Abstract
The role of endothelium-derived nitric oxide (NO) in acute inflammation is not known. Here, we examine acute inflammation in congenic endothelial NO synthase-deficient (eNOS-/-) mice. Intraplantar injection of carrageenan induces a biphasic inflammatory response. The early phase (0-6 h) is largely eliminated, and the secondary phase (24-96 h) is markedly reduced in eNOS-/- but not WT mice. Inhibition of phosphatidylinositol 3-kinase or hsp90, pathways upstream of eNOS activation, also reduces carrageenan-stimulated edema formation. To separate the ability of eNOS to regulate leukocyte trafficking vs. vascular permeability, zymosan-stimulated leukocyte infiltration and protein extravasation were assessed in WT and eNOS-/- mice. Zymosan increases inflammatory cell extravasation to the same extent in WT and eNOS-/- mice, whereas the extravasation of plasma protein is lower in eNOS-/- mice. Inhibition of phosphatidylinositol 3-kinase and hsp90 also blocks protein leakage, but not leukocyte influx. These data collectively support the critical role for eNOS in regulating the magnitude of the acute inflammatory response and show that eNOS is critical for regulating microcirculatory endothelial barrier function in vivo.
Acute inflammation is a process typical of vascularized tissues whereby interstitial fluid and white blood cells accumulate at the site of injury. Upon injury or damage, an increase in microvascular permeability is an early event that leads to edema formation during inflammation. After this change, many other mechanisms are activated, contributing to the amplification of the inflammatory response and tissue damage.
In recent years much attention has focused on the role of nitric oxide (NO) in blood vessel function as well as in the inflammatory process (1). NO is produced by the nitric oxide synthase (NOS) family of enzymes. Three forms of NOS exist: neuronal (nNOS, NOS 1), inducible (iNOS, NOS 2), and endothelial (eNOS, NOS 3). The inducible form is classically controlled by inflammatory mediators and cytokines and produces large, unregulated quantities of NO whereas nNOS and eNOS produce low levels of NO (2). The majority of studies examining the roles of NO in inflammation have examined the contribution of iNOS to acute and chronic inflammatory responses. However, considering the recent studies documenting that eNOS regulates growth factor and tumor microvascular permeability, it is intuitive that eNOS may play a role in acute inflammation (3-7).
eNOS is regulated by dynamic subcellular targeting, phosphorylation, and protein-protein interactions. In general, in the basal state, eNOS is negatively regulated by several proteins thus producing low level NO (8). When a stimulus is present, such as increased shear stress or local autacoids generated by tissue injury, eNOS activity is enhanced by positive regulators of the enzyme, thus rapidly increasing local NO levels. Among the proteins that regulate the activation state of eNOS, heat shock protein 90 (hsp90) can directly bind to eNOS and influence its calcium sensitivity and phosphorylation state (9-12). One approach to document the role of hsp90 in eNOS regulation is by the usage of the antibiotic geldanamycin (GA) (13). GA specifically binds to the ATP pocket of hsp90, inhibits ATP hydrolysis, and reduces NO release from cells, endothelium-dependent relaxations of blood vessels (9), and an edema formation in vivo (14). Another posttranslational modification that regulates eNOS activity is protein phosphorylation (15-17). eNOS is phosphorylated primarily on serine residues and to a lesser extent on tyrosine and threonine residues. eNOS is phosphorylated on Ser-1179 by many kinases, including the protein kinase Akt, and phosphorylation and NO production can be reduced by inhibitors of phosphatidylinositol 3-kinase (PI3-K) and dominant negative Akt (18, 19). However, considering the importance of eNOS-derived NO in reducing leukocyte and platelet adhesion to the endothelium on the one hand and the role of eNOS in influencing vascular leakage on the other hand, the net contribution of eNOS in the acute inflammatory response has not been unequivocally shown. Thus, we sought to investigate the role of eNOS in acute inflammation by using eNOS-deficient mice.
Materials and Methods
Animals. C57BL/6J mice were purchased from Charles River Breeding Laboratories and congenic eNOS-/- mice (20, 21) backbred at least 10 generations to C57BL/6J mice were obtained from The Jackson Laboratory.
Induction of Edema in the Mouse Paw. Male C57BL/6J and eNOS-/- mice weighing 28-30 g were separated in groups (n = 6) and lightly anesthetized with enflurane. Each group of animals received subplantar injection of 50 μl of carrageenan 1% wt/vol into the left footpad (14). Paw volume was measured by using a hydroplethismometer specially modified for small volumes (Ugo Basile, Milan, Italy) immediately before the subplantar injection and 2, 4, and 6 h thereafter. The increase in paw volume was evaluated as the difference between the paw volume at each time point and the basal paw volume. In another set of experiments, C57BL/6J were subjected to a previous subplantar injection of 20 μl of LY294002 (2-20 μg per paw), GA (2-20 μg per paw), or vehicle, 30 min before carrageenan injection. In some experiments, paws were fixed in paraformaldehyde, and frozen sections (10 μm) were stained with hematoxylin and eosin to examine paw cellularity.
Cyclooxygenase 2 (COX-2), COX-1, iNOS, and eNOS Expression in Mouse Paw of C57BL/6J Mice Treated with Carrageenan. Carrageenan-injected and noninjected paws from C57BL/6J mice killed at 2, 4, 6, and 24 h were homogenized in a 10 mM Hepes (pH 7.4) buffer containing saccharose (0.32 M), EDTA (100 μM), DTT (1 mM), phenylmethylsulfonyl fluoride (1 mg/ml), and leupeptin (10 μg/ml) by using a Polytron homogenizer (three cycles of 10 sec at maximum speed) (22). After centrifugation at 3,000 rpm for 15 min, protein supernatant content was measured by Bradford reagent, and protein concentration was adjusted to 30 μg. Protein samples were loaded on 10% SDS/PAGE and transferred onto nitrocellulose membranes for 45 min at 250 mA. Membranes were blocked in PBS-Tween 20 (0.1%) containing 5% nonfat milk and 0.1% BSA for 30 min at 4°C. Membranes were washed with PBS-Tween 20 (0.1%) at 5-min intervals for 30 min and incubated with anti-COX-2 (Cayman Chemical, Ann Arbor, MI), anti-iNOS (Cayman Chemical), or anti-eNOS (Transduction Laboratories, Lexington, KY) polyclonal Ab (dilution 1:1,000) overnight at 4°C. For COX-2 and iNOS detection, blots were washed with PBS-Tween 20 (0.1%) at 5-min intervals for 30 min and incubated with horseradish peroxidase-anti-rabbit IgG (1:20,000) for 2 h at 4°C. For eNOS detection, blots were washed with PBS-Tween 20 (0.1%) at 5-min intervals for 30 min and incubated with horseradish peroxidase-anti-mouse IgG (1:20,000) for 2 h at 4°C. The immunoreactive bands were visualized by using an enhanced chemiluminescence system (ECL; Amersham Pharmacia).
Myeloperoxidae (MPO) Measurement in Injected Paws. Mice from different groups were killed with CO2 at 6 h after carrageenan injection. Injected paws were cut, weighed, and homogenized in 1 ml of hexadecyltrimethylammonium bromide buffer by using a Polytron homogenizer (two cycles of 10 sec at maximum speed). After centrifugation of homogenates at 10,000 rpm for 2 min, supernatant fractions were assayed for MPO activity by using the method described in ref. 23. In brief, samples were mixed with phosphate buffer containing 1 mM o-dianisidine dihydrochloride and 0.001% hydrogen peroxide in a microtiter plate reader. Absorbance was measured at 450 nm, taking three readings at 30-sec intervals. Units of MPO were calculated considering that 1 unit of MPO = 1 μmol of H2O2 hydrolyzed, and 1 μmol of H2O2 gives a change in absorbance of 1.13 × 10-2 per min.
Mouse Air Pouch Model. Male C57BL/6J and eNOS knockout mice weighing 28-30 g were separated in groups (n = 6). Air pouches were developed by s.c. injection of 10 ml of sterile air into the back of mice (28-30 g). Three days later, 5 ml of sterile air was re-injected in the same cavity (24). After another 3 days (6 days after the first air injection), 1 ml of zymosan 1% wt/vol or vehicle (saline) was injected into the air pouch. At 4 h after zymosan injection, mice were killed with CO2, and the exudates in the pouches were collected with 1 ml of saline and placed in graduated tubes and centrifuged at 125 × g for 10 min. The pellet was suspended in 500 μl of saline, and total leukocyte count was evaluated by optical microscopy in the cell suspension diluted with Turk's solution. In another set of experiments C57BL/6J mice were treated with either GA (20 μg) or LY294002 (20 μg) by administering the drug into the pouch 30 min before injecting zymosan.
MPO Measurement in Air Pouch Tissue. Mice injected with zymosan were killed 2 h after injection, and portions of s.c. tissue of the air pouch were cut, weighed, and homogenized in 1 ml of hexadecyltrimethylammonium bromide buffer as described previously. Homogenates were centrifuged at 10,000 rpm for 2 min. MPO activity was estimated in aliquots of supernatant by using the method as described above.
Protein Measurement in Air Pouch Exudate. Exudates were collected from air pouch with 1 ml of saline and were centrifuged at 125 × g for 10 min. An aliquot of the supernatant was used to determinate protein content by using the Bradford reagent.
Statistical Analysis. Data are expressed as mean ± SEM. The level of statistical significance was determined by ANOVA followed by Bonferroni's t test for multiple comparisons, using GraphPad (San Diego) prism software.
Results
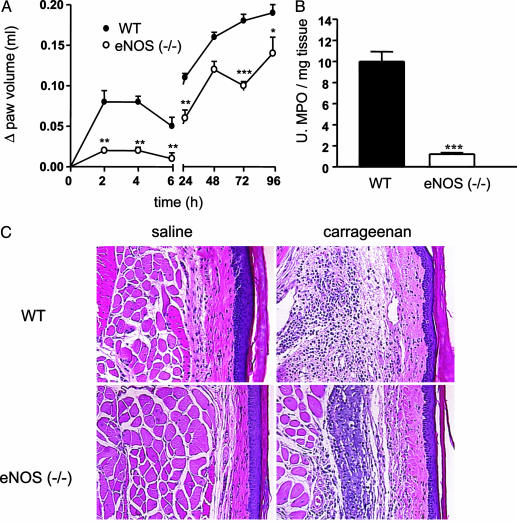
The Loss of eNOS Markedly Reduces Edema Formation. Subplantar injection of carrageenan in C57BL/6J mice caused biphasic edema formation. The first phase developed acutely within the first 6 h whereas the second phase developed after 24 h (Fig. 1A). Remarkably, edema formation was almost undetectable in eNOS-/- mice in the acute phase of edema (within the first 6 h; Fig. 1B). In addition, the infiltration of activated neutrophils and macrophage determined by MPO activity was significantly reduced at 2 h after carrageenan in eNOS-/- mice compared with WT mice (Fig. 1C). As seen in Fig. 2, there is a clear reduction in the histological appearance of interstitial edema (at 4 h) after injection of carrageenan into the paws of eNOS-/- mice.
Fig. 1.
Reduced paw edema in eNOS-/- mice. (A) eNOS-/- mice display a reduced edema. Both the first and second phases of the carrageenan-induced edema (n = 6 per group) are influenced. (B) eNOS-/- mice display a reduction in tissue MPO levels at the 2-h time point (n = 6 per group; C). Data are expressed as mean ± SEM. **, P < 0.01; ***, P < 0.001. (C) Hematoxylin and eosin staining of saline- (Left) and carrageenan-injected (Right) paws. Paws from eNOS-/- mice show a marked reduction in interstitial edema formation after carrageenan injection compared with paws from injected WT (C57BL/6J) mice.
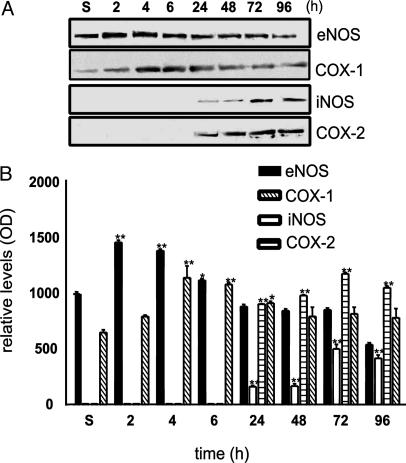
Fig. 2.
Carrageenan acutely increases eNOS and COX-1 protein levels and chronically induces both iNOS and COX-2. (A) After carrageenan injections into mouse paws, there is a significant increase in eNOS expression as compared with saline injection at 2 and 4 h. iNOS and COX-2 are not expressed until the 24-h time point (n = 8 per group). (B) Densitometric analysis of semiquantitative Western data from three separate experiments. Data are expressed as mean ± SEM. **, P < 0.01.
Carrageenan Rapidly Increases eNOS Levels Followed by iNOS and COX-2. Next, we examined the protein expression of “constitutively” expressed eNOS and COX-1 and inducibly expressed iNOS and COX-2 in the acute and chronic phases of edema. Both eNOS and COX-1 are present in saline injected paws (2 h). Injection of carrageenan increased eNOS levels at 2 and 4 h and COX-1 at 4 and 6 h. In saline-injected paws and at 2-6 h after carrageenan, the cytokine-inducible enzymes, COX-2 and iNOS, were not detectable. However, at 24 h both iNOS and COX-2 levels were increased. Thus, within the first 6 h of this response, based on the above data in eNOS-/- mice (Figs. 1 and 2) and the lack of iNOS protein, NO-dependent responses are clearly eNOS mediated.
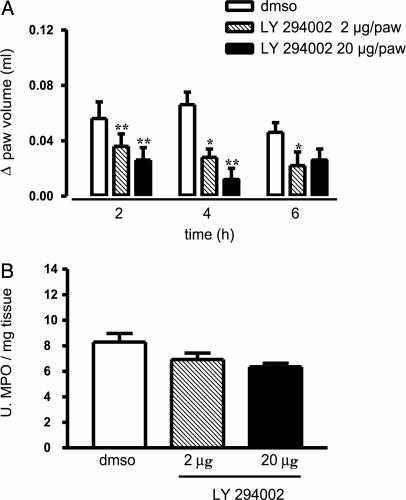
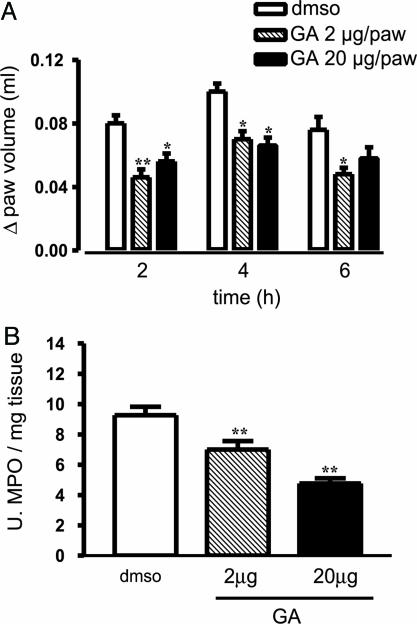
Inhibition of PI3-K and hsp90 Reduces Edema Formation. Because eNOS activity can be regulated by means of the PI3-K/Akt pathway and hsp90, we used pharmacological tools to modulate eNOS function in vivo. Subplantar injection of LY294002, an inhibitor of PI3-K, reduced carrageenan-induced paw edema in WT mice in dose-dependent fashion (Fig. 3A) without significantly affecting MPO levels (Fig. 3B). Conversely, GA given locally in the paw significantly reduced both edema formation (Fig. 4A) and MPO levels (Fig. 4B).
Fig. 3.
Inhibition of PI3-K with LY294002 reduces edema. Local intraplantar administration of LY294002 (2 and 20 μg per paw with 0.01% DMSO as a vehicle control) significantly inhibits paw edema (A) but not of tissue MPO levels (B). Data are expressed as mean ± SEM; n = 8 per group. *, P < 0.05; **, P < 0.001.
Fig. 4.
Inhibition of hsp90 function with GA reduces edema formation. Local intraplantar administration of GA (2 and 20 μg per paw with 0.01% DMSO as a vehicle control) significantly inhibits both edema (A) and MPO levels (B). Data are expressed as mean ± SEM; n = 8 per group; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
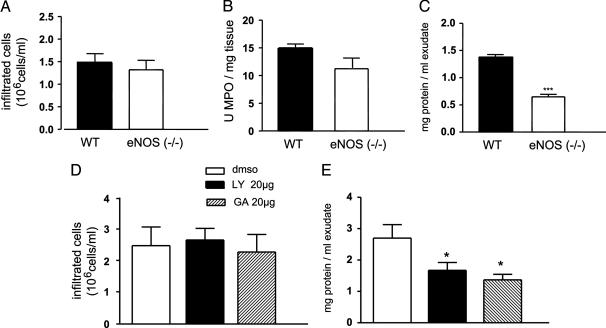
Dissecting the Role of eNOS in Regulating White Cell Infiltration vs. Microvascular Permeability. To better understand the role of the eNOS in the regulation of edema and cellular infiltration, we performed an air pouch study in WT and eNOS-/- mice, and zymosan was used as an inflammatory stimulus. As seen in Fig. 5, zymosan induced the infiltration of white cells (Fig. 5A) and associated MPO activity (Fig. 5B) in both WT and eNOS-/- mice. In contrast, the proteinaceous content of the exudates obtained from the air pouch was significantly reduced in eNOS-/- mice (Fig. 5C) consistent with the data that eNOS activation promotes microvascular leakage and is important for endothelial barrier function. Next we examined the effects of LY294002 and GA in the zymosan air-pouch model in WT mice. The local administration of LY294002 and GA did not modify the number of cells harvested from the air pouch (Fig. 5D, n = 8; not significant); however both drugs significantly reduced, to the same extent, the protein content of the exudates (Fig. 5E; n = 8; P < 0.01).
Fig. 5.
Zymosan-induced protein extravasation, but not cellular infiltrates, are reduced in eNOS-/- mice and inhibitors of eNOS activation. Zymosan treatment increased white cell infiltration (A) and MPO activity (B) to the same extent in WT and eNOS-/- mice; however, the proteinaceous content (C) of the exudates was markedly reduced in eNOS-/- mice. In WT mice, administration of LY294002 (20 μg per pouch) or GA (20 μg per pouch) did not modify zymosan-induced cellular infiltration (D) but inhibited protein extravasation (E). DMSO is used as a control as above. Data are expressed as mean ± SEM. ***, P < 0.001; n = 5 mice per group for A-C and n = 8 per group for D and E.
Discussion
Studies of NO and inflammation have focused mainly on the role of iNOS, whereas the role of eNOS has been underestimated and not well studied (1). eNOS activity can be markedly enhanced by stimuli such as increased shear stress or local autacoids generated by changes in blood flow or tissue injury (2). Here, using mice deficient in eNOS, we demonstrate that eNOS and eNOS-derived NO play key roles in regulating microvascular permeability during acute inflammation. Paw edema in the mouse is characterized by two phases: first an acute phase, rapid in onset that reaches its peak after 4 h, followed by a second late phase that takes place after 24 h. Our data show that in eNOS-/- mice the acute phase (first phase) is virtually absent. These data indicate a critical role for eNOS-derived NO in acute inflammation, in particular for the early changes in vascular permeability. The removal of eNOS-derived NO also causes a significant reduction in the second phase of the edema, suggesting that the early phase characterized by increases in blood flow and vascular permeability contributes to the development of the late phase. Our data are consistent with other studies showing that vascular endothelial growth factor-mediated changes in vascular permeability and tumor microvascular permeability are markedly reduced in eNOS-/- mice (5, 6). In addition, a cell-permeable peptide inhibitor of eNOS, derived from the negative regulator of eNOS function, caveolin-1, inhibits mouse paw edema formation as well as irritant-stimulated increases in vascular permeability in a mouse ear model (6, 7). More recently this peptide has been shown to inhibit microvascular hyperpermeability in tumors and to delay tumor progression in mice, strongly supporting the critical control of vascular permeability by eNOS (6).
There are different mechanisms involved in eNOS activation, including the phosphorylation of eNOS by the protein kinase Akt (also known as PKB) and the molecular chaperone hsp90 influencing the calcium sensitivity, phosphorylation, and coupling of eNOS, and these have been well studied (8). To gain further insights into the mechanism of action of these eNOS regulatory proteins in vivo, we have used two substances given by local administration into the paw, LY294002, an inhibitor of PI3-K, which reduces Akt-dependent eNOS phosphorylation, and GA, an inhibitor of the ATPase function of hsp90. We have shown previously that systemic administration of GA significantly reduces the second phase of mouse paw edema in a dose-dependent fashion (14). Here we demonstrate that local administration of GA reduces acute inflammation and that the inhibitory effect of LY294002 given locally is more marked than GA. These data suggest that in an acute inflammatory response, activation of the PI3-K/Akt pathway may contribute importantly to the response. On the other hand, whereas GA significantly and in a dose-dependent fashion inhibited MPO activity, an index of neutrophil infiltration and activation, LY294002 did not. These data taken together suggest that in addition to the antipermeability effects of GA and LY294002, GA may also influence neutrophil activation and emigration into the inflamed site.
To further understand the role of inflammatory cell migration in vivo, we used the mouse air pouch assay, which allows for the separate determination of cellular infiltration and protein exudation. There were no differences in the number of white cells harvested from eNOS-/- and WT mice treated with zymosan, indicating that partial or total removal of the eNOS does not inhibit or promote cell migration. This finding is further supported by the fact that MPO activity as a measure of neutrophil infiltration is not modified in eNOS-/- mice. Conversely, the protein content of the exudates was significantly reduced in eNOS-/- mice. Also, local administration of both GA and LY294002 into the air pouch reduced protein content, but similarly to eNOS-/- mice, did not modify the infiltration of inflammatory cells into the tissue. Collectively our data are consistent with the idea that the loss of eNOS blunts vascular leakage in inflamed tissue. However, in these models, the contribution of eNOS to blood flow into the inflamed site cannot be excluded. For example, histamine-mediated vasodilation in arterioles of the cremaster muscle is markedly reduced in eNOS-/- mice (25). However, there is evidence that shear stress or acetylcholine-induced changes in microvascular blood flow control are eNOS independent and likely mediated by prostanoids or endothelium-derived hyperpolarizing factor (26-29). The lack of NO contribution to microvascular blood flow control supports the concept that eNOS is critical for vascular leakage in models of acute inflammation used in this study.
In conclusion, these data demonstrate that eNOS removal abolishes the acute phase of inflammation, indicating that eNOS-derived NO plays a major role in increasing local vascular permeability. In addition, experiments in both the paw and air pouch models suggest that eNOS mainly drives changes in vascular permeability rather than white cell margination or emigration into inflamed sites. Thus, blockade of eNOS-dependent vascular permeability may be a novel strategy to reduce acute inflammation.
Author contributions: W.C.S., L.J.I., and G.C. designed research; M.B., F.R., I.P., and J.Y. performed research; F.R., L.P., W.C.S., and G.C. contributed new reagents/analytic tools; M.B., F.R., L.P., W.C.S., and L.J.I. analyzed data; and W.C.S. and G.C. wrote the paper.
Abbreviations: eNOS, endothelial nitric oxide synthase; iNOS, inducible NOS; PI3-K, phosphatidylinositol 3-kinase; GA, geldanamycin; COX, cyclooxygenase; MPO, myeloperoxidase.
References
- 1.Cirino, G., Fiorucci, S. & Sessa, W. C. (2003) Trends Pharmacol. Sci. 24, 91-95. [DOI] [PubMed] [Google Scholar]
- 2.Sessa, W. C. (1994) J. Vasc. Res. 31, 131-143. [DOI] [PubMed] [Google Scholar]
- 3.He, P., Liu, B. & Curry, F. E. (1997) Am. J. Physiol. 272, H176-H185. [DOI] [PubMed] [Google Scholar]
- 4.Lal, B. K., Varma, S., Pappas, P. J., Hobson II, R. W. & Duran, W. N. (2001) Microvasc. Res. 62, 252-262. [DOI] [PubMed] [Google Scholar]
- 5.Fukumura, D., Gohongi, T., Kadambi, A., Izumi, Y., Ang, J., Yun, C. O., Buerk, D. G., Huang, P. L. & Jain, R. K. (2001) Proc. Natl. Acad. Sci. USA 98, 2604-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gratton, J. P., Lin, M. I., Yu, J., Weiss, E. D., Jiang, Z. L., Fairchild, T. A., Iwakiri, Y., Groszmann, R., Claffey, K. P., Cheng, Y. C., et al. (2003) Cancer Cell 4 31-39. [DOI] [PubMed] [Google Scholar]
- 7.Bucci, M., Gratton, J. P., Rudic, R. D., Acevedo, L., Roviezzo, F., Cirino, G. & Sessa, W. C. (2000) Nat. Med. 6, 1362-1367. [DOI] [PubMed] [Google Scholar]
- 8.Fulton, D., Gratton, J. P. & Sessa, W. C. (2001) J. Pharmacol. Exp. Ther. 299, 818-824. [PubMed] [Google Scholar]
- 9.Garcia-Cardena, G., Fan, R., Shah, V., Sorrentino, R., Cirino, G., Papapetropoulos, A. & Sessa, W. C. (1998) Nature 392, 821-824. [DOI] [PubMed] [Google Scholar]
- 10.Gratton, J. P., Fontana, J., O'Connor, D. S., Garcia-Cardena, G., McCabe, T. J. & Sessa, W. C. (2000) J. Biol. Chem. 275, 22268-22272. [DOI] [PubMed] [Google Scholar]
- 11.Brouet, A., Sonveaux, P., Dessy, C., Balligand, J. L. & Feron, O. (2001) J. Biol. Chem. 276, 32663-32669. [DOI] [PubMed] [Google Scholar]
- 12.Fontana, J., Fulton, D., Chen, Y., Fairchild, T. A., McCabe, T. J., Fujita, N., Tsuruo, T. & Sessa, W. C. (2002) Circ. Res. 90, 866-873. [DOI] [PubMed] [Google Scholar]
- 13.Pratt, W. B. & Toft, D. O. (2003) Exp. Biol. Med. 228, 111-133. [DOI] [PubMed] [Google Scholar]
- 14.Bucci, M., Roviezzo, F., Cicala, C., Sessa, W. C. & Cirino, G. (2000) Br. J. Pharmacol. 131, 13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michel, T., Li, G. K. & Busconi, L. (1993) Proc. Natl. Acad. Sci. USA 90, 6252-6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Cardena, G., Fan, R., Stern, D. F., Liu, J. & Sessa, W. C. (1996) J. Biol. Chem. 271, 27237-27240. [DOI] [PubMed] [Google Scholar]
- 17.Corson, M. A., James, N. L., Latta, S. E., Nerem, R. M., Berk, B. C. & Harrison, D. G. (1996) Circ. Res. 79, 984-991. [DOI] [PubMed] [Google Scholar]
- 18.Fulton, D., Gratton, J. P., McCabe, T. J., Fontana, J., Fujio, Y., Walsh, K., Franke, T. F., Papapetropoulos, A. & Sessa, W. C. (1999) Nature 399, 597-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimmeler, S., Fleming, I., Fisslthaler, B., Hermann, C., Busse, R. & Zeiher, A. M. (1999) Nature 399, 601-605. [DOI] [PubMed] [Google Scholar]
- 20.Huang, P. L., Huang, Z., Mashimo, H., Bloch, K. D., Moskowitz, M. A., Bevan, J. A. & Fishman, M. C. (1995) Nature 377, 239-242. [DOI] [PubMed] [Google Scholar]
- 21.Shesely, E. G., Maeda, N., Kim, H. S., Desai, K. M., Krege, J. H., Laubach, V. E., Sherman, P. A., Sessa, W. C. & Smithies, O. (1996) Proc. Natl. Acad. Sci. USA 93, 13176-13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knowles, R. G., Merrett, M., Salter, M. & Moncada, S. (1990) Biochem. J. 270, 833-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradley, P. P., Priebat, D. A., Christensen, R. D. & Rothstein, G. (1982) J. Invest. Dermatol. 78, 206-209. [DOI] [PubMed] [Google Scholar]
- 24.Edwards, J. C., Sedgwick, A. D. & Willoughby, D. A. (1981) J. Pathol. 134, 147-156. [DOI] [PubMed] [Google Scholar]
- 25.Payne, G. W., Madri, J. A., Sessa, W. C. & Segal, S. S. (2003) Am. J. Physiol. 285, H493-H498. [DOI] [PubMed] [Google Scholar]
- 26.Godecke, A., Decking, U. K., Ding, Z., Hirchenhain, J., Bidmon, H. J., Godecke, S. & Schrader, J. (1998) Circ. Res. 82, 186-194. [DOI] [PubMed] [Google Scholar]
- 27.Sun, D., Huang, A., Smith, C. J., Stackpole, C. J., Connetta, J. A., Shesely, E. G., Koller, A. & Kaley, G. (1999) Circ. Res. 85, 288-293. [DOI] [PubMed] [Google Scholar]
- 28.Huang, A., Sun, D., Smith, C. J., Connetta, J. A., Shesely, E. G., Koller, A. & Kaley, G. (2000) Am. J. Physiol. 278, H762-H768. [DOI] [PubMed] [Google Scholar]
- 29.Scotland, R. S., Chauhan, S., Vallance, P. J. & Ahluwalia, A. (2001) Hypertension 38, 833-839. [DOI] [PubMed] [Google Scholar]