Abstract
The cervical auscultation refers to the observation and analysis of sounds or vibrations captured during swallowing using either a stethoscope or acoustic/vibratory detectors. Microphones and accelerometers have recently become two common sensors used in modern cervical auscultation methods. There are open questions about whether swallowing signals recorded by these two sensors provide unique or complementary information about swallowing function; or whether they present interchangeable information. The aim of this study is to present a broad comparison of swallowing signals recorded by a microphone and a tri-axial accelerometer from 72 patients (mean age 63.94 ± 12.58 years, 42 male, 30 female), who underwent videofluoroscopic examination. The participants swallowed one or more boluses of thickened liquids of different consistencies, including thin liquids, nectar-thick liquids, and pudding. A comfortable self-selected volume from a cup or a controlled volume by the examiner from a 5ml spoon was given to the participants. A comprehensive set of features was extracted in time, information-theoretic, and frequency domains from each of 881 swallows presented in this study. The swallowing sounds exhibited significantly higher frequency content and kurtosis values than the swallowing vibrations. In addition, the Lempel-Ziv complexity was lower for swallowing sounds than those for swallowing vibrations. To conclude, information provided by microphones and accelerometers about swallowing function are unique and these two transducers are not interchangeable. Consequently, the selection of transducer would be a vital step in future studies.
Keywords: cervical auscultation, swallowing difficulties, tri-axial accelerometry, microphone, signal processing
1. Introduction
Cervical auscultation (CA), the observation of swallowing sounds or vibrations during deglutition, has been used in the screening of swallowing disorders [1, 2, 3, 4, 5, 6, 7, 8, 9, 10]. The acoustic information observed during swallowing has not yet been clearly delineated to represent specific physiologic events occurring during swallowing because the signals are so complex, and numerous events are taking place simultaneously during a single swallow. In CA, a stethoscope or an electronic acoustic/vibratory detector, such as a microphone or an accelerometer, is placed on the patient's anterior neck on the skin in the region of the larynx to listen to or record the swallowing acoustics or vibration signals [1, 2, 3, 5, 7, 8, 11, 12]. It is believed that information captured by this method corresponds to the movement of the hyolaryngeal structure during the act of deglutition [4, 5, 7, 13, 14, 15, 16, 17], which is a crucial component of the swallowing function, though there is only preliminary evidence to support this belief.
A stethoscope is originally used in CA to listen to the acoustic sounds as by-products of the swallowing process [1, 11, 18]. However, performance of the CA method by stethoscope alone, as a clinical screening method for the detection and management of swallowing difficulties, remains weak because of the subjective methods of individual interpretation of the observed sounds and the lack of solid methodological standardization of this method [1, 18]. Therefore, there has been a growing interest in the development of other devices, that could provide more discrete observations of acoustic and vibratory correlates of swallowing physiology, enable advanced and more precise signal processing analyses to elucidate the sources of the signals, and which could possibly be used as substitutes for the stethoscope, which could be deployed by minimally trained individuals.
Microphones and accelerometers are two common detectors that have been recently used to record the swallowing signals [1, 2, 3, 6, 7, 8, 9, 10, 12, 14, 18, 19, 20, 21, 22, 23, 24]. These approaches are based on the transduction of vibrations and sounds recorded from the upper aerodigestive tract structure during the act of swallowing into a voltage signal [5, 7, 13]. One of the basic mechanisms of the transduction in accelerometers and condenser microphones is based on the changes in the capacitance between internally fixed capacitive plates and free plates that move as acceleration forces or sound waves act upon the sensor. As sound waves or vibrations hit the free plates, the distance between the free plates and fixed plates changes, which alters the capacitances between the plates and results in the charge and discharge of the capacitors. Microphones are required to be open to the atmosphere, since they work by sensing the pressure waves on either side of a free plate (diaphragm), while the accelerometers can be sealed off from the atmosphere, since they measure the acceleration in one, two, or three axes based on inertial effects (the measurement of vibrations). In addition, these transducers differ in other factors such as size, weight, and environmental characteristics (e.g., temperature and humidity) [25, 26]. Consequently, the swallowing signals recorded by these two transducers might differ in content, and need to be analyzed in order to discern more about their natures.
Numerous investigations have been recently performed on swallowing signals recorded by microphones and accelerometers in an attempt to discern more about nature of the signals in relation to swallowing function. The focus of these investigations can be categorized into several main topics, such as the physiological sources of the signals [4, 5, 13, 14, 15, 16, 17, 27], the best placement site of microphones and accelerometers on the neck [1, 7, 27, 28], the best preprocessing methods for signals [20, 27, 29, 30, 31], characterization of the recorded signals [1, 2, 3, 6, 8, 9, 12, 18, 21, 23, 27, 32], segmentation of the swallowing signals [20, 22, 27, 33, 34, 35, 36], and classification of an abnormal swallow from a normal swallow [10, 27, 37, 38, 39].
Researchers have tried to characterize the swallowing sounds and vibrations separately by extracting different features in the various domains including time, frequency, and time-frequency [1, 2, 3, 6, 7, 8, 10, 12, 18, 21, 22, 23, 27, 37]. The results have provided some evidence that using microphones or dual-axial accelerometers may be a valid approach for detecting some swallowing difficulties. In addition, the results for swallowing vibrations have demonstrated that signals generated in the anterior-posterior (A-P) and superior-interior (S-I) directions provide unique information about the upward and forward movements of the hyolaryngeal structure during the swallowing function [10, 12, 21, 22, 23, 27, 37]. Moreover, we presented in our previous research [40] employing the third axis, medial-lateral (M-L), would be of interest since it offers complementary information about the swallowing functions.
For CA methods using electronic detectors to be considered as a practical clinical tool, its deployment must be standardized in a way that dysphagia screening for each patient is consistent between different clinics. The first step to reach this standardization is to find out which type of detectors, microphone or accelerometer, would provide better information about swallowing difficulties. To answer this question, we need first to investigate whether these two detectors provide similar or unique information about swallows by comparing the characteristics and features extracted from recorded swallowing signals by these two detectors. In the case that they would provide unique information about swallows, we must investigate which features are different as this information would be helpful in future studies exploring swallow classification methods. On the other hand, if they provide similar and equally accurate information, selection of the preferred technology would be based on feasibility and cost of deployment. While researchers have investigated the practicality of microphones and accelerometers separately in several studies, four studies with varying methods have compared swallowing signals recorded by these two sensors simultaneously on the same subjects and under the same conditions [7, 9, 24, 28]. The results from two studies were equivocal, showing that the information provided by these two sensors are interchangeable [7, 28]. One of them supported accelerometers in preference to microphones as they showed that the accelerometers had a flatter frequency response than microphones[28], while the other pointed in the opposite direction [7] and supported microphones versus accelerometers as microphones had a better signal-to-noise ratio than the accelerometers. Other two studies simply demonstrated the differences between recorded signals by the accelerometer and the microphone [9, 24]. It should be noted that these studies utilized different hardware and procedural methods, which limits the ability to compare their results. Thus, prior studies do not agree as to whether one technology or the other is superior because their methods and instrumentation were different; therefore their results are equivocal. In addition, some studies faultily assumed swallowing sounds and vibrations are equivalent [6, 28]. They considered swallowing signals recorded by accelerometers as swallowing sounds. Therefore, interchangeability of the information provided by these two sensors, or superiority of one of the sensors over the other is still controversial.
The aim of this study was to systematically and objectively characterize the swallowing sounds and vibrations recorded by a microphone and a tri-axial accelerometer in order to investigate whether swallowing signals recorded by these two sensors differ from each other or carry unique information about swallowing function. The results of this study aim to answer the following question: would employing a combination of a microphone and an accelerometer for recording swallowing sounds be beneficial in the screening of swallowing disorders? To answer this question, we did a broad comparison between features extracted from swallowing sounds and swallowing vibrations. The result of this study could help to make one step toward standardization of the CA method using electronic devices for dysphagia assessment, as it would clear if the logic for combining two types of detector makes sense.
2. Methodology
2.1. Data Acquisition
The total number of 881 swallows were recorded from 72 patients who underwent routine videofluoroscopic examinations of swallowing function at the Presbyterian Hospital of the University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania). A tri-axial accelerometer (ADXL 327, Analog Devices, Norwood, Massachusetts) with a sensitivity of 420 mV/g, resolution of 250 μg/√Hzrms, and an acceleration measurement of a minimum full-scale range of ±2g. The accelerometer was taped to the participants' anterior neck at the level of the cricoid cartilage, such that the sensitive axes of the accelerometer were aligned to the anatomical anterior-posterior (A-P), superior-inferior (S-I) and medial-lateral (M-L) axes, as shown in Fig.1. Signals from the A-P, S-I, and M-L axes were bandpass filtered from 0.1 to 3000 Hz, as previous studies have shown that the predominant acoustic energy of swallowing vibrations are below 3.5 kHz [2, 4, 11, 18], and then amplified (model P55, Grass Technologies, Warwick, Rhode Island) prior to storage on a research computer. The voltage signals for each axis of the accelerometer were fed into a National Instruments 6210 DAQ and recorded at 20 kHz by the LabView program Signal Express (National Instruments, Austin, Texas). A microphone (model C 411L, AKG, Vienna, Austria) with the sensitivity of 2 mV/Pa was attached below the accelerometer and angled slightly towards the right lateral side of the trachea on order to avoid contact between the two sensors and prevent obstruction of the radiographic view of the upper airway, but to still enable it to record events from approximately the same location. The microphone was powered by a power supply (model B29L, AKG, Vienna, Austria) and set to line impedance with a volume of 9 while the resulting voltage signal was sent to the previously mentioned DAQ. This signal was left unfiltered, as an upper limit to the bandwidth of swallowing sounds has not yet been found. Instead we recorded the entire dynamic range of our microphone signal (10 Hz to 20 kHz) to ensure that we did not lose any important components of our signal. Concurrent with the microphone and the accelerometer recordings, the biomechanical activity and the bolus flow in the upper aerodigestive tract was captured by the x-ray machine (Ultimax system, Toshiba, Tustin, CA) at 30 pulses per second, videofluoroscopic images were captured at 60 frames per second by a video card (AccuStream Express HD, Foresight Imaging, Chelmsford, MA), and information was recorded on hard drive with the same Labview program.
Figure 1.
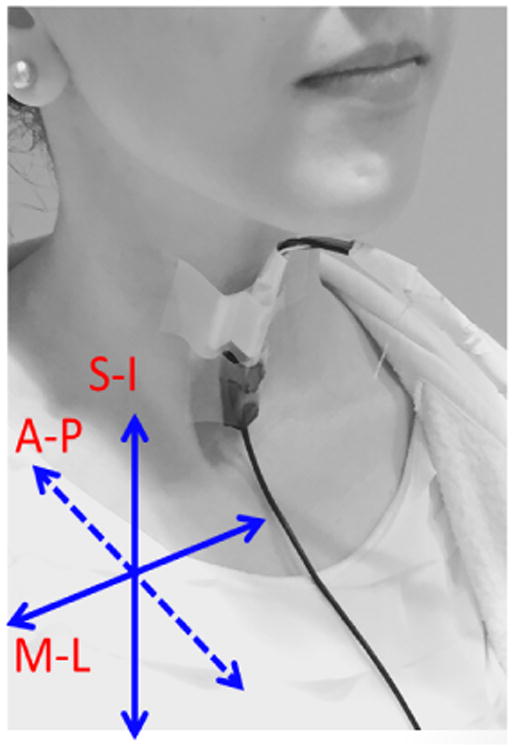
Location of the microphone and the accelerometer on a participant's neck and orientation of the three anatomical axes relative to the sensor.
Among 72 participants (mean age 63.94 ± 12.58 years, 42 male, 30 female) in this study, 20 participants had a history of stroke and the remaining were stroke free. As a part of their examinations, the participants swallowed one or more liquids with different consistencies, including thin liquid (Varibar Thin Liquid with < 5 cp viscosity), nectar-thick liquid (Varibar Nectar with ≈ 300 cP viscosity), and a semi-solid pudding (Varibar Pudding with ≈ 5000 cP viscosity). The test was performed in a neutral head position and, in some cases, in a head-flexion (chin-tuck) position, which has been used in some patients with dysphagia to manage aspiration during swallowing [41, 42, 43, 44]. Swallows with any other swallow maneuvers used to manage the patient's swallowing disorder such as the supraglottic swallow, Mendelsohn maneuver, and effortful swallow were excluded from the data set. All participants signed informed consent and the data collection protocol was approved by the University of Pittsburgh Institutional Review Board.
2.2. Data Preprocessing
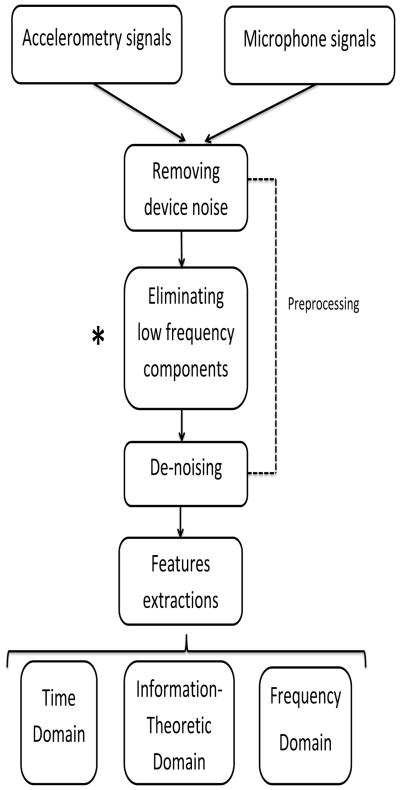
The time-marked sound and vibration signals were collected with the microphone and the tri-axial accelerometer in the S-I, A-P, and M-L directions. The time-linked onsets and offsets of each swallow were obtained via the frame-by-frame temporal analysis of videofluoroscopic images by a trained and experienced speech-language pathologist, whose inter- and intra-rater reliability in the judgment of these parameters was established a priori. The onset of the swallow segments was defined as the time at which the leading edge of the presented bolus intersected with the shadow cast on the x-ray image by the posterior border of the ramus of the mandible. The offset was the time that the hyoid bone completed motion associated with swallowing-related pharyngeal activity and clearance of the bolus from the video image. After segmentation of the swallow signals, swallow signals were grouped based on the two factors including the viscosity of the liquids participants were swallowing and whether the participants have had a stroke history, since dysphagia following a stroke and the effect of viscosity on the swallowing signals have been well-documented [6, 45, 46, 47, 48, 49, 50]. Then, each swallow signal was pre-processed as follows. First, the device noise was eliminated from the signals by applying microphone/axial-specific finite impulse response (FIR) filters [12]. Next, the low frequency components associated with head movement were eliminated from only the accelerometry signals using a least-squares spline approximation algorithm [29, 30]. This stage of pre-processing was applied for only swallowing accelerometry, because there is no information about whether low frequency contents of swallowing sounds recorded by the microphone carry important information about swallowing function. Finally, the filtered signals were denoised via a ten-level discrete wavelet transform using the Meyer wavelet with a soft thresholding. The level of decomposition was determined by minimizing noise in the signals, while retaining the interesting detail of the signals [31].
2.3. Feature Extraction
To capture key statistical differences between swallowing vibrations in the A-P, S-I, and M-L axes, several features in multiple domains were extracted from each of the axes of each swallow signal. In this study, 8 features in time, information-theoretic, and frequency domains were evaluated. Furthermore, the practicality and the validity of these features has been demonstrated in previous swallowing studies [10, 12, 21, 37]. The computational details for each of these features are described in the following subsections.
2.3.1. Time Domain Features
In this study, a number of time domain features were calculated for the swallowing vibrations to determine whether the physics of signal behavior differs between the axes of accelerometer and microphone. Consider a signal X = {x1, x2, …xn}, from which the following features are extracted:
-
The unbiased estimation of the standard deviation is obtained by
(1) where μX denotes the mean of the signal. The standard deviation reflects how the signal fluctuates around the mean value. Here the important parameter is the ac signal power represented by the deviation from the mean. The bigger the standard deviation, the wider the distribution of data points.
-
To quantify the symmetry of the amplitude distribution of swallow signals recorded by the microphone and accelerometer, skewness was computed.
This feature can be calculated as follows:
(2) A symmetrical distribution has a skewness of zero. However, an asymmetrical distribution with a long tail to the right or left has either a positive or negative skew, respectively.
-
To measure the degree of the peakedness of the amplitude distribution, kurtosis was computed. A high kurtosis value indicates a distribution with a sharp, narrow peak, declines rather rapidly, and has heavy tails, while a low kurtosis value signifies a distribution with a flattened peak and thin tails [51]. This feature is computed as:
(3)
2.3.2. Information-Theoretic Features
The different types of information-theoretic features were calculated to characterize the swallowing signals in this study.
-
The complexity or randomness of the swallow signals was measured by the Lempel-Ziv complexity (LZC), whose practicality for biological signals has been proved by previous studies [52, 53]. To compute the LZC, a signal must be first transformed into a symbolic sequence, since LZC measures the complexity through an estimated number of distinct patterns obtained by parsing a symbolic sequence [53]. Quantization is a typical method used to convert biomedical signals into a binary sequence. The signal is converted to 100 symbols by comparing it to 99 thresholds. After the quantized signal is obtained, it can be decomposed into the k blocks; then the LZC is calculated as:
(4) The logarithm of n to the base 100 is used in the above formula because the signal is quantized into 100 symbols.
-
The entropy rate is a valuable measurement to compare the regularity of biological signals [54, 55]. Zero and one values of entropy rate indicate a periodic repetition of the same pattern and aperiodic dynamics, respectively. First, to normalize values of X, μX is subtracted from X and divided by σX. Then, it is quantized into 10 equally spaced levels. In the next step, sequences of consecutive points in quantized signal X̂ of length H, 10 ≤ H ≤ 30, are coded as a series of integers, ϒH = {u1, u2, …un−H+1}, by means of the following formula:
(5) Now the normalized entropy rate is computed as
(6) where perc(H) is the percent of unique occurrence of coded integers in the sequence of H and ∆(H) is the calculated Shannon entropy of the sequence, which is obtained as follows:
(7) Finally, the entropy rate is presented as:
(8)
2.3.3. Frequency Domain Features
The computed features in frequency domain for this study are listed below.
-
Peak frequency is simply the frequency corresponding to maximum power and is calculated as follows:
(9) where fmax = 100Hz.
-
The spectral centroid points to where the “center of mass” of the spectrum is located and is defined as:
(10) -
The bandwidth of the signal is defined as follows:
(11) Table 1 contains the summary of all features and their definitions.
Table 1. Summary of all features.
| Time Domain Features | |
|---|---|
| Standard deviation (σ) | Reflects how a signal fluctuates around the mean value of the signal. |
| Skewness (ξ) | Describes the asymmetry of the amplitude distribution. Negative and positive skewness indicates the distribution of the signal with a long tail, higher values, to the left/ right side. |
| Kurtosis (γ) | Describes peaked/flat amplitude distribution relative to a normal distribution. High/low kurtosis values specify a signal distribution with a sharp/flattened peak. |
| Information-Theoretic Domain Features | |
| Lempel-Ziv Complexity (LZC) | Evaluates the randomness of a signal. Higher/lower values indicate more/less randomness in the signal. |
| Entropy rate (ρ) | Evaluates the degree of regularity of the signal distribution. One and zero value of the entropy rate indicates a periodic repetition of the same pattern and aperiodic dynamics, respectively. |
| Frequency Domain Features | |
| Peak frequency (f) | Describes the frequency corresponding to the maximum spectral power. |
| Spectral centroid (f̂) | Indicates the “ center of mass” of the frequency spectrum of a signal. |
| Bandwidth (BW) | The difference between the uppermost and lowermost frequencies in a signal. |
2.4. Statistical Analysis
To account for multiple trials from the same participants, linear mixed models with random effects were employed as an analytic strategy in this study. First, the anatomical direction differences were computed between the three axes and microphone for all the extracted features in each of the trials. Then, an intercept-only model was fitted to assess statistical significance of each of the said differences in the presence of multiple trials per participant. Next, in order to identify the correlation of the extracted features, another series of linear mixed models with each of the extracted features and their between differences (if applicable) as the dependent variable were fitted; each of the individual potentials correlates age and sex and collectively correlates them as fixed effect(s) of interest; and a participant random effect. The statistical significance of the parameters of the fixed effects solution with interpretation analogous to regression coefficients was used as evidence of significant association. Due to the large number of extracted features examined and the opportunity for inflated type I error due to multiplicity, the false discovery rate (FDR) methodology was used to make multiplicity corrections to the p-values in all analyses. SAS version 9.3 (SAS Institute, Inc., Cary, North Carolina) was used for all statistical analyses in this study. The benchmark for statistical significance was established at p < 0.05.
3. Results
The microphone and accelerometer data were presented in this study for 72 participants and 881 swallows. The extracted features were separated based on the participants' stroke history and viscosity of the fluids. A total number of 98 comparisons were made between each of the three axes of the accelerometer, the A-P, S-I, and M-L directions, and the microphone for all extracted features. Here, we summarize statistically significant results.
3.1. Time Domain Analysis
Table 2 displays the result of the time domain features calculated in this study for each of the accelerometer axes (A-P, S-I, and M-L), and microphone grouped by viscosity and participants' stroke history. Generally, all three axes and microphone demonstrated low values for standard deviations and skewness and high values of kurtosis. In particular, A-P and S-I axes demonstrated a higher rate of standard deviation, σ, than the microphone, for all the viscosities in the participants with stroke history and for only the thin and nectar viscosities in the participants without stroke history (p < 0.05). For only one case, thin liquid swallows in the non-stroke group, the microphone showed a higher standard deviation than the M-L axis (p = 0.017). While considering the asymmetry of swallows vibration and sound, ξs, the S-I amplitude distributions for thin swallows in both the stroke and non-stroke participants were slightly negatively skewed; however, the microphone amplitude distributions had a tendency to be positively skewed (p < 0.05). Lastly, the microphone signals were significantly more leptokurtic(p < 0.005), which resulted in a higher peak than for all three anatomical directions of the accelerometer signals for all the groups of study, except for the A-P axis for all the viscosities within the stroke group.
Table 2. Statistical features extracted from swallowing sounds and vibrations grouped by participants' stroke history and viscosity of fluids.
| Stroke | Non-Stroke | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
|
|
ξs |
|
|
ξns |
|
||||||
| Thin | A-P | 0.16 ± 0.13† | 0.55 ± 4.88 | 1.66 ± 7.92 | 0.19 ± 0.19 | 0.72 ± 2.55 | 0.37 ± 0.75† | ||||
| S-I | 0.29 ± 0.22† | -0.47 ± 1.60† | 0.18 ± 0.30† | 0.31 ± 0.21† | -0.57 ± 2.69† | 0.42 ± 1.50† | |||||
| M-L | 0.06 ± 0.04 | 0.71 ± 3.53 | 0.30 ± 1.75† | 0.07 ± 0.07† | 0.31 ± 3.42 | 0.61 ± 4.46† | |||||
| Mic | 0.12 ± 0.18 | 1.16 ± 9.37 | 6.08 ± 1.08 | 0.18 ± 0.47 | 0.78 ± 6.60 | 4.00 ± 6.34 | |||||
|
| |||||||||||
| Nectar | A-P | 0.15 ± 0.20† | -0.09 ± 4.57 | 1.48 ± 8.37 | 0.16 ± 0.20 | 0.73 ± 2.24 | 0.49 ± 1.10† | ||||
| S-I | 0.24 ± 0.19† | -0.30 ± 1.34 | 0.15 ± 0.24† | 0.28 ± 0.23† | 0.14 ± 4.37 | 0.71 ± 2.90† | |||||
| M-L | 0.06 ± 0.04 | 0.13 ± 1.14 | 0.13 ± 0.33† | 0.06 ± 0.05 | 0.64 ± 3.57 | 0.78 ± 3.79† | |||||
| Mic | 0.08 ± 0.17 | 1.72 ± 8.89 | 4.98 ± 8.54 | 0.14 ± 0.32 | 1.17 ± 7.32 | 4.91 ± 6.93 | |||||
|
| |||||||||||
| Pudding | A-P | 0.10 ± 0.05† | -1.40 ± 4.79 | 1.83 ± 7.96 | 0.18 ± 0.14 | 1.12 ± 3.21 | 0.55 ± 0.99† | ||||
| S-I | 0.20 ± 0.11† | -0.13 ± 0.74 | 0.06 ± 0.07† | 0.27 ± 0.18 | -0.05 ± 1.87 | 0.24 ± 0.46† | |||||
| M-L | 0.05 ± 0.03 | 0.20 ± 1.07 | 0.08 ± 0.21† | 0.08 ± 0.10 | 0.00 ± 0.00 | 0.41 ± 1.58† | |||||
| Mic | 0.04 ± 0.08 | 1.29 ± 8.56 | 6.83 ± 8.85 | 0.21 ± 0.44 | -0.04 ± 1.45 | 6.03 ± 8.95 | |||||
σ = Standard deviation, ξ = Skewness, γ = Kurtosis
Denotes multiplication by 10−2
Denotes multiplication by 102
Statistical difference between axis and Mic (p-value < 0.05)
3.2. Information-Theoretic Analysis
The summary of information-theoretic features is presented in Table 3. While considering the evaluation of randomness in swallow signals, even though signals in all three axes and microphone showed low rate of LZC (less than 0.07), the statistical analysis for LZC presented statistical difference between all three anatomical directions and the microphone for all the groups of study (p < 0.001). Furthermore, both the microphone and the accelerometer in all three axes presented high values for the entropy rate, ρ, (more than 0.98). Pudding swallows in the stroke group presented slightly higher values of entropy rate for the microphone than the S-I and M-L axes (p < 0.005).
Table 3. Information-theoretic features extracted from swallowing sounds and vibrations grouped by the participants' stroke history and viscosity of fluids.
| Stroke | Non-Stroke | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| LZCs |
|
LZCns |
|
||||
| Thin | A-P | 0.05 ± 0.02† | 9.86 ± 0.01 | 0.06 ± 0.02† | 9.86 ± 0.01 | ||
| S-I | 0.06 ± 0.02† | 9.87 ± 0.01 | 0.07 ± 0.02† | 9.88 ± 0.00 | |||
| M-L | 0.06 ± 0.02† | 9.89 ± 0.00 | 0.06 ± 0.02† | 9.89 ± 0.00 | |||
| Mic | 0.03 ± 0.02 | 9.88 ± 0.97 | 0.03 ± 0.02 | 9.87 ± 0.01 | |||
|
| |||||||
| Nectar | A-P | 0.06 ± 0.03† | 9.89 ± 0.01 | 0.06 ± 0.02† | 9.88 ± 0.01 | ||
| S-I | 0.07 ± 0.02† | 9.89 ± 0.00 | 0.06 ± 0.03† | 9.89 ± 0.00 | |||
| M-L | 0.06 ± 0.02† | 9.89 ± 0.00 | 0.06 ± 0.02† | 9.90 ± 0.00 | |||
| Mic | 0.03 ± 0.02 | 9.90 ± 0.65 | 0.03 ± 0.02 | 9.90 ± 0.01 | |||
|
| |||||||
| Pudding | A-P | 0.06 ± 0.03† | 9.90 ± 0.00 | 0.05 ± 0.02† | 9.88 ± 0.00 | ||
| S-I | 0.07 ± 0.02† | 9.89 ± 0.00† | 0.07 ± 0.03† | 9.89 ± 0.00 | |||
| M-L | 0.05 ± 0.02† | 9.90 ± 0.00† | 0.06 ± 0.02† | 9.89 ± 0.00 | |||
| Mic | 0.02 ± 0.01 | 9.93 ± 0.33 | 0.03 ± 0.02 | 9.88 ± 0.01 | |||
LZC= Lempel-Ziv Complexity, ρ= Entropy rate
Denotes multiplication by 10−1
Statistical difference between axis and Mic (p-value < 0.05)
3.3. Frequency Domain Analysis
It is necessary to explain that bandpass filtering of swallowing vibrations (from 0.1 to 3000 Hz) would not affect the comparison between swallow sounds and vibrations in frequency domain as the upper band of recorded swallow sounds and vibrations in this study was less than 1 kHz. Table 4 represents the summary of frequency features considered in the current study. The results showed significant dissimilarity between the microphone and the three axes of the swallowing accelerometry signals in frequency features in this study (p < 0.05) for all groups of the study. The exceptions include the A-P signals of thin swallows in the stroke group for centroid frequency feature as well as the three axes of the pudding swallows and the A-P signals of the nectar swallows in the stroke group for the peak frequency feature. The microphone demonstrated considerably higher values for peak frequency, centroid frequency, and bandwidth than the A-P, S-I, and M-L signals.
Table 4. Frequency features extracted from swallowing sounds and vibrations grouped by the participants' stroke history and viscosity of fluids.
| Stroke | Non-Stroke | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
|
|
|
|
|
|
|
||||||||
| Thin | A-P | 0.20 ± 0.68† | 0.41 ± 1.15 | 0.42 ± 5.75† | 0.15 ± 0.42† | 1.19 ± 2.12† | 2.10 ± 3.53† | ||||||
| S-I | 0.11 ± 0.11† | 0.08 ± 0.07† | 0.15 ± 1.46† | 0.12 ± 0.27† | 0.96 ± 3.91† | 1.45 ± 3.38† | |||||||
| M-L | 0.06 ± 0.11† | 0.04 ± 0.10† | 0.10 ± 1.61† | 0.08 ± 0.23† | 0.95 ± 4.66† | 1.16 ± 3.30† | |||||||
| Mic | 2.72 ± 4.91 | 1.19 ± 1.23 | 0.82 ± 0.82 | 2.75 ± 4.47 | 0.88 ± 0.85 | 5.90 ± 6.10 | |||||||
|
| |||||||||||||
| Nectar | A-P | 1.06 ± 8.04 | 0.29 ± 0.96† | 0.34 ± 6.12† | 0.08 ± 0.20† | 1.72 ± 3.42† | 3.22 ± 5.33† | ||||||
| S-I | 0.12 ± 0.28† | 0.04 ± 0.06† | 0.10 ± 1.42† | 0.12 ± 0.43† | 1.16 ± 3.98† | 1.90 ± 3.92† | |||||||
| M-L | 0.06 ± 0.13† | 0.03 ± 0.06† | 0.08 ± 1.02† | 0.06 ± 0.08† | 1.01 ± 4.80† | 1.24 ± 3.95† | |||||||
| Mic | 1.70 ± 4.06 | 1.00 ± 1.02 | 0.80 ± 0.82 | 2.56 ± 4.60 | 0.95 ± 1.01 | 6.49 ± 7.12 | |||||||
|
| |||||||||||||
| Pudding | A-P | 0.03 ± 0.02 | 0.29 ± 1.22† | 0.25 ± 6.25† | 0.12 ± 0.36† | 1.41 ± 2.28† | 2.67 ± 3.62† | ||||||
| S-I | 0.06 ± 0.02 | 0.03 ± 0.04† | 0.08 ± 1.57† | 0.09 ± 0.09† | 0.96 ± 2.07† | 2.06 ± 4.07† | |||||||
| M-L | 0.05 ± 0.03 | 0.02 ± 0.03† | 0.13 ± 1.93† | 0.08 ± 0.18† | 1.19 ± 4.17† | 2.05 ± 5.66† | |||||||
| Mic | 0.72 ± 2.35 | 1.58 ± 1.65 | 1.29 ± 0.99 | 2.35 ± 4.14 | 1.15 ± 1.11 | 8.69 ± 8.38 | |||||||
f = Peak frequency, f̂ = Centroid frequency, BW = Bandwidth
denotes multiplication by 102
denotes multiplication by 103
Statistical difference between axis and Mic (p-value < 0.05)
3.4. Summary of All Findings
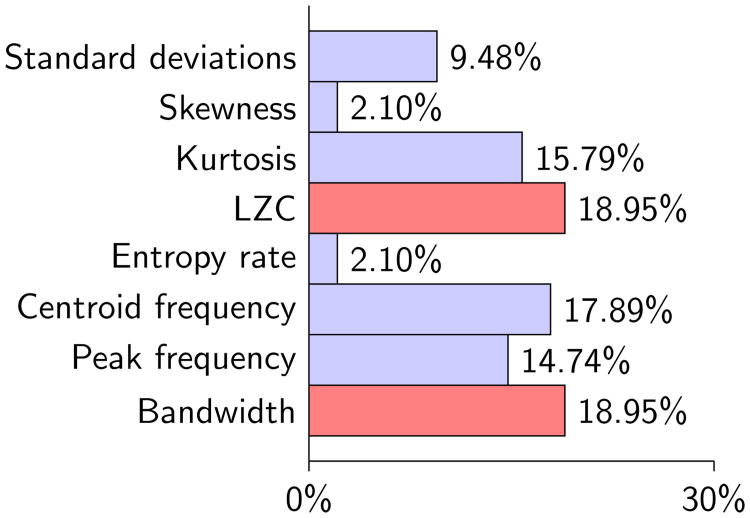
A total number of 144 comparisons were performed between the features calculated for swallowing signals of the microphone and three axes of the swallowing accelerometer and grouped by the viscosity of fluids and participants' stroke history. Among 144 comparison cases, 95 cases showed statistically significant differences, which is ≈ 66% of total number of comparisons. As we can see in figure 3, most of the percentage of dissimilarities was related to the computed features of the LZC (18.95%), bandwidth (18.95%), centroid frequency (17.89%), kurtosis (15.79%), and peak frequency (14.74%).
Figure 3.
Percentages of dissimilarities between computed features from swallowing signals recorded by the tri-axial accelerometer and microphone
Table 5 summarizes all our findings. Each circle in Table 5 specifies existing differences between values of computed features for two directions. Red/black circles indicate that the axis/Mic in the “Comparison couple” column has the higher value in a specific feature mentioned in the “Features” column than the Mic/axis. The column highlighted in light green and five rows highlighted in light orange indicate viscosity group and features that present the largest number of dissimilarities. First, we consider the features that give us the largest numbers of directional difference among the various groups of study. The results obtained for LZC, bandwidth, centroid frequency, kurtosis, and peak frequency showed 18, 18, 17, 15, and 14 cases of dissimilarity, respectively, representing ≈ 86% of the total dissimilarities. 28%, 39% and 33% of dissimilarities were found in comparisons between the microphone & A-P, microphone & S-I, and microphone & M-L, respectively. Generally, microphone signals demonstrated higher values in kurtosis, centroid frequency, peak frequency, and bandwidth and lower values in LZC when compared with the three axes of the swallowing accelerometry signals in the most groups of the study. Second, considering the viscosity and participants' stroke history, all groups in the study showed a minimum of 15 cases of dissimilarity in measured features. The thin swallows in the non-stroke group demonstrated the most cases of dissimilarity among all the groups, highlighted in light green in Table 5.
Table 5.
Summary of differences between swallowing sounds and vibrations among study groups. All dots (red and black dots) indicate the existence of dissimilarities between swallowing sounds and vibrations at the p<0.05 level. Red dots indicate that axis has a higher value of the computed feature in “Features” column than the mic, while black dots indicate the mic has a higher value than the axis. The column highlighted in light green and five rows highlighted in light orange indicate group and features with the largest number of dissimilarities, respectively.
| Stroke | Non-stroke | ||||||
|---|---|---|---|---|---|---|---|
| Features | Comparison couple | Thin | Nectar | pudding | Thin | Nectar | Pudding |
| Standard Deviation | A-P & Mic |

|

|

|
|||
| S-I & Mic |

|

|

|

|

|
||
| M-L & Mic |

|
||||||
|
| |||||||
| Skewness | A-P & Mic | ||||||
| S-I & Mic |

|

|
|||||
| M-L & Mic | |||||||
|
| |||||||
| Kurtosis | A-P & Mic |

|

|

|
|||
| S-I & Mic |

|

|

|

|

|

|
|
| M-L & Mic |

|

|

|

|

|

|
|
|
| |||||||
| LZC | A-P & Mic |

|

|

|

|

|

|
| S-I & Mic |

|

|

|

|

|

|
|
| M-L & Mic |

|

|

|

|

|

|
|
|
| |||||||
| Entropy Rate | A-P & Mic | ||||||
| S-I & Mic |

|
||||||
| M-L & Mic |

|
||||||
|
| |||||||
| Centroid Frequency | A-P & Mic |

|

|

|

|

|
|
| S-I & Mic |

|

|

|

|

|

|
|
| M-L & Mic |

|

|

|

|

|

|
|
|
| |||||||
| Peak Frequency | A-P & Mic |

|

|

|

|
||
| S-I & Mic |

|

|

|

|

|
||
| M-L & Mic |

|

|

|

|

|
||
|
| |||||||
| Bandwidth | A-P & Mic |

|

|

|

|

|

|
| S-I & Mic |

|

|

|

|

|

|
|
| M-L & Mic |

|

|

|

|

|

|
|
4. Discussion
The present study provides a quantitative characterization of swallowing signals recorded by a tri-axial accelerometer, as well as, a microphone during videofluoroscopy exam in patients with swallowing difficulties. Multiple features in time, information-theoretic, frequency, and time-frequency domains were extracted from swallowing signals for each the microphone and A-P, S-I, and M-L directions of the swallowing accelerometer.
Generally, swallowing sounds and vibrations showed low values of standard deviation and skewness and high values of kurtosis, which indicates a dense symmetry distribution with decreasing amplitude values rapidly below and above the mean. The A-P signals had higher values of standard deviation than the microphone within the stroke group for all viscosities, and within the non-stroke group for thin and nectar swallows. The S-I and A-P signals also showed higher values of standard deviation than the microphone within the stroke group for all viscosities. These results suggest that swallowing vibration related to the forward and upward movements of the hyolaryngeal structure were actually more variable in amplitude than swallowing sounds during the act of swallowing in the previously mentioned groups of study. There was only one group of study, thin swallows in the non-stroke group, presented higher values of standard deviation for the microphone than for the M-L axis, which indicates that the swallowing sounds were more variable in the amplitude than the vibration related to the sideways movement of the hyolaryngeal structure. In addition, the amplitude of S-I signals of thin swallows in the both stroke and non-stroke groups were slightly negatively skewed, while the microphone amplitude distributions had a tendency to be positively skewed. These results mean that the intensity of the forces of swallowing signals recorded by the microphone were smaller for longer periods of a swallow than the recorded swallowing vibration of upward movement. Lastly, in the time-domain analysis, kurtosis values for swallowing sounds were higher than swallowing vibrations in all three directions in all groups of the study, which indicates a peaked distribution of swallowing sounds compared to swallowing vibrations.
Based on the results of information-theoretic features, swallowing signals recorded by the microphone and the accelerometer for the three anatomical axes demonstrated high values for the entropy rate (around 0.99) and low values for the LZC (ranges from 0.03 to 0.07), indicating predictable and regular behavior of the swallowing signals. However, the degree of predictability (LZC) marginally differed between swallowing sounds and swallowing vibrations in all three anatomical directions in all groups of the study. Swallowing vibrations in the A-P, S-I, and M-L directions had a slightly higher rates for the LCZ than swallowing sounds, which indicates less predictable behavior of swallowing vibrations. Considering the dissimilarities in regularity of swallowing signals (entropy rate) found between swallowing sounds and vibrations, only the S-I and M-L vibrations of pudding swallows in the stroke group had lower entropy rates than swallowing sounds.
The frequency domain showed the most dissimilarities between swallowing signals recorded by the axes of the accelerometer and the microphone, which indicated that the energy distribution over the range of the frequencies is considerably different among swallowing sounds and vibrations. The results showed that swallowing sounds presented higher values for centroid frequency, peak frequency, and bandwidth than all three axes of the swallowing accelerometer for most groups of the study. It suggests that the swallowing sounds had a maximum power and most concentrated energy in higher frequencies, and a wider spread of frequency components than the swallowing vibrations in all three directions. In addition, the observed higher frequency component in the recorded swallowing signals by only one of the transducers, the microphone, highlights the importance of the difference between the mechanism of recording signals with the possible same source in the microphones and accelerometers. Accelerometers work based on the recording directional vibrations caused by the swallowing function, while microphones record pressure waves generated by the same source of vibration.
To conclude, although the swallowing sounds and swallowing vibrations might have the same physiological sources, swallowing signals recorded by the microphone and the accelerometer differ from each other in the time and frequency domains. This finding disagrees with the previous studies showed that information provided from swallowing sounds and vibrations are equivalent [6, 28] or interchangeable and considered just one of the sensors to be an optimal acoustic detector [7, 28]. In the current study, we showed that each of swallowing sounds and swallowing vibrations could provide useful information about the swallowing function. Therefore, based on the desired goal of future studies, one of the transducers or a combination of them should be considered for swallowing signal analysis. In this study, a variety of the features were extracted from swallowing sounds and vibrations which could be helpful for future studies in order to choose which transducer would be most beneficial to be consider.
Here in this study, we only investigated whether there was a difference between the swallowing signals recorded by the microphone and the accelerometer, without answering the question of which one is more successful to provide information about the important details of the swallowing function in order to classify healthy swallows from unhealthy swallows. Therefore, future studies should focus on applying the classification and pattern recognition algorithms on the swallowing signals recorded by these two transducers to see which instrument is capable of higher accuracy at detecting swallowing difficulties. There is also a need in future works to analyze the videofluoroscopy images simultaneously with the swallowing sounds and vibrations to determine whether either technology is capable of detecting, and possibly measuring the physiological events observed in the swallowing signals. In addition, the manual segmentation of swallowing signals in this study increases the rate of human error due to fatigue and decreases the consistency in segmentation criteria. Therefore, investigating a swallow segmentation algorithm that can extract each swallow from the recorded signals with high accuracy would be an important contribution. Finally, it would be good to consider the participants' head position in future studies, since previous previous studies showed the possible effects of the neutral head-neck posture and the head-neck flexion (chin-tuck) position on swallowing signals in the A-P and S-I axes [41, 42, 43, 44, 45].
5. Conclusion
In this study, we comprehensively compared the swallowing signals recorded by a microphone and a tri-axial accelerometer, in both stroke and non-stroke groups among various viscosities, including thin liquid, nectar-tick liquid, and pudding-tick liquid. We considered eight features from 881 swallowing accelerometer signals in various domains including time, information-theoretic, and frequency domains. The results indicated that swallowing signals recorded by the microphone differed from the each of the three directions of the swallowing accelerometer. Some major dissimilarities were observed between most groups of study. First, swallowing sounds exhibited higher values for features in the frequency domain than the swallowing vibrations in cases. Second, the LZC was greater for swallowing vibrations than swallowing sounds. Third, swallowing sounds had demonstrated higher kurtosis values than swallowing vibrations. To summarize, we concluded that information provided by the swallowing sounds and swallowing vibrations are not interchangeable. As a result, identifying the transducer used to collect such information and assessing its recording capabilities should be an important methodological step in future work.
Figure 2.
Flowchart outlining the preprocessing and features extraction steps.
* This stage of preprocessing was not applied for the swallowing signals recorded by the microphone
Highlights.
Cervical auscultation refers to sounds or vibrations captured during swallowing.
Microphones and accelerometers are common sensors used for cervical auscultation.
Open questions exist about information provided by the two sensors about the swallowing function.
We investigated these questions in the current manuscript.
Details provided by microphones and accelerometers about the swallowing function are unique and these two transducers are not interchangeable.
The selection of transducer would be a vital step in future studies.
Acknowledgments
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under Award Number R01HD074819. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Santamato A, Panza F, Solfrizzi V, Russo A, Frisardi V, Megna M, Ranieri M, Fiore P. Acoustic analysis of swallowing sounds: a new technique for assessing dysphagia. Journal of Rehabilitation Medicine. 2009;41(8):639–645. doi: 10.2340/16501977-0384. [DOI] [PubMed] [Google Scholar]
- 2.Cichero JA, Murdoch BE. Acoustic signature of the normal swallow: characterization by age, gender, and bolus volume. Annals of Otology, Rhinology and Laryngology. 2002;111(7):623–632. doi: 10.1177/000348940211100710. [DOI] [PubMed] [Google Scholar]
- 3.Almeida ST, Ferlin EL, Parente MAM, Goldani HA. Assessment of swallowing sounds by digital cervical auscultation in children. Annals of Otology, Rhinology and Laryngology. 2008;117(4):253–258. doi: 10.1177/000348940811700403. [DOI] [PubMed] [Google Scholar]
- 4.Cichero JA, Murdoch BE. The physiologic cause of swallowing sounds: answers from heart sounds and vocal tract acoustics. Dysphagia. 1998;13(1):39–52. doi: 10.1007/PL00009548. [DOI] [PubMed] [Google Scholar]
- 5.Zenner PM, Losinski DS, Mills RH. Using cervical auscultation in the clinical dysphagia examination in long-term care. Dysphagia. 1995;10(1):27–31. doi: 10.1007/BF00261276. [DOI] [PubMed] [Google Scholar]
- 6.Youmans SR, Stierwalt JA. An acoustic profile of normal swallowing. Dysphagia. 2005;20(3):195–209. doi: 10.1007/s00455-005-0013-1. [DOI] [PubMed] [Google Scholar]
- 7.Cichero JA, Murdoch BE. Detection of swallowing sounds: methodology revisited. Dysphagia. 2002;17(1):40–49. doi: 10.1007/s00455-001-0100-x. [DOI] [PubMed] [Google Scholar]
- 8.Yadollahi A, Moussavi Z. The 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Lyon, FR: 2007. Feature selection for swallowing sounds classification; pp. 3172–3175. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds EW, Vice FL, Gewolb IH. Variability of swallow-associated sounds in adults and infants. Dysphagia. 2009;24(1):13–19. doi: 10.1007/s00455-008-9160-5. [DOI] [PubMed] [Google Scholar]
- 10.Nikjoo M, Steele C, Sejdić E, Chau T. Automatic discrimination between safe and unsafe swallowing using a reputation-based classifier. Biomedical Engineering Online. 2011;10(100):1–17. doi: 10.1186/1475-925X-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamlet S, Penney DG, Formolo J. Stethoscope acoustics and cervical auscultation of swallowing. Dysphagia. 1994;9(1):63–68. doi: 10.1007/BF00262761. [DOI] [PubMed] [Google Scholar]
- 12.Sejdić E, Komisar V, Steele CM, Chau T. Baseline characteristics of dual-axis cervical accelerometry signals. Annals of Biomedical Engineering. 2010;38(3):1048–1059. doi: 10.1007/s10439-009-9874-z. [DOI] [PubMed] [Google Scholar]
- 13.Zoratto D, Chau T, Steele CM. Hyolaryngeal excursion as the physiological source of accelerometry signals during swallowing. Physiological Measurement. 2010;31(6):843–855. doi: 10.1088/0967-3334/31/6/008. [DOI] [PubMed] [Google Scholar]
- 14.Sazonov E, Schuckers S, Lopex-Meyer P, Makeyev O, Sazonova N, Melanson E, Neuman M. Non-invasive monitoring of chewing and swallowing for objective quantification of ingestive behavior. Physiological Measurement. 2008;29(5):525–541. doi: 10.1088/0967-3334/29/5/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy NP, Katakam A, Gupta V, Unnikrishnan R, Narayanan J, Canilang EP. Measurements of acceleration during videofluorographic evaluation of dysphagic patients. Medical Engineering and physics. 2000;22(6):405–412. doi: 10.1016/s1350-4533(00)00047-3. [DOI] [PubMed] [Google Scholar]
- 16.Vice FL, Heinz JM, Giuriati G, Hood M, Bosma JF. Cervical auscultation of suckle feeding in newborn infants. Developmental Medicine and Child Neurology. 1990;32(9):760–768. doi: 10.1111/j.1469-8749.1990.tb08479.x. [DOI] [PubMed] [Google Scholar]
- 17.Soentgen M, Pierce L, Brenman H. Mouthing activities in the human neonatal sucking act. Archives of Oral Biology. 1969;14(10):1159–1167. doi: 10.1016/0003-9969(69)90155-1. [DOI] [PubMed] [Google Scholar]
- 18.Logan WJ, Kavanagh JF, Wornall A. Sonic correlates of human deglutition., Journal of Applied Physiology. 1967;23(2):279–284. doi: 10.1152/jappl.1967.23.2.279. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K, Groher ME, Michi Ki. Symmetry and reproducibility of swallowing sounds. Dysphagia. 1994;9(3):168–173. doi: 10.1007/BF00341261. [DOI] [PubMed] [Google Scholar]
- 20.Aboofazeli M, Moussavi Z. The 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. New York, NY: 2006. Automated extraction of swallowing sounds using a wavelet-based filter; pp. 5607–5610. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Steele C, Chau T. Time and time-frequency characterization of dual-axis swallowing accelerometry signals. Physiological Measurement. 2008;29(9):1105–1120. doi: 10.1088/0967-3334/29/9/008. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Steele CM, Chau T. Swallow segmentation with artificial neural networks and multi-sensor fusion. Medical Engineering and Physics. 2009;31(9):1049–1055. doi: 10.1016/j.medengphy.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Sejdić E, Steele CM, Chau T. Understanding the statistical persistence of dual-axis swallowing accelerometry signals. Computers in Biology and Medicine. 2010;40(11):839–844. doi: 10.1016/j.compbiomed.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Dudik JM, Jestrović I, Luan B, Coyle JL, Sejdić E. A comparative analysis of swallowing accelerometry and sounds during saliva swallows. Biomedical Engineering Online. 2015;14(3):1–15. doi: 10.1186/1475-925X-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Analog Devices, ADXL322: Small and Thin 2g Accelerometer Data Sheet. 6th. 2005. [Google Scholar]
- 26.AKG Acoustics, MicroMic, The Original: C411. 1st. 1991. [Google Scholar]
- 27.Dudik JM, Coyle JL, Sejdic E. Dysphagia screening: contributions of cervical auscultation signals and modern signal-processing techniques. IEEE Transaction on Human-Machine Systems. 2015;45(4):465–477. doi: 10.1109/THMS.2015.2408615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi K, Groher ME, Michi Ki. Methodology for detecting swallowing sounds. Dysphagia. 1994;9(1):54–62. doi: 10.1007/BF00262760. [DOI] [PubMed] [Google Scholar]
- 29.Sejdić E, Steele CM, Chau T. A method for removal of low frequency components associated with head movements from dual-axis swallowing accelerometry signals. PLoS ONE. 2012;7(3):1–8. doi: 10.1371/journal.pone.0033464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sejdić E, Steele CM, Chau T. The effects of head movement on dual-axis cervical accelerometry signals. BioMedical Central Research Notes. 2010;3(1):269–1–6. doi: 10.1186/1756-0500-3-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sejdić E, Steele CM, Chau T. A procedure for denoising dual-axis swallowing accelerometry signals. Physiological Measurement. 2010;31(1):N1–N9. doi: 10.1088/0967-3334/31/1/N01. [DOI] [PubMed] [Google Scholar]
- 32.Fontana JM, Melo PL, Sazonov ES. Proc of 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC, 2011) Boston, MA, USA: 2011. Swallowing detection by sonic and subsonic frequencies: A comparison; pp. 6890–6893. [DOI] [PubMed] [Google Scholar]
- 33.Sejdić E, Steele CM, Chau T. Segmentation of dual-axis swallowing accelerometry signals in healthy subjects with analysis of anthropometric effects on duration of swallowing activities. IEEE Transactions on Biomedical Engineering. 2009;56(4):1090–1097. doi: 10.1109/TBME.2008.2010504. [DOI] [PubMed] [Google Scholar]
- 34.Damouras S, Sejdić E, Steele CM, Chau T. An online swallow detection algorithm based on the quadratic variation of dual-axis accelerometry. IEEE Transactions on Signal Processing. 2010;58(6):3352–3359. [Google Scholar]
- 35.Sejdić E, Falk TH, Steele CM, Chau T. Vocalization removal for improved automatic segmentation of dual-axis swallowing accelerometry signals. Medical Engineering and Physics. 2010;32(6):668–672. doi: 10.1016/j.medengphy.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Dudik JM, Kurosu A, Coyle JL, Sejdić E. A comparative analysis of dbscan, k-means, and quadratic variation algorithms for automatic identification of swallows from swallowing accelerometry signals. Computers in Biology and Medicine. 2015;59:10–18. doi: 10.1016/j.compbiomed.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, Steele CM, Chau T. Classification of healthy and abnormal swallows based on accelerometry and nasal airflow signals. Artificial Intelligence in Medicine. 2011;52(1):17–25. doi: 10.1016/j.artmed.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Sejdić E, Steele CM, Chau T. Classification of penetration-aspiration versus healthy swallows using dual-axis swallowing accelerometry signals in dysphagic subjects. IEEE Transactions on Biomedical Engineering. 2013;60(7):1859–1866. doi: 10.1109/TBME.2013.2243730. [DOI] [PubMed] [Google Scholar]
- 39.Steele CM, Sejdić E, Chau T. Noninvasive detection of thin-liquid aspiration using dual-axis swallowing accelerometry. Dysphagia. 2013;28(1):105–112. doi: 10.1007/s00455-012-9418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Movahedi F, Kurosu A, Coyle JL, Perera S, Sejdić E. Anatomical directional dissimilarities in tri-axial swallowing accelerometry signals. IEEE Transactions on Neural Systems and Rehabilitation Engineering. doi: 10.1109/TNSRE.2016.2577882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shanahan TK, Logemann JA, Rademaker AW, Roa Pauloski B. Chin-down posture effect on aspiration in dysphagic patients. Archives of Physical Medicine and Rehabilitation. 1993;74(7):736–739. doi: 10.1016/0003-9993(93)90035-9. [DOI] [PubMed] [Google Scholar]
- 42.Bülow M, Olsson R, Ekberg O. Supraglottic swallow, effortful swallow, and chin tuck did not alter hypopharyngeal intrabolus pressure in patients with pharyngeal dysfunction. Dysphagia. 2002;17(3):197–201. doi: 10.1007/s00455-002-0050-y. [DOI] [PubMed] [Google Scholar]
- 43.Ertekin C, Keskin A, Kiylioglu N, Kirazli Y, On AY, Tarlaci S, Aydoğdu I. The effect of head and neck positions on oropharyngeal swallowing: a clinical and electrophysiologic study. Archives of Physical Medicine and Rehabilitation. 2001;82(9):1255–1260. doi: 10.1053/apmr.2001.25156. [DOI] [PubMed] [Google Scholar]
- 44.Bülow M, Olsson R, Ekberg O. Videomanometric analysis of supraglottic swallow, effortful swallow, and chin tuck in healthy volunteers. Dysphagia. 1999;14(2):67–72. doi: 10.1007/PL00009589. [DOI] [PubMed] [Google Scholar]
- 45.Jestrović I, Dudik J, Luan B, Coyle J, Sejdić E. The effects of increased fluid viscosity on swallowing sounds in healthy adults. Biomedical Engineering Online. 2013;12(90):1–17. doi: 10.1186/1475-925X-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steele CM, Alsanei WA, Ayanikalath S, Barbon CE, Chen J, Cichero JA, Coutts K, Dantas RO, Duivestein J, Giosa L, et al. The influence of food texture and liquid consistency modification on swallowing physiology and function: A systematic review. Dysphagia. 2014;30(1):2–26. doi: 10.1007/s00455-014-9578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson RD, Howe EC. A cost-effectiveness analysis of screening methods for dysphagia after stroke. Physical Medicine and Rehabilitation Journal. 2012;4(4):273–282. doi: 10.1016/j.pmrj.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Holas MA, DePippo KL, Reding MJ. Aspiration and relative risk of medical complications following stroke. Archives of Neurology. 1994;51(10):1051–1053. doi: 10.1001/archneur.1994.00540220099020. [DOI] [PubMed] [Google Scholar]
- 49.Smithard D, Smeeton N, Wolfe C. Long-term outcome after stroke: does dysphagia matter? Age and Ageing. 2007;36(1):90–94. doi: 10.1093/ageing/afl149. [DOI] [PubMed] [Google Scholar]
- 50.Lee J, Sejdić E, Steele CM, Chau T. Effects of liquid stimuli on dual-axis swallowing accelerometry signals in a healthy population. Biomedical Engineering Online. 9(7) doi: 10.1186/1475-925X-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeCarlo LT. On the meaning and use of kurtosis. Psychological Methods. 1997;2(3):292–307. [Google Scholar]
- 52.Aboy M, Hornero R, Abásolo D, Álvarez D. Interpretation of the lempelziv complexity measure in the context of biomedical signal analysis. IEEE Transactions on Biomedical Engineering. 2006;53(11):2282–2288. doi: 10.1109/TBME.2006.883696. [DOI] [PubMed] [Google Scholar]
- 53.Hu J, Gao J, Principe JC. Analysis of biomedical signals by the lempel-ziv complexity: the effect of finite data size. IEEE Transactions on Biomedical Engineering. 2006;53(12):2606–2609. doi: 10.1109/TBME.2006.883825. [DOI] [PubMed] [Google Scholar]
- 54.Porta A, Guzzetti S, Montano N, Furlan R, Pagani M, Malliani A, Cerutti S. Entropy, entropy rate, and pattern classification as tools to typify complexity in short heart period variability series. IEEE Transactions on Biomedical Engineering. 2001;48(11):1282–1291. doi: 10.1109/10.959324. [DOI] [PubMed] [Google Scholar]
- 55.Porta A, Baselli G, Liberati D, Montano N, Cogliati C, Gnecchi-Ruscone T, Malliani A, Cerutti S. Measuring regularity by means of a corrected conditional entropy in sympathetic outflow. Biological Cybernetics. 1998;78(1):71–78. doi: 10.1007/s004220050414. [DOI] [PubMed] [Google Scholar]