Abstract
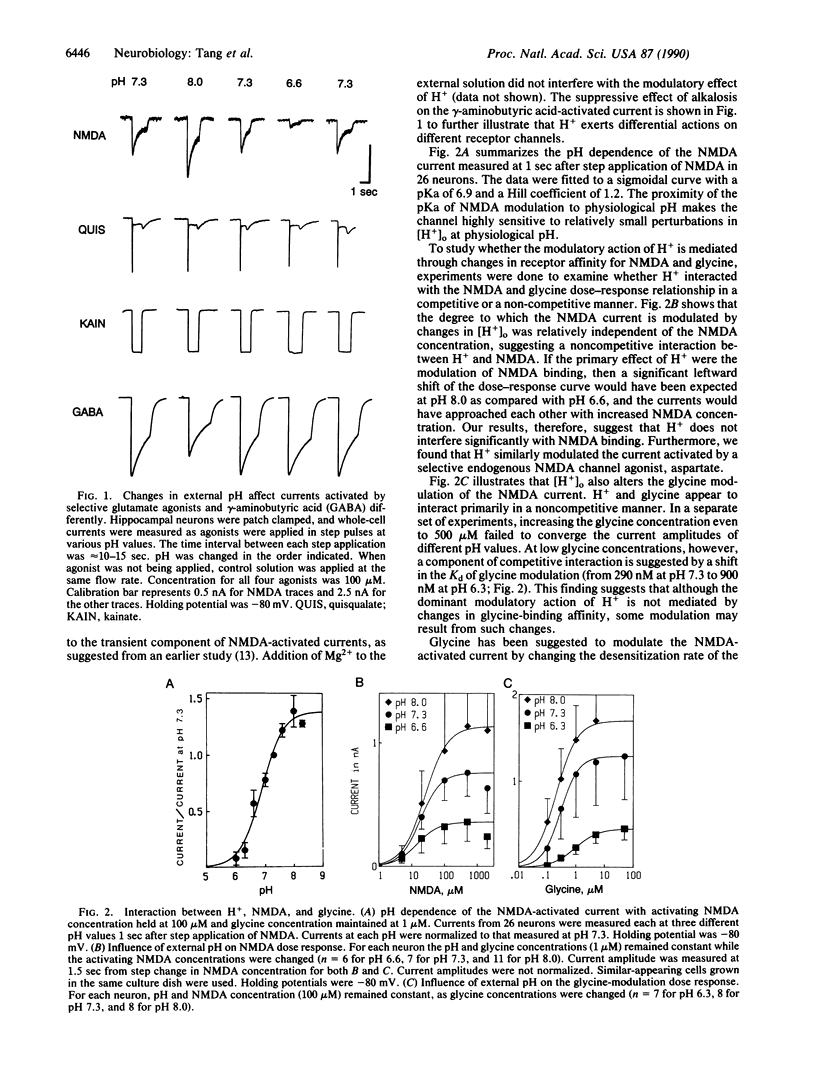
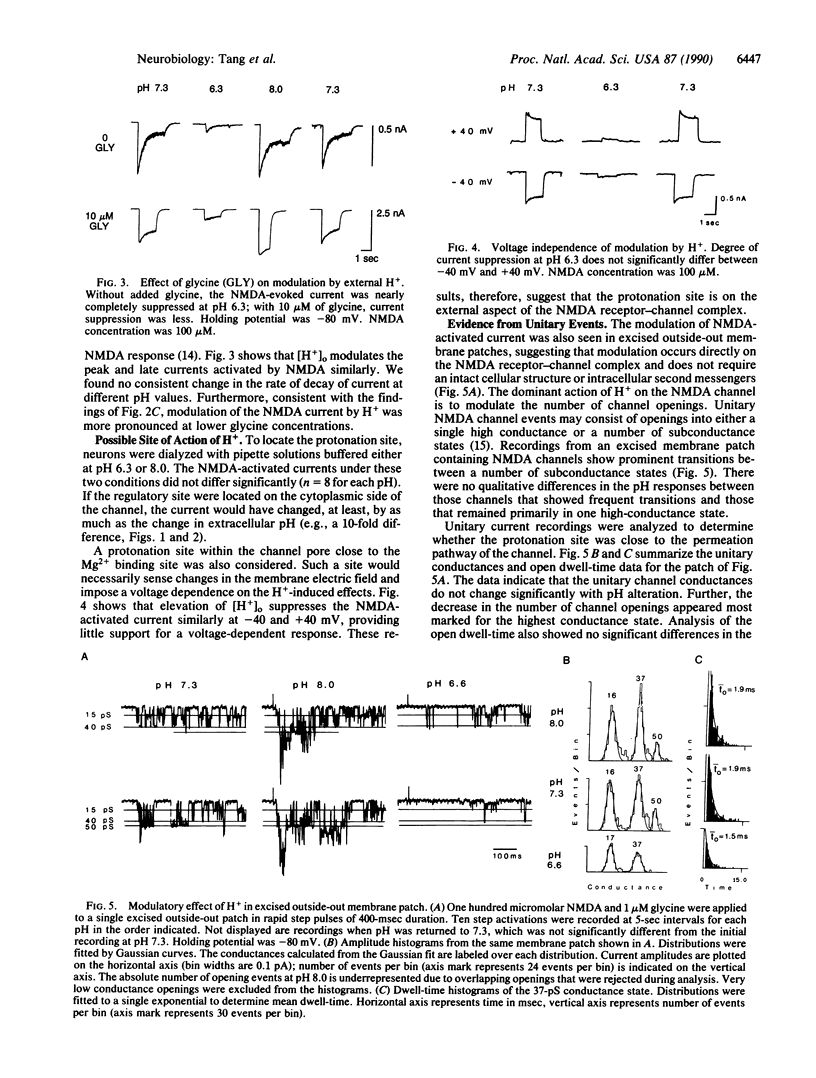
The influence of external [H+] on whole-cell and single-channel currents activated by glutamate agonists was studied in rat hippocampal neurons. In the pH range between 6.6 and 8.0, changes in external [H+] had negligible influence on the amplitude and kinetics of the monovalent ion-carrying currents activated by the agonists quisqualate and kainate. The divalent ion-carrying N-methyl-D-aspartate (NMDA)-activated current, on the other hand, was strongly modulated by extracellular [H+]. Increased external [H+] suppressed, whereas decreased external [H+] enhanced, the NMDA-activated current. Changes in internal [H+] had little or no effect on the NMDA-activated current. Modulation of the NMDA-activated current resulted primarily from changes in the number of channel openings. Neither the unitary conductance nor the individual open dwell-times were significantly affected. These results suggest that the protonation site is on the external aspect of the channel and is far removed from the channel permeation pathway. Because interactions between H+, NMDA, and glycine in activating the current were predominantly noncompetitive, our results suggest that the modulatory effect of H+ was not associated with changes in receptor-agonist affinities. These results suggest that modulation of the NMDA-receptor channel by [H+] may be an intrinsic protective mechanism by which calcium influx into neurons is regulated, particularly in hypoxic/ischemic conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers G. W., Goldberg M. P., Choi D. W. N-methyl-D-aspartate antagonists: ready for clinical trial in brain ischemia? Ann Neurol. 1989 Apr;25(4):398–403. doi: 10.1002/ana.410250412. [DOI] [PubMed] [Google Scholar]
- Aram J. A., Lodge D. Epileptiform activity induced by alkalosis in rat neocortical slices: block by antagonists of N-methyl-D-aspartate. Neurosci Lett. 1987 Dec 29;83(3):345–350. doi: 10.1016/0304-3940(87)90112-1. [DOI] [PubMed] [Google Scholar]
- Balestrino M., Somjen G. G. Concentration of carbon dioxide, interstitial pH and synaptic transmission in hippocampal formation of the rat. J Physiol. 1988 Feb;396:247–266. doi: 10.1113/jphysiol.1988.sp016961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Moody W. J. Membrane currents of internally perfused neurones of the snail, Lymnaea stagnalis, at low intracellular pH. J Physiol. 1986 Jul;376:477–491. doi: 10.1113/jphysiol.1986.sp016165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M. The regulation and modulation of pH in the nervous system. Prog Neurobiol. 1990;34(5):401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- Cheung J. Y., Bonventre J. V., Malis C. D., Leaf A. Calcium and ischemic injury. N Engl J Med. 1986 Jun 26;314(26):1670–1676. doi: 10.1056/NEJM198606263142604. [DOI] [PubMed] [Google Scholar]
- Christine C. W., Choi D. W. Effect of zinc on NMDA receptor-mediated channel currents in cortical neurons. J Neurosci. 1990 Jan;10(1):108–116. doi: 10.1523/JNEUROSCI.10-01-00108.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantyn R., Lux H. D. Similarity and mutual exclusion of NMDA- and proton-activated transient Na+-currents in rat tectal neurons. Neurosci Lett. 1988 Jun 29;89(2):198–203. doi: 10.1016/0304-3940(88)90381-3. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989 Mar 30;338(6214):425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- McDonald J. W., Penney J. B., Johnston M. V., Young A. B. Characterization and regional distribution of strychnine-insensitive [3H]glycine binding sites in rat brain by quantitative receptor autoradiography. Neuroscience. 1990;35(3):653–668. doi: 10.1016/0306-4522(90)90336-3. [DOI] [PubMed] [Google Scholar]
- Meldrum B. S., Croucher M. J., Badman G., Collins J. F. Antiepileptic action of excitatory amino acid antagonists in the photosensitive baboon, Papio papio. Neurosci Lett. 1983 Aug 19;39(1):101–104. doi: 10.1016/0304-3940(83)90172-6. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Prod'hom B., Pietrobon D., Hess P. Direct measurement of proton transfer rates to a group controlling the dihydropyridine-sensitive Ca2+ channel. Nature. 1987 Sep 17;329(6136):243–246. doi: 10.1038/329243a0. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K., von Hanwehr R., Nergelius G., Nevander G., Ingvar M. Extra- and intracellular pH in the brain during seizures and in the recovery period following the arrest of seizure activity. J Cereb Blood Flow Metab. 1985 Mar;5(1):47–57. doi: 10.1038/jcbfm.1985.7. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981 Feb 13;211(4483):712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]



