCN proteins in immune cells remains largely unexplored. NOV (alias CCN3) is of particular interest because of its functional differences from the other CCN family members, with the loss of CCN3 exacerbating vascular disease. CCN3 was first isolated from nephroblastoma tissue in newborn chicks infected with the MAV-1(N) avian retrovirus.8 Since then, studies have shown that CCN3 can regulate endothelial cell inflammation and neointimal hyperplasia,9, 10 as well as exert atheroprotective effects on diet-induced mouse models.11 Of significance, in contrast to CCN1 and CCN2 knockout mice, CCN3 knockout mice are viable and develop to adulthood with a normal vasculature and ECM composition.10 In this study, using the CCN3 knockout model, we investigated the role of macrophage CCN3 in the pathogenesis of atherosclerosis. Our findings described herein offer novel insights into the regulation of macrophage function by CCN3.
Materials and Methods
Animals
All animal protocols were approved by the Institutional Animal Care and Use Committee at Case Western Reserve University, which is certified by the American Association of Accreditation for Laboratory Animal Care. Ccn3−/− mice on a C57BL/6 background were generated, as previously described.10 Apoe−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME), also on a C57BL/6 background.
Ccn3−/− mice were mated to Apoe−/− mice to generate Apoe−/−; Ccn3−/− mice. Mice were housed under standard light-dark conditions (12 hours:12 hours) and allowed ad libitum access to standard rodent chow and water. Accelerated atherosclerosis was induced by feeding male mice a high-fat diet (HFD) containing 1.25% added cholesterol (Research Diets, New Brunswick, NJ; D-12108) for a total of 15 weeks, starting at 6 weeks of age.
Bone Marrow Transplantation
Eight-week-old male recipient Apoe−/− and Apoe−/−; Ccn3−/− mice were lethally irradiated (11 Gy) 4 hours before transplantation. Bone marrow was collected from the femurs and tibias of donor Apoe−/− and Apoe−/−; Ccn3−/− deficient mice by flushing with sterile Dulbecco's phosphate-buffered saline containing 2% fetal bovine serum (Atlantic Biologicals, Miami, FL). Each recipient mouse was injected with 2 × 106 of unfractionated bone marrow cells through the lateral tail vein. Mice were then fed a HFD for 15 weeks beginning 4 weeks after bone marrow (BM) transplantation.
Atherosclerotic Lesion Analysis
After 15 weeks on a HFD, mice were anesthetized and sacrificed. The left ventricles were perfused with 20 mL of phosphate-buffered saline, followed by 10 mL of 4% paraformaldehyde. After 48 hours of fixation in 4% paraformaldehyde, the adventitia were carefully removed. For en face preparation, the aortae were stained with Sudan IV (Sigma-Aldrich, St. Louis, MO) for 15 minutes and differentiated in ethanol. The stained aortas were then opened longitudinally and pinned onto plain black wax plates. Digital images of Sudan IV–stained aortas were quantified using ImageJ software version 1.49 (NIH, Bethesda, MD; http://imagej.nih.gov/ij). Results were expressed as the percentage of the positive Sudan IV color to the total aortic area. For aortic root quantification of atherosclerosis, paraffin-embedded sections of the aortic root were serially sectioned through the aorta at the origins of aortic valve leaflets. Every eighth section (80-μm interval) from the aortic sinus, one slide was chosen and stained with hematoxylin and eosin for quantitation of the lesion areas with ImageJ. The aortic lesion size of each animal was obtained by the averaging of lesion areas in five slides.
Foam Cell Formation and Macrophage Lipid Uptake
Peritoneal macrophages were isolated from 8- to 10-week-old mice 3 days after an i.p. injection of 2 mL of 3% thioglycollate (Sigma-Aldrich). Cells were suspended in RPMI 1640 culture media (Life Technologies, Carlsbad, CA) supplemented with 5% fetal bovine serum and plated onto a 12-well tissue culture plate. After 2 hours, nonadherent cells were removed and macrophages were incubated in fresh RPMI 1640 media for 24 hours at 37°C with 5% CO2. For foam cell formation assessment, cells were incubated with oxidized low-density lipoprotein (oxLDL; 50 μg/mL; Alfa Aesar, Haverhill, MA) for 24 hours. Cells were stained with 0.5% oil red O (Sigma-Aldrich) in isopropanol (60%) for 60 minutes and photographs were taken. Cells that contained lipids that covered greater than one-third of the cytoplasm were categorized as foam cells. For fluorescently labeled oxLDL (DiI-oxLDL) uptake, cells were incubated with 10 μg/mL DiI-oxLDL for 2 hours at 37°C, then were washed and suspended in phosphate-buffered saline, followed by flow cytometric analysis.
Protein Isolation and Immunoblotting
Thioglycollate-elicited peritoneal macrophages were treated with or without oxLDL for 24 hours at 37°C and 5% CO2. Total protein was harvested by radioimmunoprecipitation assay buffer supplemented with protease and phosphatase inhibitor tablets (Roche Diagnostics, Indianapolis, IN). Aortic tissue was snap frozen in liquid nitrogen, then homogenized using a pellet pestle motor (Sigma-Aldrich). Protein samples were extracted using a Total Protein Extraction Kit with protease and phosphatase inhibitors (EMD Millipore, Billerica, MA), according to the manufacturer's instructions. Protein concentrations were determined using a bicinchoninic acid protein assay kit (Pierce Biotechnology, Waltham, MA). Lysates containing 60 μg (tissue) or 30 μg (cells) of protein were analyzed by SDS-PAGE and immunoblotting. Primary antibodies used were the following: ABCA-1 and ABCG-1 (Santa Cruz Biotechnology, Dallas, TX), CD36 and SRAI (R&D Systems, Minneapolis, MN), and ACAT1 (Novus Biologicals, Littleton, CO). Membranes were reprobed with antibodies against β-actin (Santa Cruz Biotechnology) to confirm equal loading. Protein levels were quantified using ImageJ.
Histology and Immunohistochemistry
Aortic tissue from euthanized mice was harvested, rinsed in phosphate-buffered saline, fixed in 4% paraformaldehyde for 48 hours, embedded in paraffin, and serial sections (5 μm thick) were cut. Aortic paraffin sections were stained for MAC3 (BD Biosciences, San Jose, CA), CD68 (Bio-Rad Laboratories, Hercules, CA), monocyte chemoattractant protein-1 (Santa Cruz), and vascular cell adhesion molecule 1 (Santa Cruz). Immunostained sections were quantified as the percentage of positive staining area per total area using ImageJ.
Quantitative PCR
mRNA was isolated from peritoneal macrophages from wild-type (WT) and Apoe−/− mice using an mRNA isolation kit (Qiagen), following the standard protocol, and cDNA was made from 1 μg mRNA template using iScript Reverse Transcriptase (Bio-Rad Laboratories). Quantitative PCRs were performed using Power SYBR Green master mix (Applied Biosystems, Foster City, CA).
Lipid Analysis
Animals were fasted for 12 to 15 hours, and blood was collected from the tail vein. Measurement of total plasma cholesterol, triglyceride levels, and the lipid profile assessment by fast performance liquid chromatography was performed by the Vanderbilt NIH Mouse Metabolic Phenotyping Core.
Statistical Analysis
Data are expressed as means ± SEM. Either the t-test or U-test was used, where appropriate, when comparing the difference between two groups. For comparison of the effects of two variables, two-way analysis of variance, followed by Bonferroni post hoc correction, was used. P < 0.05 was considered significant.
Results
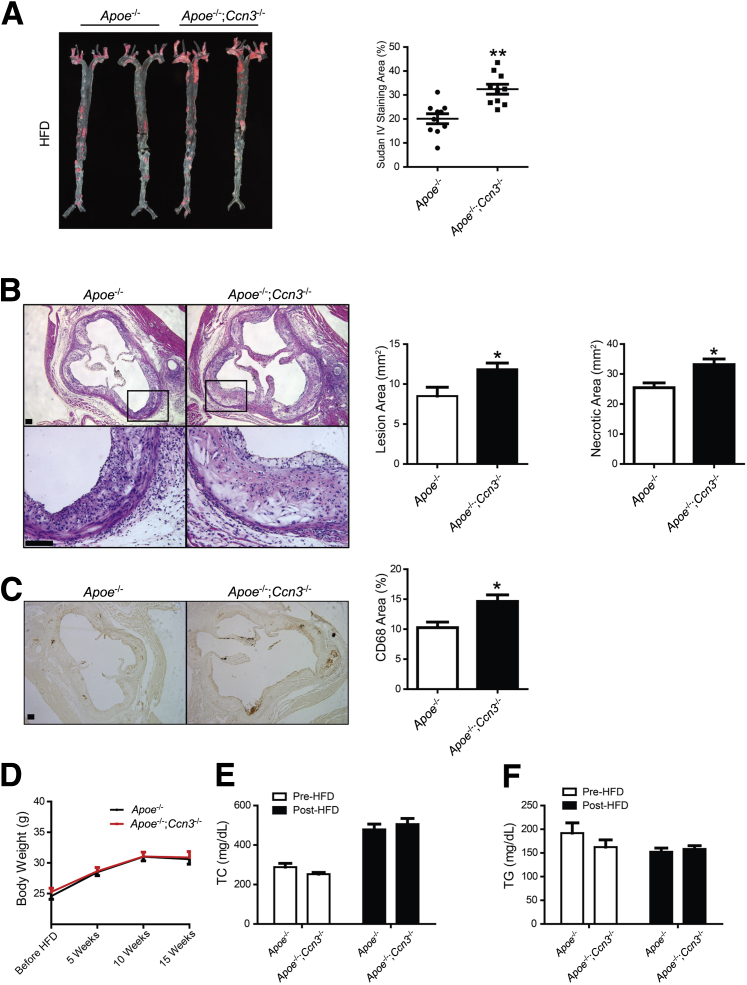
Our recent observations provided the inaugural demonstration of the role of CCN3 in mitigating the formation and progression of abdominal aortic aneurysm. Reduced incidence and severity of abdominal aortic aneurysm was attributed to the pleiotropic effects of CCN3 on the aortic vasculature: dampening vascular inflammation, oxidative stress, matrix metalloproteinase proteolytic activity, and cellular apoptosis.5 Because many of these processes are important for accelerated plaque development, data from our previous report combined with data generated by Liu et al,11 showing CCN3 overexpression inhibits atherosclerotic development within Apoe-deficient mice, invite speculation that CCN3 deficiency may also affect the development of (exacerbate) atherosclerosis. To test this hypothesis, we performed studies using the well-established ApoE-null atherosclerosis model. Apoe−/− and Apoe−/−; Ccn3−/− mice were subjected to a HFD, as described above, and the degree of atherosclerosis was determined by the quantitation of sudanophilic en face lesions in pinned aortas. Aortas of Apoe−/−; Ccn3−/− mice displayed enhanced atherosclerotic plaque formation, approximately 50% greater than observed in Apoe−/−mice (Figure 1A). Hematoxylin and eosin staining of the aortic root showed a significantly greater area of lipid-rich plaques in Apoe−/−; Ccn3−/− mice when compared to Apoe−/− mice, with an average lesional area increase from 0.86 to 1.19 mm2 (Figure 1B). This was concomitant with an increase of localized necrosis (Figure 1B) as well as macrophage infiltration, as shown by CD68 staining (Figure 1C). The same trend was observed when aortas were examined from both groups fed only a normal chow diet (Supplemental Figure S1A), although, predictably, total lesion areas were substantially less when compared to aortas from mice on a HFD. Notably, although CCN3 expression was increased in Apoe−/−mice on normal chow and HFD (Supplemental Figure S2), no significant differences were observed in body weight (Figure 1D), cholesterol levels (Figure 1E), and plasma triglycerides (Figure 1F) either before or after high-fat diet feeding. Lipoprotein profiles between the two groups of mice were also unaffected (Supplemental Figure S1, B and C, and Supplemental Figure S3). Consistent with increased lesion severity, a heightened inflammatory response was seen in the aortic tissues from Apoe−/−; Ccn3−/− mice, as illustrated by enhanced macrophage infiltration (Supplemental Figure S4A), as well as increased monocyte chemoattractant protein-1 and vascular cell adhesion molecule 1 expression (Supplemental Figure S4, B and C). Taken together, the genetic loss of CCN3 increases aortic atherogenesis independently of the plasma lipid profiles in Apoe−/−; Ccn3−/− mice.
Figure 1.
CCN3 deficiency accelerates atherosclerosis in Apoe-null mice after 15 weeks of high-fat diet (HFD) feeding. A:Left panel: Representative image of Sudan IV staining of whole aortas from Apoe−/− and Apoe−/−; Ccn3−/− mice. Right panel: atheroma quantitation is shown. B:Left panels: Representative examples of cross sections from the aortic sinus stained with hematoxylin and eosin. Right panels: Quantitation of atheroma in the root is shown. Boxed areas are shown at higher magnification below. C:Left panels: Representative examples of cross sections from the aortic sinus stained with CD68. Right panel: Quantitation of necrosis is shown. D: Body weight of Apoe−/− and Apoe−/−; Ccn3−/− mice before and after 15 weeks on a HFD. E and F: Fasting total cholesterol (TC; E) and triglyceride levels (TG; F) of Apoe−/−and Apoe−/−; Ccn3−/− mice before and after 15 weeks on a HFD. n = 10 per group (A); n = 5 per group (B and C); n = 17 to 18 (D); n = 9 to 10 (E and F). ∗P < 0.05, ∗∗P < 0.005 versus Apoe−/−. Scale bars = 100 μm (B and C). Original magnifications: ×5 (B, top panels); ×20 (B, bottom panels).
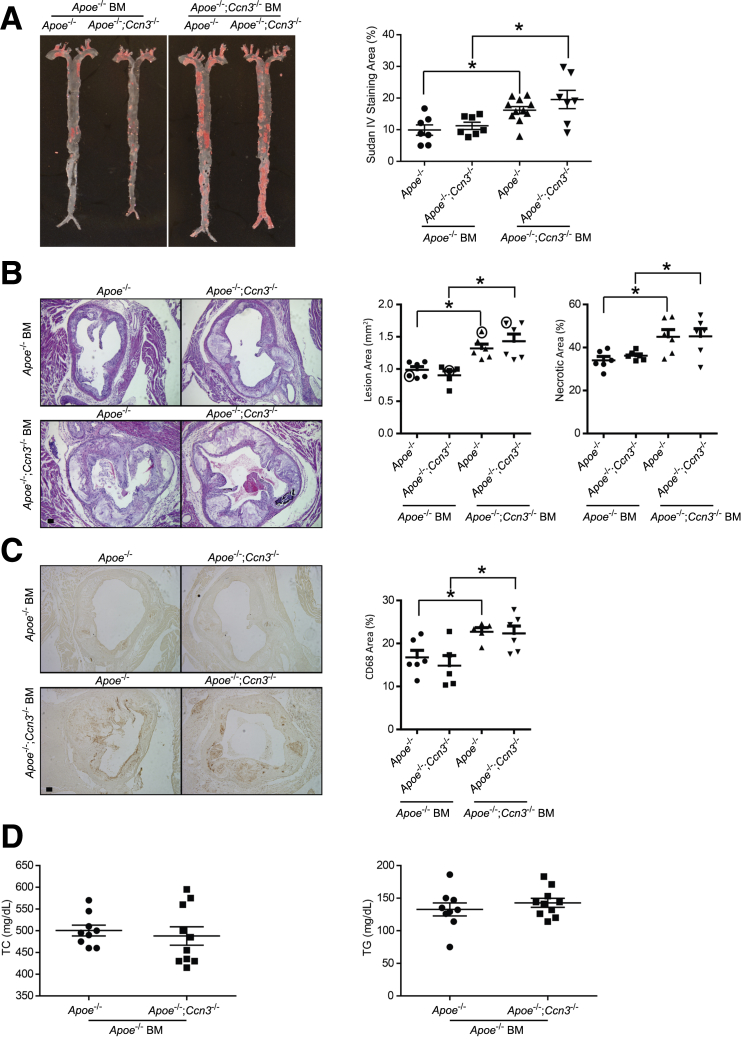
To determine whether CCN3 deficiency in myeloid cells contributes to atherogenesis, we performed reciprocal BM transplant experiments. As measured by quantification of en face aortic lesion area, transplantation of Apoe−/− BM into Apoe−/−; Ccn3−/− mice caused a reduction in total aortic lesion area to a comparable level, as seen in the Apoe−/− recipient (Sudan IV–stained aortas) (Figure 2A). Consistent with this observation, transplantation of Apoe−/−; Ccn3−/− BM into Apoe−/− mice resulted in a significant increase of total lesion area, recapitulating the disease severity seen in Apoe−/−; Ccn3−/− controls (Figure 2A). When the aortic root is compared between the two groups, the identical trend is observed. Apoe−/−; Ccn3−/− BM recipients show increased lesion area and enhanced necrosis when compared to Apoe−/− BM recipients (Figure 2B). In agreement with the macrophage data presented in Figure 1C, there was increased macrophage content (CD68 staining) in aortae from mice transplanted with Apoe−/−; Ccn3−/− BM, whereas putting Apoe−/− BM back into Apoe−/−; Ccn3−/− mice returned macrophage numbers to Apoe−/− baseline levels (Figure 2C). Analysis of total plasma cholesterol and triglyceride levels from BM-transplanted mice (Apoe−/− as donor) after HFD administration revealed no significant difference between the Apoe−/−; Ccn3−/− and Apoe−/− groups (Figure 2D). Combined, these data strongly suggest that CCN3 deficiency in BM contributes to atherogenesis and its expression in BM is atheroprotective.
Figure 2.
Deficiency of CCN3 in the bone marrow results in increased atherosclerosis development in Apoe−/− mice. A:Left panels: Representative image of Sudan IV staining of whole aortas from Apoe−/− and Apoe−/−; Ccn3−/− chimeras receiving bone marrow (BM) from Apoe−/− or Apoe−/−; Ccn3−/− mice. Right panel: Lesion quantitation is shown. B:Left panels: Representative hematoxylin and eosin–stained images of cross sections from the aortic sinus. Right panels: Quantitation is shown. Data points in circles correspond to shown images. C: Left panels: Representative CD68-stained images of cross sections from the aortic sinus. Right panel: Quantitation is shown. D: Fasting total cholesterol (TC) and triglyceride (TG) levels from Apoe−/− and Apoe−/−; Ccn3−/− mice transplanted with Apoe−/− marrow after high-fat diet. n = 7 to 10 per group (A); n = 5 to 6 per group (B and C); n = 9 to 10 per group (D). ∗P < 0.05. Scale bars = 100 μm (B and C).
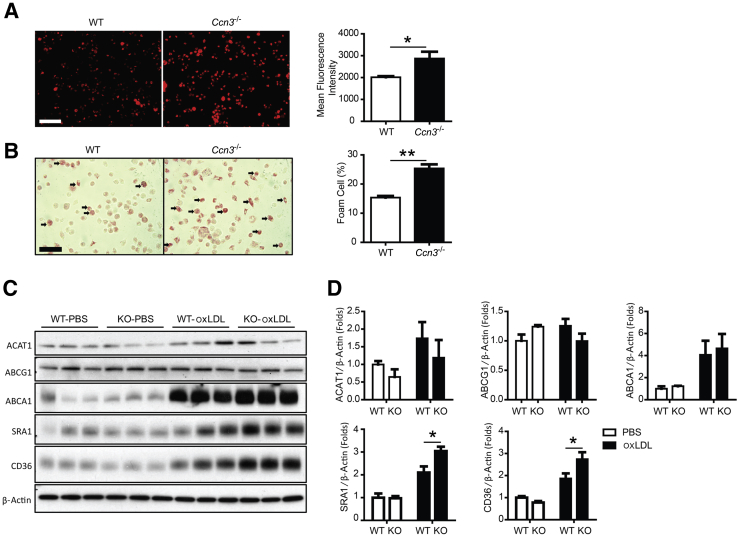
Given the central importance of macrophages in the pathogenesis of atherosclerosis, we focused on the role of CCN3 in regulating macrophage function. First, we assessed the effect of CCN3 deficiency on foam cell formation—a crucial step in atherogenesis. On exposure to modified lipoproteins, such as oxidized LDL, macrophages differentiate into foam cells. Thus, we investigated peritoneal macrophage uptake of DiI-oxLDL. Approximately a 50% increase of Dil-oxLDL uptake was observed in CCN3-deficient macrophages when compared to Dil-oxLDL uptake in WT macrophages, findings confirmed and quantified by flow cytometric analysis (Figure 3A). Consistent with increased oxLDL uptake in CCN3−/− macrophages, increased foam cell formation was also observed when compared to WT control (Figure 3B). The same trend was observed when peritoneal macrophages from Apoe−/− and Apoe−/−; Ccn3−/− mice were tested for foam cell formation (Supplemental Figure S5, A and B). Although there was an overall increase in foam cell numbers in both groups, as expected, the absence of CCN3 led to an even further increase (compare Apoe−/− at 33% to Apoe−/−; Ccn3−/− at 48%), in agreement with what was observed in WT and Ccn3−/− macrophages. (Figure 3, A and B). In addition, to determine whether the enhanced foam cell formation because of the absence of CCN3 can be reversed, we added oxLDL to macrophages from WT, Ccn3+/−, and Ccn3−/− mice and quantified foam cell numbers. Ccn3+/− macrophages still produced significantly more foam cells than WT macrophages (Supplemental Figure S5C), yet less than seen in Ccn3−/− macrophages. Therefore, addition of one WT CCN3+ allele was not sufficient to completely rescue the phenotype.
Figure 3.
CCN3 deficiency in macrophages promotes lipid uptake and foam cell formation. A: Representative image of fluorescently labeled oxidized low-density lipoprotein (DiI-oxLDL) uptake in macrophages. Quantitation of DiI-oxLDL in wild-type (WT) and Ccn3−/− macrophages by flow cytometry. B: Foam cell formation in WT and Ccn3−/− peritoneal macrophages after incubation with oxLDL for 24 hours. Arrows indicate foam cells. Quantitation of foam cell formation. C: Western blot of ACAT1, ABCG1, ABCA1, SRAI, and CD36 in WT and Ccn3−/− macrophages treated with and without oxLDL for 24 hours. D: Quantitation of Western blotting in C. n = 5 (A); n = 6 (B); n = 3 to 6 (D). ∗P < 0.05, ∗∗P < 0.005. Scale bars = 100 μm (A and B). KO, CCN3 knockout mouse macrophages; PBS, phosphate-buffered saline.
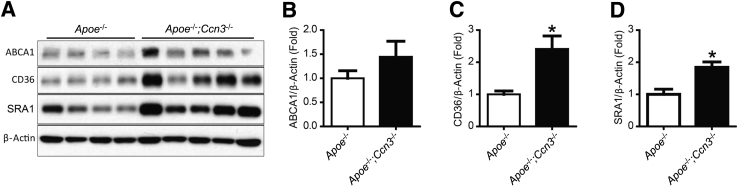
To understand how CCN3 deficiency might enhance lipid accumulation and foam cell formation in macrophages, we surveyed the expression of several key factors involved in cholesterol esterification and efflux. No significant difference in protein levels was observed for ABCA1 and ABCG1, two major factors involved in cholesterol efflux from macrophage foam cells (Figure 3, C and D). Similarly, loss of CCN3 did not alter the expression of ACAT-1, an enzyme responsible for cholesterol esterification (Figure 3, C and D). These results suggest that CCN3 deficiency does not influence the cholesterol esterification or the cholesterol efflux machinery. Next, we determined the expression of two key scavenger receptors involved in lipid uptake, CD36 and SRA1. CCN3 deficiency caused significant up-regulation of CD36 and SRA1 expression in oxLDL-treated macrophages (Figure 3, C and D), findings recapitulated in aortic tissues from HFD-fed mice (Figure 4). These findings point to the potential importance of scavenger receptors CD36 and SRA1 in mediating the enhanced lipid uptake in CCN3-deficient macrophages.
Figure 4.
Increase of CD36 and SRA1 expression in Apoe−/−; Ccn3−/− aortas. A: Western blot of ABCA1, CD36, and SRA1 protein in mouse aortic tissue from Apoe−/−and Apoe−/−; Ccn3−/− mice after 15 weeks on a high-fat diet. B–D: Quantitation of ABCA1 (B), CD36 (C), and SRA1 (D). n = 4 to 5 aortic samples per group (B–D). ∗P < 0.05 versus Apoe−/−.
Discussion
Atherosclerosis progression is a complicated process that results in a buildup of fats, cholesterol, immunocytes, and other substances within arteries, leading to plaque formation, blood flow restriction, and potential clot formation. The key findings of this study show that atherosclerosis progression is exacerbated when CCN3 expression is lost. Increased lesion incidence and severity was observed when CCN3 was removed from mice on both the WT and the proatherogenic, Apoe−/− background, and that CCN3 expression in bone marrow–derived cells, and not resident cells within the vasculature, drives its atheroprotective role. Interestingly, we observed a significant increase in CCN3 expression in Apoe−/− mice under both normal chow and HFD conditions. This up-regulation supports CCN3 as atheroprotective, in agreement with overexpression studies conducted by Liu et al.11 In vitro studies using peritoneal macrophages indicate that CCN3 deficiency leads to a marked enhancement of foam cell formation, a crucial event required for atherosclerosis development. At the molecular level, our mechanistic studies suggest increased scavenger receptor CD36 and SRA1 expression in CCN3-deficient macrophages may account for the enhanced uptake of modified lipoproteins and subsequent foam cell formation.
Results of the current study unequivocally support the hypothesis that CCN3 expression in bone marrow–derived cells plays a dominant role against atherosclerosis formation. At the cellular level, we have initially focused our studies on macrophages because of their central importance in regulation of foam cell formation, a crucial event in the pathogenesis of atheroscerlosis. Loss of CCN3 resulted in significant increases in lipid uptake and foam cell formation within macrophages when compared to control. We cannot rule out, however, the contribution of other cell types located in or around the vessel wall. Atheroprotective CCN3, as well as the proatherosclerotic family members, CCN1 and CCN2, are differentially and temporally expressed within the vasculature. To further pinpoint the importance of macrophage-, endothelial cell–, and vascular smooth muscle cell–expressing CCN3 in atherogenesis, studies using cell type–specific CCN3 knockout mice are required. Given the known contributions of other immune cell types in the hematopoietic compartment (ie, neutrophils and T cells) to atherosclerosis formation and progression,12 future studies are also warranted to determine the roles of other bone marrow–derived cell types in the formation of the severe plaque buildup observed in Apoe−/−; Ccn3−/− aorta.
The inhibitory effects of CCN3 have also been shown in inhibition of vascular smooth muscle cell migration and proliferation after photochemically induced thrombosis,10 and suppression of abdominal aortic aneurysm formation.5 This suggests that CCN3 plays a prominent role in both acute (injury) and chronic (atherosclerosis, aneurysm formation) vascular pathologies. A recent report that supports our observations suggests an inhibitory role of CCN3 overexpression within the perivascular carotid artery collar–induced atherosclerosis model.11 However, this study differs with ours in regard to the lipid profile results. We observed no significant alterations in lipid profiles between control Apoe−/− and Apoe−/−; Ccn3−/− mice after high-fat diet feeding, whereas previous observations showed an inhibition of total cholesterol and triglycerides by CCN3 overexpression.11 Differential effects observed because of protein overexpression versus protein ablation are not unusual. As seen with intercellular adhesion molecule-1, overexpression of CCN3 in human umbilical vein endothelial cells decreased intercellular adhesion molecule-1 expression, whereas CCN3 knockdown in these same cells had no effect on intercellular adhesion molecule-1 protein levels.9 In vivo, transgenic expression in mice of uncoupling protein 2 within the liver affected metabolic processing of a safflower oil HFD differently than the uncoupling protein 2 systemic knockout animal.13 It is possible that exogenous overexpression of CCN3 alters certain functions within other somatic tissues, such as the liver, which plays an important role in cholesterol metabolism. Although the underlying mechanisms appear somewhat divergent, these two studies reached similar conclusions on the atheroprotective role of CCN3, with our study showing that CCN3 deficiency plays a causal role in atherogenesis.
The role of scavenger receptors CD36 and SRA1 in oxLDL uptake has been well established,14, 15 yet their contributions to cardiovascular disease are less clear. CD36 is generally thought to be proatherogenic but not essential for atherosclerotic plaque development,16 with the absence of CD36 atheroprotective,17, 18, 19 whereas SRA1 has been shown to be either proatherogenic or antiatherogenic, depending on the experimental system studied.18, 20, 21 In our studies, both CD36 and SRA1 protein levels were significantly increased in Apoe−/−; Ccn3−/− mice under proatherosclerotic conditions when compared to levels in Apoe−/− mice, concomitant with increased macrophage infiltration within the lesional area. This resulted in increased lipid uptake and enhanced foam cell formation, ultimately exacerbating lesion development. It is possible that the absence of CCN3 enhances the proatherogenic functions of these scavenger receptors, through either direct interaction or regulation of protein expression. Another possibility is that CCN3 deficiency within macrophages increases intrinsic endoplasmic reticulum stress, perhaps because of increased CCN1 protein production.22 CCN1 has been detected in atherosclerotic plaque,23, 24, 25 and CCN3 can regulate CCN1 expression.11 Increased CCN1 within the vessel could increase oxLDL uptake as well as stress-related cell signaling, leading to increased CD36 and SRA1 protein expression.26, 27 Therefore, enhanced oxLDL uptake in Apoe−/−; Ccn3−/− may be because of enhanced endoplasmic reticulum stress signals, facilitating increased M1-to-M2 macrophage transitions and increased scavenger receptor expression. More work is needed to determine the exact role of CCN3 in these processes.
In summary, these loss-of-function studies provide the most cogent evidence in support of the atheroprotective role of endogenous CCN3. These findings, coupled with the inhibitory effects of CCN3 on abdominal aortic aneurysm, strongly speak to the importance of CCN3 in maintaining vascular homeostasis. Thus, CCN3 may be an attractive therapeutic target for vascular disease.
Footnotes
Supported by NIH grants HL087595, HL117759, and AA021390 (all to Z.L.), the National Natural Science Foundation of China grant 81600345 (C.Z.), and the National Key Research and Development Program of China Stem Cell and Translational Research grant 2016YFA0101100 (N.D.).
H.S. and C.Z. contributed equally to this work.
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2017.01.020.
Contributor Information
Nianguo Dong, Email: dongnianguo@hotmail.com.
Zhiyong Lin, Email: zhiyong.lin@case.edu.
Supplemental Data
Loss of CCN3 increases aortic lesion formation in Apoe−/− mice on normal chow. A:Left panel: Representative image of Sudan IV staining of whole aortas from Apoe−/− and Apoe−/−; Ccn3−/− mice fed a normal chow diet. Right panel: Sudan IV quantitation of lesion area. B: Triglycerides profile of Apoe−/− and Apoe−/−; Ccn3−/− mice fed a normal chow diet. C: Total cholesterol profile of Apoe−/− and Apoe−/−; Ccn3−/− mice fed a normal chow diet. Very low density lipoprotein is located in fractions 12 to 24, low-density lipoprotein in fractions 25 to 40, and high-density lipoprotein in fractions 44 to 53. n = 4 per group (A). ∗P < 0.05 versus Apoe−/−.
CCN3 expression increases in Apoe−/− mice. Quantitation of CCN3 mRNA expression levels from tissue isolated from wild-type (WT) [normal chow (NC)], Apoe−/− (NC), and Apoe−/− [high-fat diet (HFD)] mice. n = 4. ∗P < 0.05 when compared to Apoe−/− (NC); ∗∗∗P < 0.001 versus WT.
Lipoprotein profiles from Apoe−/− and Apoe−/−; Ccn3−/− mice after 15 weeks on a high-fat diet. A: Triglyceride. B: Cholesterol. Equivalent to that seen in Supplemental Figure S1, very low density lipoprotein is located in fractions 12 to 24, low-density lipoprotein in fractions 25 to 40, and high-density lipoprotein in fractions 44 to 53.
Deficiency of CCN3 augments the inflammatory response in the aorta. Representative immunohistochemical staining for macrophages (MAC3; A), monocyte chemoattractant protein-1 (B), and vascular cell adhesion molecule 1 (VCAM-1; C) staining in wild-type versus CCN3-deficient aortae after 15 weeks of high-fat diet feeding. n = 5 (A–C). Scale bars = 100 μm (A–C).
Loss of CCN3 increases macrophage foam cell formation in multiple backgrounds. A: Representative images of oil red O–stained peritoneal macrophages from Apoe−/− and Apoe−/−; Ccn3−/− mice. B: Quantitation of foam cell formation. Arrows indicate representative foam cells. C: Quantitation of foam cells after oxLDL treatment of peritoneal macrophages from wild-type (WT), Ccn3+/−, and Ccn3−/− mice. n = 3 for all groups. ∗P < 0.05, ∗∗P < 0.005, and ∗∗∗P < 0.001 versus WT. Scale bar = 100 μm.
References
- 1.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore K.J., Sheedy F.J., Fisher E.A. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kubota S., Takigawa M. CCN family proteins and angiogenesis: from embryo to adulthood. Angiogenesis. 2007;10:1–11. doi: 10.1007/s10456-006-9058-5. [DOI] [PubMed] [Google Scholar]
- 4.Jun J.I., Lau L.F. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C., van der Voort D., Shi H., Zhang R., Qing Y., Hiraoka S., Takemoto M., Yokote K., Moxon J.V., Norman P., Rittie L., Kuivaniemi H., Atkins G.B., Gerson S.L., Shi G.P., Golledge J., Dong N., Perbal B., Prosdocimo D.A., Lin Z. Matricellular protein CCN3 mitigates abdominal aortic aneurysm. J Clin Invest. 2016;126:1282–1299. doi: 10.1172/JCI82337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai T., Chen C.C., Lau L.F. Matricellular protein CCN1 activates a proinflammatory genetic program in murine macrophages. J Immunol. 2010;184:3223–3232. doi: 10.4049/jimmunol.0902792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jun J.I., Kim K.H., Lau L.F. The matricellular protein CCN1 mediates neutrophil efferocytosis in cutaneous wound healing. Nat Commun. 2015;6:7386. doi: 10.1038/ncomms8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joliot V., Martinerie C., Dambrine G., Plassiart G., Brisac M., Crochet J., Perbal B. Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1-induced nephroblastomas. Mol Cell Biol. 1992;12:10–21. doi: 10.1128/mcb.12.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Z., Natesan V., Shi H., Hamik A., Kawanami D., Hao C., Mahabaleshwar G.H., Wang W., Jin Z.G., Atkins G.B., Firth S.M., Rittie L., Perbal B., Jain M.K. A novel role of CCN3 in regulating endothelial inflammation. J Cell Commun Signal. 2010;4:141–153. doi: 10.1007/s12079-010-0095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimoyama T., Hiraoka S., Takemoto M., Koshizaka M., Tokuyama H., Tokuyama T., Watanabe A., Fujimoto M., Kawamura H., Sato S., Tsurutani Y., Saito Y., Perbal B., Koseki H., Yokote K. CCN3 inhibits neointimal hyperplasia through modulation of smooth muscle cell growth and migration. Arterioscler Thromb Vasc Biol. 2010;30:675–682. doi: 10.1161/ATVBAHA.110.203356. [DOI] [PubMed] [Google Scholar]
- 11.Liu J., Ren Y., Kang L., Zhang L. Overexpression of CCN3 inhibits inflammation and progression of atherosclerosis in apolipoprotein E-deficient mice. PLoS One. 2014;9:e94912. doi: 10.1371/journal.pone.0094912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libby P., Lichtman A.H., Hansson G.K. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuboyama-Kasaoka N., Sano K., Shozawa C., Osaka T., Ezaki O. Studies of UCP2 transgenic and knockout mice reveal that liver UCP2 is not essential for the antiobesity effects of fish oil. Am J Physiol Endocrinol Metab. 2008;294:E600–E606. doi: 10.1152/ajpendo.00551.2007. [DOI] [PubMed] [Google Scholar]
- 14.Silverstein R.L. Inflammation, atherosclerosis, and arterial thrombosis: role of the scavenger receptor CD36. Cleve Clin J Med. 2009;76 Suppl 2:S27–S30. doi: 10.3949/ccjm.76.s2.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben J., Zhu X., Zhang H., Chen Q. Class A1 scavenger receptors in cardiovascular diseases. Br J Pharmacol. 2015;172:5523–5530. doi: 10.1111/bph.13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manning-Tobin J.J., Moore K.J., Seimon T.A., Bell S.A., Sharuk M., Alvarez-Leite J.I., de Winther M.P., Tabas I., Freeman M.W. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore K.J., Kunjathoor V.V., Koehn S.L., Manning J.J., Tseng A.A., Silver J.M., McKee M., Freeman M.W. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuchibhotla S., Vanegas D., Kennedy D.J., Guy E., Nimako G., Morton R.E., Febbraio M. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc Res. 2008;78:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Febbraio M., Podrez E.A., Smith J.D., Hajjar D.P., Hazen S.L., Hoff H.F., Sharma K., Silverstein R.L. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babaev V.R., Gleaves L.A., Carter K.J., Suzuki H., Kodama T., Fazio S., Linton M.F. Reduced atherosclerotic lesions in mice deficient for total or macrophage-specific expression of scavenger receptor-A. Arterioscler Thromb Vasc Biol. 2000;20:2593–2599. doi: 10.1161/01.atv.20.12.2593. [DOI] [PubMed] [Google Scholar]
- 21.de Winther M.P., Gijbels M.J., van Dijk K.W., van Gorp P.J., Suzuki H., Kodama T., Frants R.R., Havekes L.M., Hofker M.H. Scavenger receptor deficiency leads to more complex atherosclerotic lesions in APOE3Leiden transgenic mice. Atherosclerosis. 1999;144:315–321. doi: 10.1016/s0021-9150(98)00332-3. [DOI] [PubMed] [Google Scholar]
- 22.Borkham-Kamphorst E., Steffen B.T., Van de Leur E., Haas U., Tihaa L., Friedman S.L., Weiskirchen R. CCN1/CYR61 overexpression in hepatic stellate cells induces ER stress-related apoptosis. Cell Signal. 2016;28:34–42. doi: 10.1016/j.cellsig.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Hilfiker A., Hilfiker-Kleiner D., Fuchs M., Kaminski K., Lichtenberg A., Rothkotter H.J., Schieffer B., Drexler H. Expression of CYR61, an angiogenic immediate early gene, in arteriosclerosis and its regulation by angiotensin II. Circulation. 2002;106:254–260. doi: 10.1161/01.cir.0000021426.87274.62. [DOI] [PubMed] [Google Scholar]
- 24.Schober J.M., Chen N., Grzeszkiewicz T.M., Jovanovic I., Emeson E.E., Ugarova T.P., Ye R.D., Lau L.F., Lam S.C. Identification of integrin alpha(M)beta(2) as an adhesion receptor on peripheral blood monocytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2): immediate-early gene products expressed in atherosclerotic lesions. Blood. 2002;99:4457–4465. doi: 10.1182/blood.v99.12.4457. [DOI] [PubMed] [Google Scholar]
- 25.Sigala F., Georgopoulos S., Papalambros E., Chasiotis D., Vourliotakis G., Niforou A., Kotsinas A., Kavantzas N., Patsouris E., Gorgoulis V.G., Bastounis E. Heregulin, cysteine rich-61 and matrix metalloproteinase 9 expression in human carotid atherosclerotic plaques: relationship with clinical data. Eur J Vasc Endovasc Surg. 2006;32:238–245. doi: 10.1016/j.ejvs.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Oh J., Weng S., Felton S.K., Bhandare S., Riek A., Butler B., Proctor B.M., Petty M., Chen Z., Schechtman K.B., Bernal-Mizrachi L., Bernal-Mizrachi C. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–698. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh J., Riek A.E., Weng S., Petty M., Kim D., Colonna M., Cella M., Bernal-Mizrachi C. Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation. J Biol Chem. 2012;287:11629–11641. doi: 10.1074/jbc.M111.338673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Loss of CCN3 increases aortic lesion formation in Apoe−/− mice on normal chow. A:Left panel: Representative image of Sudan IV staining of whole aortas from Apoe−/− and Apoe−/−; Ccn3−/− mice fed a normal chow diet. Right panel: Sudan IV quantitation of lesion area. B: Triglycerides profile of Apoe−/− and Apoe−/−; Ccn3−/− mice fed a normal chow diet. C: Total cholesterol profile of Apoe−/− and Apoe−/−; Ccn3−/− mice fed a normal chow diet. Very low density lipoprotein is located in fractions 12 to 24, low-density lipoprotein in fractions 25 to 40, and high-density lipoprotein in fractions 44 to 53. n = 4 per group (A). ∗P < 0.05 versus Apoe−/−.
CCN3 expression increases in Apoe−/− mice. Quantitation of CCN3 mRNA expression levels from tissue isolated from wild-type (WT) [normal chow (NC)], Apoe−/− (NC), and Apoe−/− [high-fat diet (HFD)] mice. n = 4. ∗P < 0.05 when compared to Apoe−/− (NC); ∗∗∗P < 0.001 versus WT.
Lipoprotein profiles from Apoe−/− and Apoe−/−; Ccn3−/− mice after 15 weeks on a high-fat diet. A: Triglyceride. B: Cholesterol. Equivalent to that seen in Supplemental Figure S1, very low density lipoprotein is located in fractions 12 to 24, low-density lipoprotein in fractions 25 to 40, and high-density lipoprotein in fractions 44 to 53.
Deficiency of CCN3 augments the inflammatory response in the aorta. Representative immunohistochemical staining for macrophages (MAC3; A), monocyte chemoattractant protein-1 (B), and vascular cell adhesion molecule 1 (VCAM-1; C) staining in wild-type versus CCN3-deficient aortae after 15 weeks of high-fat diet feeding. n = 5 (A–C). Scale bars = 100 μm (A–C).
Loss of CCN3 increases macrophage foam cell formation in multiple backgrounds. A: Representative images of oil red O–stained peritoneal macrophages from Apoe−/− and Apoe−/−; Ccn3−/− mice. B: Quantitation of foam cell formation. Arrows indicate representative foam cells. C: Quantitation of foam cells after oxLDL treatment of peritoneal macrophages from wild-type (WT), Ccn3+/−, and Ccn3−/− mice. n = 3 for all groups. ∗P < 0.05, ∗∗P < 0.005, and ∗∗∗P < 0.001 versus WT. Scale bar = 100 μm.